Structural Characterization of New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues
Abstract
:1. Introduction
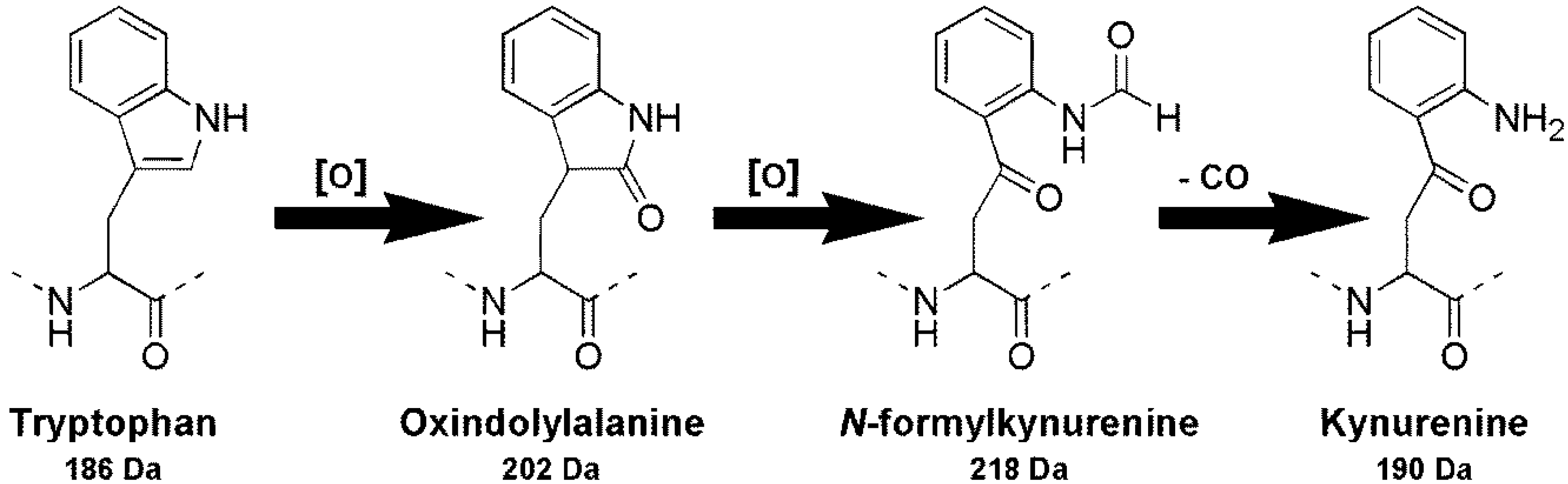
2. Results and Discussion
2.1. Liquid Chromatography-Tandem Mass Spectrometric Identification New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues
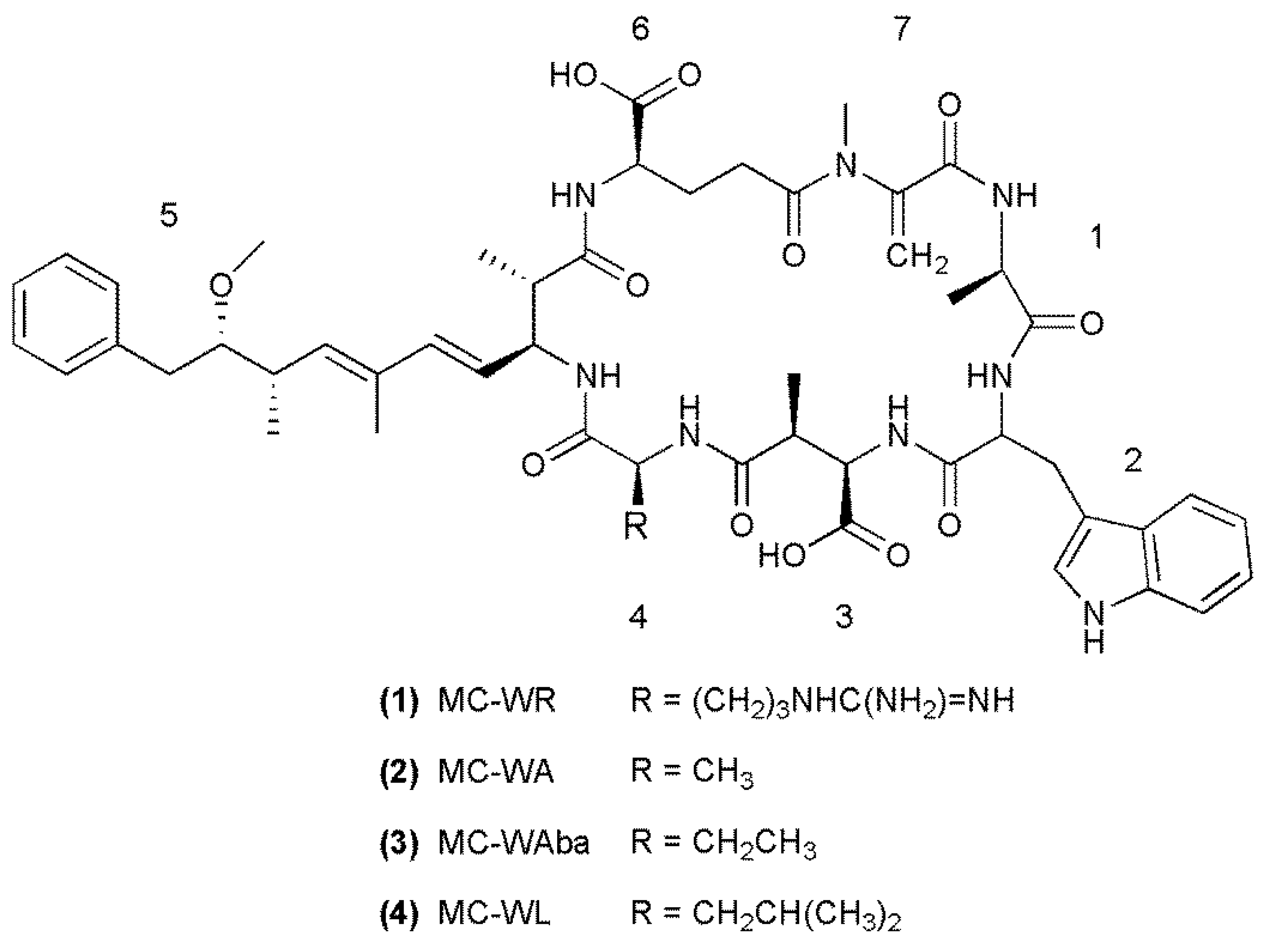
| Fragment Assignment a | MC-WA (2) | MC-WAba (3) | MC-WL (4) |
|---|---|---|---|
| M + H | 983 | 997 | 1025 |
| M − H2O + H | 965 | 979 | 1007 |
| M − Mdha − H2O + H | 882 | 896 | |
| M − Masp or Glu + H | 854 | 896 | |
| M − Adda sidechain + H | 849 | 863 | 891 |
| M − Adda sidechain − H2O + H | 831 | 845 | 873 |
| M − Adda + H | 670 | 684 | 712 |
| M − Adda − H2O + H | 652 | 666 | 694 |
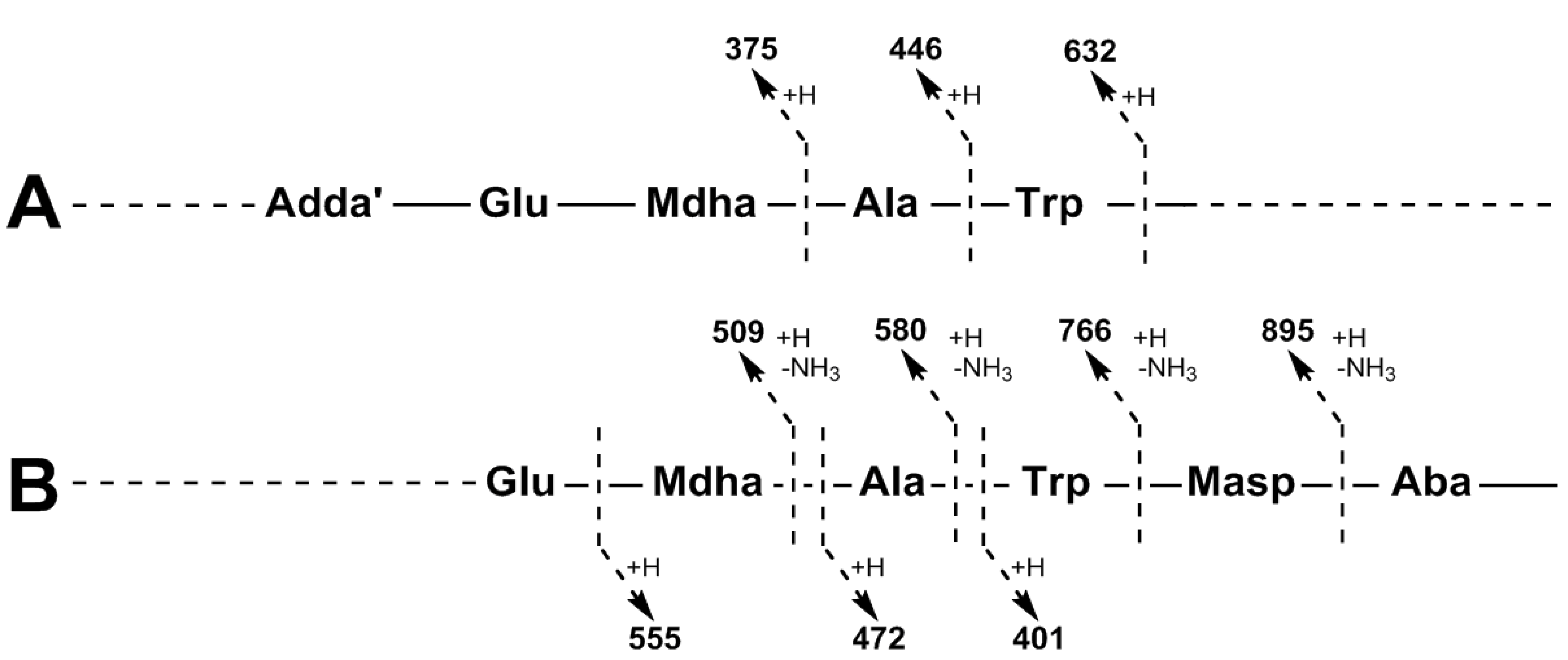
| Adda-Glu-Mdha-Ala-Trp-Masp − NH3 + H | 895 | 895 | |
| Adda-Glu-Mdha-Ala-Trp − NH3 + H | 766 | 766 | 766 |
| Adda-Glu-Mdha-Ala − NH3 + H | 580 | 580 | 580 |
| Adda-Glu-Mdha − NH3 + H | 509 | 509 | 509 |
| Adda′-Glu-Mdha-Ala-Trp + H | 632 | 632 | 632 |
| Adda-Glu-Mdha-Ala + H | 446 | 446 | 446 |
| Adda′-Glu-Mdha + H | 375 | 375 | 375 |
| Mdha-Ala-Trp-Masp-Z + NH4 | 558 | 572 | 600 |
| Ala-Trp-Masp-Z + NH4 | 475 | 489 | 517 |
| Trp-Masp-Z + NH4 | 404 | 418 | 446 |
| Mdha-Ala-Trp-Masp-Z + H | 541 | 555 | 583 |
| Ala-Trp-Masp-Z + H | 458 | 472 | 500 |
| Trp-Masp-Z + H | 387 | 401 | 429 |
| Microcystin | Mr a (Da) | Rt b (min) | X | Z | |
|---|---|---|---|---|---|
| MC-XR | MC-WR (1) | 1067.5 | 7.60 | 186 Da | Arg |
| MC-1071 (5) | 1071.5 | 7.45 | 190 Da | Arg | |
| MC-1083 (6) | 1083.5 | 7.37 | 202 Da | Arg | |
| MC-1099 (7) | 1099.5 | 7.32 | 218 Da | Arg | |
| MC-XA | MC-WA (2) | 982.5 | 9.54 | 186 Da | Ala |
| MC-986 (8) | 986.5 | 9.41 | 190 Da | Ala | |
| MC-998 (9) | 998.5 | 9.31 | 202 Da | Ala | |
| MC-1014 (10) | 1014.5 | 9.07 | 218 Da | Ala | |
| MC-XAba | MC-WAba (3) | 996.5 | 10.24 | 186 Da | Aba |
| MC-1000 (11) | 1000.5 | 9.80 | 190 Da | Aba | |
| MC-1012 (12) | 1012.5 | 9.58 | 202 Da | Aba | |
| MC-1028 (13) | 1028.5 | 9.46 | 218 Da | Aba | |
 | |||||
| Fragment Assignment a | MC-WR (1) | MC-KynR (5) | MC-OiaR (6) | MC-NfkR (7) |
|---|---|---|---|---|
| M + H | 1068 | 1072 | 1084 | 1100 |
| M − H2O + H | 1050 | 1054 | 1066 | 1082 |
| M − Ala + H | 997 | 1001 | 1013 | 1029 |
| M − CH2NHCN2H3 + H | 996 | 1000 | 1012 | 1028 |
| M − Glu or Masp + H | 939 | 943 | 955 | 971 |
| M − Adda sidechain + H | 934 | 938 | 950 | 966 |
| M − Adda + H | 755 | 759 | 771 | 787 |
| Masp-Arg-Adda − CO + H or Arg-Adda-Glu − CO + H | 571 | 571 | 571 | 571 |
| Masp-Arg-Adda-Glu + H | 728 | 728 | 728 | 728 |
| Masp-Arg-Adda + H or Arg-Adda-Glu + H | 599 | 599 | 599 | 599 |
| Arg-Adda + H | 470 | 470 | 470 | 470 |
| Arg-Adda-Glu − NH3 + H | 582 | 582 | 582 | 582 |
| Arg-Adda − NH3 + H | 453 | 453 | 453 | 453 |
| Mdha-Ala-X-Masp-Arg + H | 626 | 630 | 642 | 658 |
| Ala-X-Masp-Arg + H | 543 | 547 | 559 | 575 |
| X-Masp-Arg + H | 472 | 476 | 488 | 504 |
| Adda′-Glu-Mdha-Ala + H | 446 | 446 | 446 | 446 |
| Adda′-Glu-Mdha + H | 375 | 375 | 375 | 375 |
| Mdha-Ala-X-Masp + H | 470 | 474 | 486 | 502 |
| Mdha-Ala-X + H | 341 | 345 | 357 | 373 |
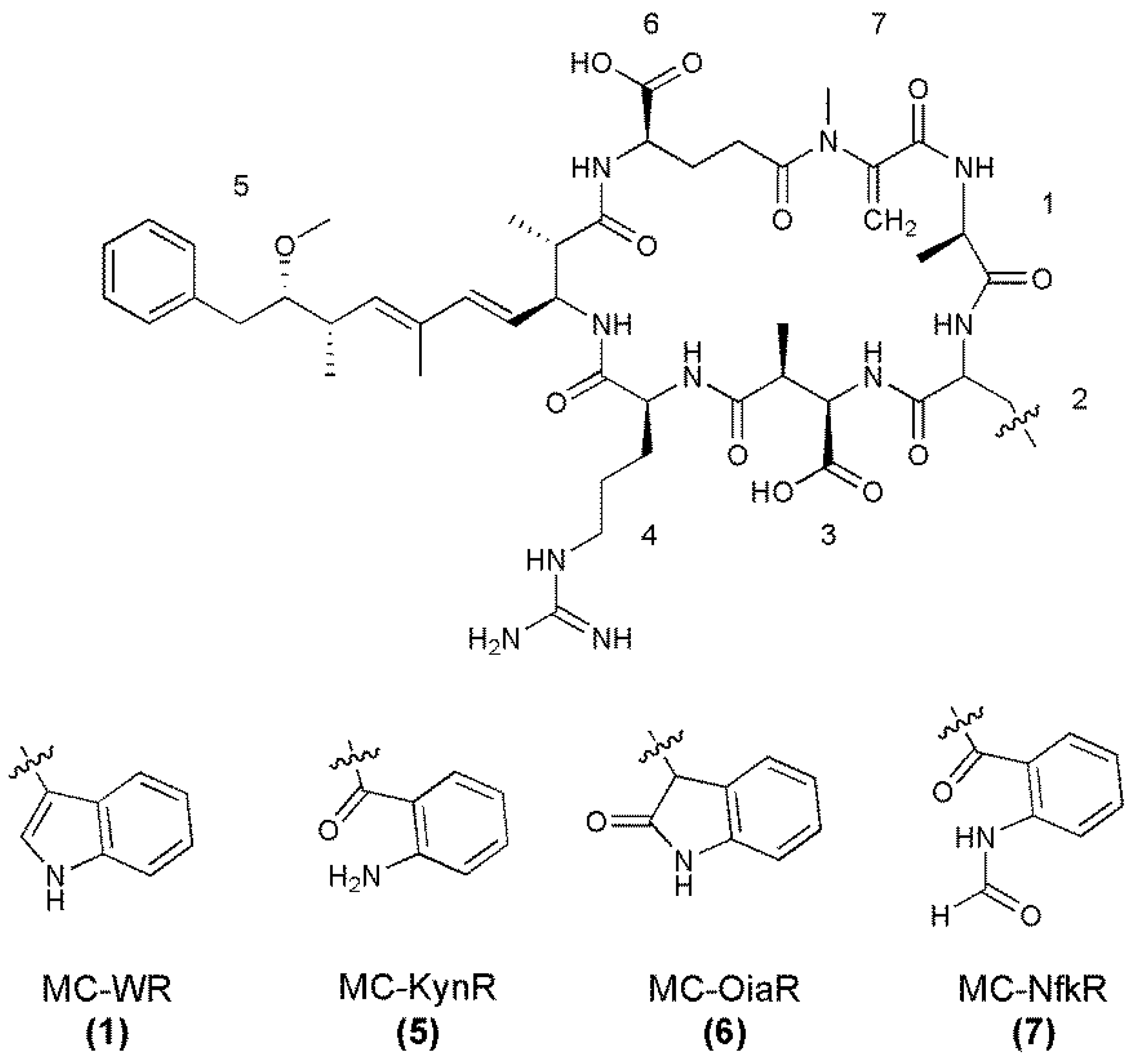
| Microcystin | Measured MH+ | Molecular Formula (MH+) | Calculated MH+ | Deviation (ppm) |
|---|---|---|---|---|
| MC-WR (1) | 1068.5465 | C54H74N11O12 | 1068.5513 | −4.5 |
| MC-OiaR (6) | 1084.5449 | C54H74N11O13 | 1084.5462 | −1.2 |
| MC-NfkR (7) | 1100.5449 | C54H74N11O14 | 1100.5411 | +3.4 |
| MC-KynR (5) | 1072.5431 | C53H74N11O13 | 1072.5462 | −2.9 |
| Fragment Assignment a | MC-WA (2) | MC-KynA (8) | MC-OiaA (9) | MC-NfkA (10) |
|---|---|---|---|---|
| M + H | 983 | 987 | 999 | 1015 |
| M − H2O + H | 965 | 969 | 981 | 997 |
| M − Mdha − H2O + H | 882 | 886 | 898 | 914 |
| M − Adda sidechain + H | 849 | 853 | 865 | 881 |
| M − Adda sidechain − H2O + H | 831 | 835 | 847 | 863 |
| M − Adda + H | 670 | 674 | 702 | |
| M − Adda − H2O + H | 652 | 656 | 668 | 684 |
| Adda-Glu-Mdha-Ala-X-Masp − NH3 + H | 895 | 899 | 911 | 927 |
| Adda-Glu-Mdha-Ala-X − NH3 + H | 766 | 770 | 782 | 798 |
| Adda-Glu-Mdha-Ala − NH3 + H | 580 | 580 | 580 | 580 |
| Adda-Glu-Mdha − NH3 + H | 509 | 509 | 509 | 509 |
| Adda′-Glu-Mdha-Ala-X + H | 632 | 636 | 648 | 664 |
| Adda′-Glu-Mdha-Ala + H | 446 | 446 | 446 | 446 |
| Adda′-Glu-Mdha + H | 375 | 375 | 375 | 375 |
| Mdha-Ala-X-Masp-Ala + H | 541 | 545 | 557 | 573 |
| Ala-X-Masp-Ala + H | 458 | 462 | 474 | 490 |
| X-Masp-Ala + H | 387 | 391 | 419 | |
| Mdha-Ala-X + H | 341 | 345 | 357 | 373 |

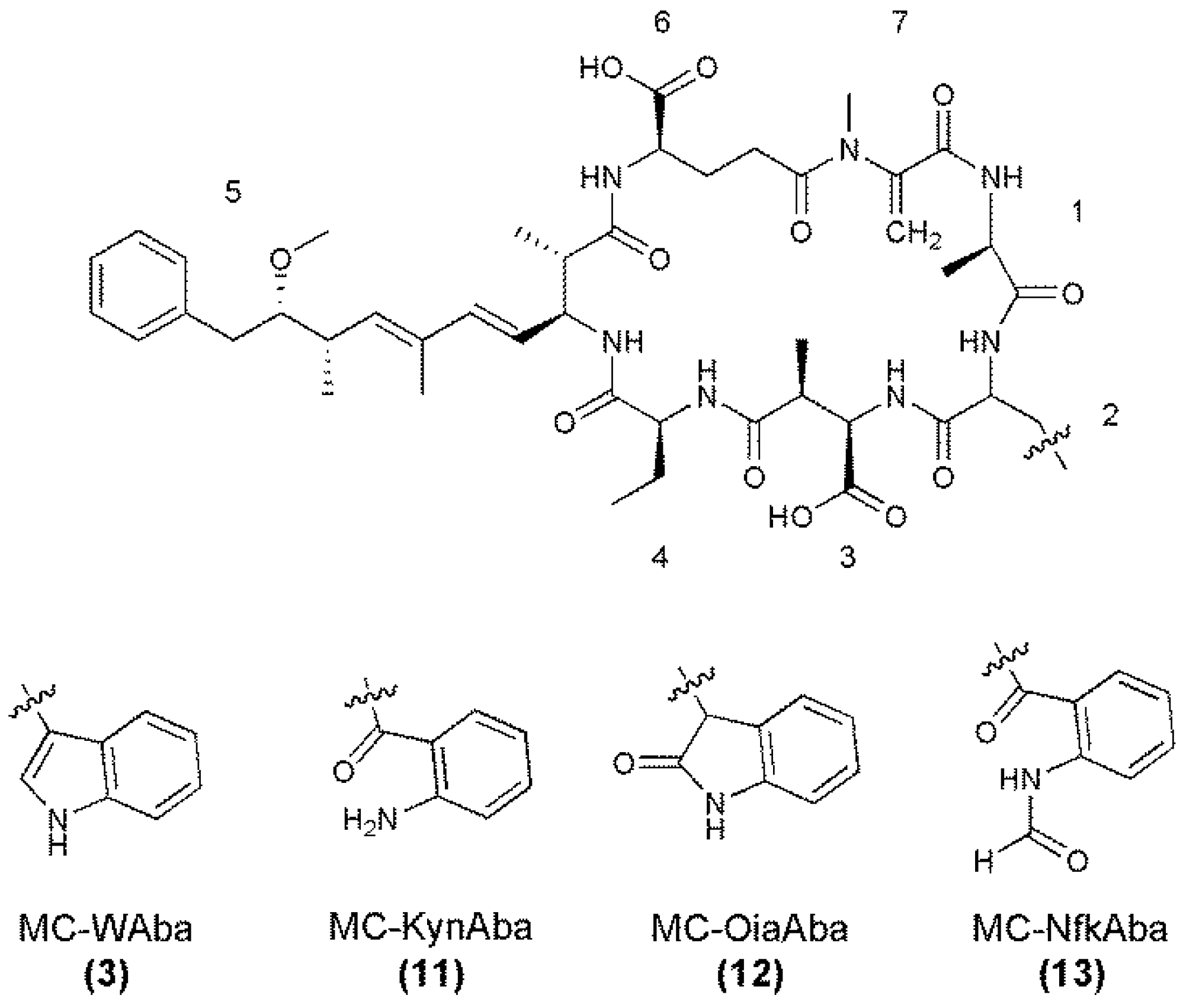
| Fragment Assignment a | MC-WAba (3) | MC-OiaAba (12) | MC-NfkAba (13) |
|---|---|---|---|
| M + H | 997 | 1013 | 1029 |
| M − H2O + H | 979 | 995 | 1011 |
| M − Mdha − H2O + H | 896 | 928 | |
| M − Aba − H2O + H | 910 | ||
| M − Glu or Masp − H2O + H | 850 | 866 | 878 |
| M − Adda sidechain + H | 863 | 879 | 895 |
| M − Adda sidechain − H2O + H | 845 | 861 | 877 |
| M − Adda + H | 684 | 700 | 716 |
| M − Adda − H2O + H | 666 | 698 | |
| Adda-Glu-Mdha-Ala-X-Masp − NH3 + H | 895 | 911 | |
| Adda-Glu-Mdha-Ala-X − NH3 + H | 766 | 782 | 798 |
| Adda-Glu-Mdha-Ala − NH3 + H | 580 | 580 | 580 |
| Adda-Glu-Mdha − NH3 + H | 509 | 509 | 509 |
| Adda′-Glu-Mdha-Ala-X + H | 632 | 648 | 664 |
| Adda′-Glu-Mdha-Ala + H | 446 | 446 | 446 |
| Adda′-Glu-Mdha + H | 375 | 375 | 375 |
| Mdha-Ala-X-Masp-Aba + H | 555 | 571 | 587 |
| Ala-X-Masp-Aba + H | 472 | 504 | |
| X-Masp-Aba + H | 401 | 417 | 433 |
2.2. Nuclear Magnetic Resonance Spectroscopy of MC-NfkA
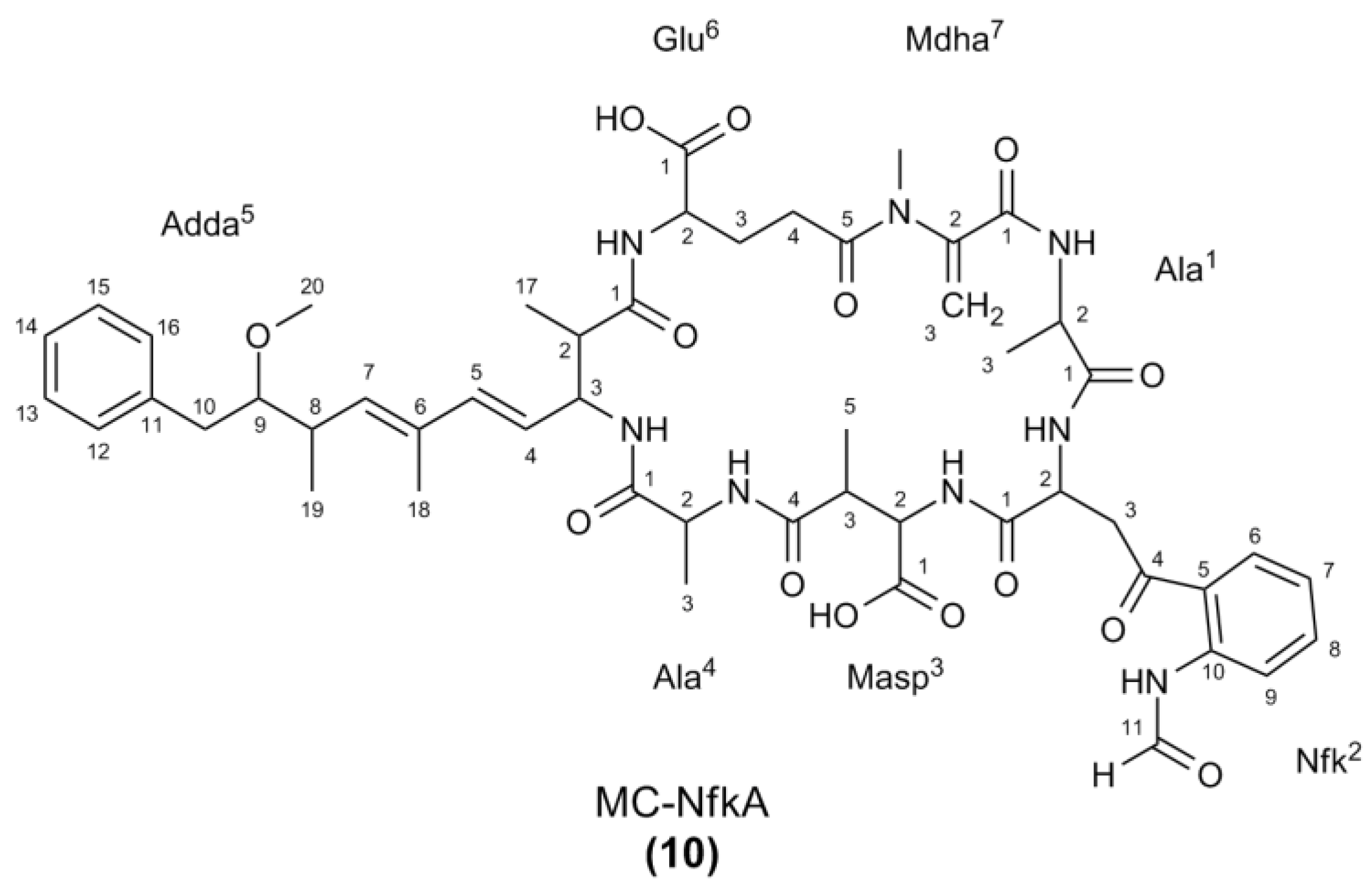
| Position a | δC | δH (J in Hz) | COSY | HMBC b | ROESY | |
|---|---|---|---|---|---|---|
| Ala1 | 1 | 175.7 | ||||
| 2 | 49.9 | 4.54, m | Ala1-3, NH | Ala1-1 | Ala1-3 | |
| 3 | 17.1 | 1.40, d (7.3) | Ala1-2 | Ala1-1, 2 | Ala1-2 | |
| NH | - | 8.22, d (8.5) | Ala1-2 | |||
| Nfk2 | 1 | Nd | ||||
| 2 | 55.9 | 4.26, m | Nfk-3, NH | |||
| 3 | 29.1 | 2.02 (2H), m | Nfk-2 | |||
| 4 | Nd | |||||
| 5 | 124.9 | |||||
| 6 | 132.4 | 8.06, d (7.2) d | Nfk-7 | Nfk-8, 10 | ||
| 7 | 125.5 | 7.24, dd (7.2, 8.6) d | Nfk-6, 8 | Masp-NH | ||
| 8 | 134.6 | 7.49, dd (8.0, 8.6) | Nfk-7, 9 | |||
| 9 | 122.2 | 8.49, d (8.0) | Nfk-8 | Nfk-5 | ||
| 10 | 139.5 | |||||
| 11 | 162.5 | 8.43, s | Nfk-10 | Nfk-CHO-NH | ||
| CHO-NH | - | 8.93, br s | Nfk-11 | |||
| NH | - | 8.32, d (6.8) | Nfk-2 | |||
| Masp3 | 1 | Nd | ||||
| 2 | 58.5 | 4.36, m | Masp-3, NH | Masp-5 | ||
| 3 | 42.3 | 3.14, m | Masp-2, 5 | Masp-5; Ala4-NH | ||
| 4 | 177.8 | - | ||||
| 5 | 15.8 | 1.06, d (6.9) | Masp-3 | Masp-2, 3, 4 | Masp-2, 3 | |
| NH | - | 8.06, d (8.3) | Masp-2 | |||
| Ala4 | 1 | 172.5 | - | |||
| 2 | 49.0 c | 4.41, m | Ala4-3, NH | Ala4-1 | Ala4-3; Adda-NH | |
| 3 | 17.5 | 1.26, d (7.3) | Ala4-2 | Ala4-1, 2 | Ala4-2, NH; Adda-NH | |
| NH | - | 8.72, d (9.2) | Ala4-2 | Ala4-3; Adda-NH; Masp-3 | ||
| Adda5 | 1 | 176.5 | - | |||
| 2 | 44.6 | 3.16, m | Adda-3, 17 | Glu-NH | ||
| 3 | 56.7 | 4.57, m | Adda-2, 4, NH | Adda-17 | Adda-17, NH | |
| 4 | 127.3 | 5.58, dd (9.3, 15.5) | Adda-3, 5 | Adda-5, 6 | Adda-18, NH | |
| 5 | 138.6 | 6.24, d (15.5) | Adda-4 | Adda-3, 6, 7, 18 | Adda-7 | |
| 6 | 136.0 | - | ||||
| 7 | 136.4 | 5.41, d (10.0) | Adda-8, 18 | Adda-5, 6, 8, 9 | Adda-5, 19 | |
| 8 | 37.7 | 2.59, m | Adda-7, 9, 19 | Adda-6 | Adda-19, 20 | |
| 9 | 88.4 | 3.25, m c | Adda-8, 10A, 10B | Adda-11, 19, 20 | Adda-19 | |
| 10 | 39.3 | 2.82, dd (4.7, 14.0) | Adda-9, 10B | Adda-8, 9, 11, 12/16 | Adda-10B, 12/16, 19 | |
| 2.68, dd (7.4, 14.0) | Adda-9, 10A | Adda-8, 9, 11, 12/16 | Adda-10A, 12/16, 19 | |||
| 11 | 140.3 | - | ||||
| 12/16 | 130.1 | 7.19, d (7.8) | Adda-13/15 | Adda-10, 14 | Adda-10A, 10B | |
| 13/15 | 128.7 | 7.24, dd (7.4, 7.8) | Adda-12/16, 14 | Adda-11 | ||
| 14 | 126.9 | 7.16, t (7.4) | Adda-13/15 | Adda-12/16 | ||
| 17 | 15.9 | 1.07, d (6.7) | Adda-2 | Adda-1, 3 | Adda-3 | |
| 18 | 12.9 | 1.64, s | Adda-7 | Adda-6, 7 | Adda-4 | |
| 19 | 16.4 | 1.00, d (6.9) | Adda-8 | Adda-7, 8, 9 | Adda-7, 8, 9, 10A, 10B | |
| 20 | 58.7 | 3.24, s | Adda-9 | Adda-8 | ||
| NH | - | 8.01, d (9.2) | Adda-3 | Adda-3, 4; Ala4-2, 3, NH | ||
| Glu6 | 1 | Nd | ||||
| 2 | 56.5 | 4.06, m | Glu-3A, 3B, NH | |||
| 3 | 29.7 | 2.22, m | Glu-2, 4A, 4B | Glu-3-B | ||
| 1.93, m | Glu-2, 4A, 4B | Glu-3A | ||||
| 4 | 33.5 | 2.66, m | Glu-3A, 3B, 4B | Glu-4B | ||
| 2.48, m | Glu-3A, 3B, 4A | Glu-4A | ||||
| 5 | 177.1 | |||||
| NH | - | 8.39, d (7.2) | Glu-2 | Adda-2 | ||
| Mdha7 | 1 | 165.9 | ||||
| 2 | 146.3 | |||||
| 3 | 113.6 | 5.71, s | Mdha-3B | Mdha-1 | Mdha-3B | |
| 5.27, s | Mdha-3A | Mdha-1, 2 | Mdha-3A | |||
| N-CH3 | 38.2 | 3.28, s c | Mdha-2; Glu-5 | |||
2.3. Oxidation of Tryptophan-Containing Microcystins
2.4. Presence of Intracellular Oxidized Tryptophan Microcystins
2.5. Implications of These Findings
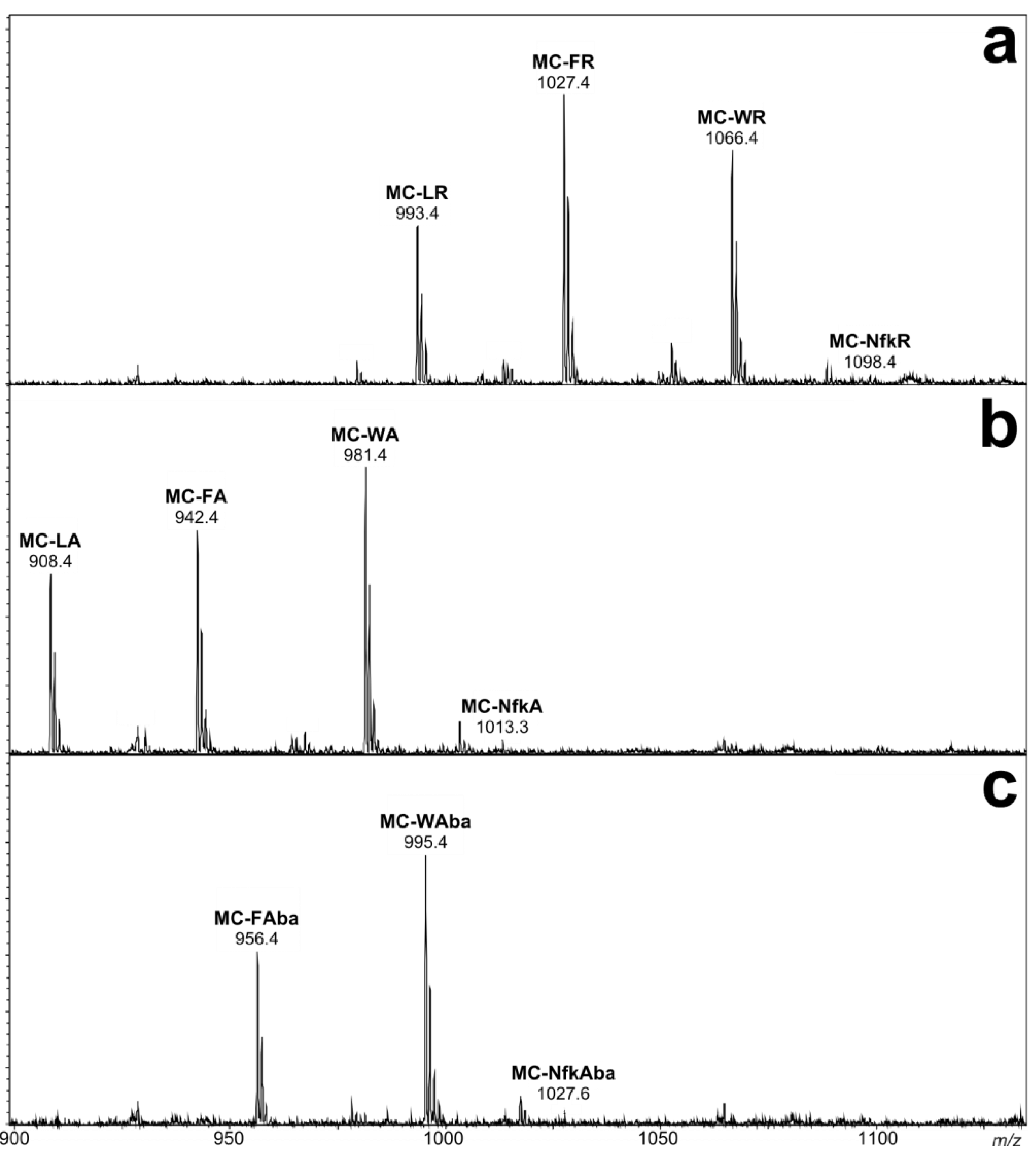
3. Experimental Section
3.1. General Experimental Procedures
3.2. Liquid Chromatography-Mass Spectrometry Analysis
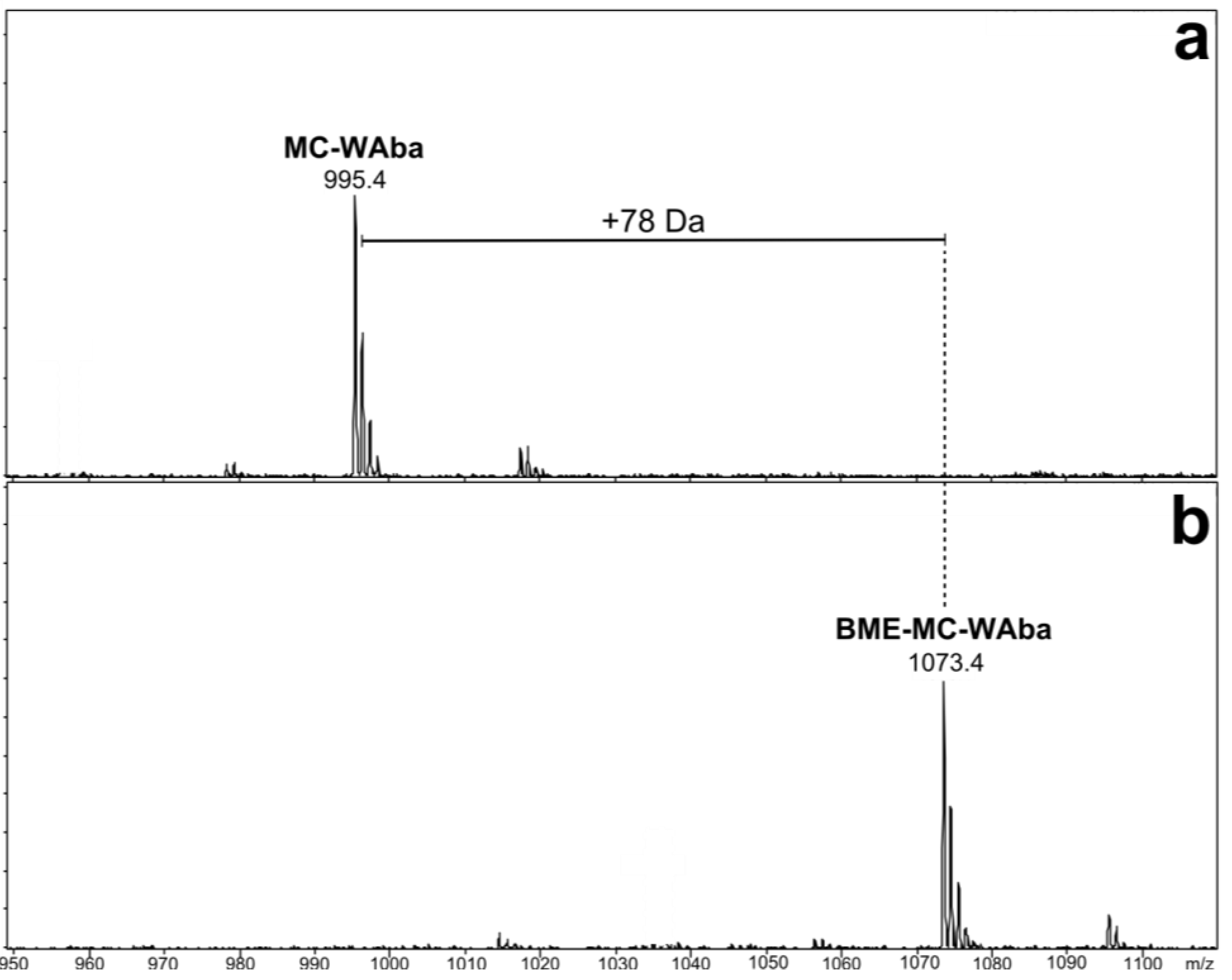
3.3. β-Mercaptoethanol Derivatization for Mdha/Dhb Determination
3.4. Isolation of the MC-XA Oxidized Tryptophan Congeners
3.5. Mild Extraction of Microcystis CAWBG11
3.6. Oxidation of Tryptophan-Containing Microcystins
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- An, J.; Carmichael, W.W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 1994, 32, 1495–1507. [Google Scholar] [CrossRef]
- Welker, M.; von Döhren, H. Cyanobacterial peptides: Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef]
- Rinehart, K.; Namikoshi, M.; Choi, B. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J. Appl. Phycol. 1994, 6, 159–176. [Google Scholar] [CrossRef]
- Hopkins, F.G.; Cole, S.W. A contribution for the chemistry of the protein materials. Part II. The constitution of the tryptophan and the effect of the bacteria on the latter. J. Physiol. 1903, 29, 451–466. [Google Scholar]
- Kotake, Y.; Iwao, J. Studies on the intermediary metabolism of tryptophan. I. Kynurenine, an intermediary metabolic product of tryptophan. Z. Physiol. Chem. 1931, 195, 139–145. [Google Scholar] [CrossRef]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharmacol. 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Taylor, S.W.; Fahy, E.; Murray, J.; Capaldi, R.A.; Ghosh, S.S. Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins. J. Biol. Chem. 2003, 278, 19587–19590. [Google Scholar]
- Simat, T.J.; Steinhart, H. Oxidation of free tryptophan and tryptophan residues in peptides and proteins. J. Agric. Food. Chem. 1998, 46, 490–498. [Google Scholar] [CrossRef]
- Finley, E.L.; Dillon, J.; Crouch, R.K.; Schey, K.L. Identification of tryptophan oxidation products in bovine α-crystallin. Protein Sci. 1998, 7, 2391–2397. [Google Scholar] [CrossRef]
- Bienvenut, W.V.; Déon, C.; Pasquarello, C.; Campbell, J.M.; Sanchez, J.-C.; Vestal, M.L.; Hochstrasser, D.F. Matrix-assisted laser desorption/ionization-tandem mass spectrometry with high resolution and sensitivity for identification and characterization of proteins. Proteomics 2002, 2, 868–876. [Google Scholar] [CrossRef]
- Lushchak, V. Free radical oxidation of proteins and its relationship with functional state of organisms. Biochemistry (Moscow) 2007, 72, 809–827. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef]
- Kuroda, M.; Sakiyama, F.; Narita, K. Oxidation of tryptophan in lysozyme by ozone in aqueous solution. J. Biochem. 1975, 78, 641–651. [Google Scholar]
- Namikoshi, M.; Rinehart, K.L.; Sakai, R.; Stotts, R.R.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W.; Evans, W.R. Identification of 12 hepatotoxins from a Homer Lake bloom of the cyanobacteria Microcystis aeruginosa, Microcystis viridis, and Microcystis wesenbergii: Nine new microcystins. J. Org. Chem. 1992, 57, 866–872. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Cary, S.C.; Hamilton, D.P.; Wilkins, A.L. Isolation and structure determination of two new hydrophobic microcystins from Microcystis sp. (CAWBG11). Phytochem. Lett. 2013. [Google Scholar] [CrossRef]
- Robillot, C.; Vinh, J.; Puiseux-Dao, S.; Hennion, M.-C. Hepatotoxin production kinetics of the cyanobacterium Microcystis aeruginosa PCC 7820, as determined by HPLC-mass spectrometry and protein phosphatase bioassay. Environ. Sci. Technol. 2000, 34, 3372–3378. [Google Scholar] [CrossRef]
- Bateman, K.P.; Thibault, P.; Douglas, D.J.; White, R.L. Mass spectral analyses of microcystins from toxic cyanobacteria using on-line chromatographic and electrophoretic separations. J. Chromatogr. A 1995, 712, 253–268. [Google Scholar] [CrossRef]
- Sano, T.; Kaya, K. Microcystin-AW, A new microcystin variant isolated from cyanobacterial waterbloom in Thailand. In Proceedings of the 9th International Conference on Harmful Algal Blooms, Tasmania, Australia, 7–11 February 2000.
- Lee, T.-H.; Chou, H.-N. Isolation and identification of seven microcystins from a cultured M.TN-2 strain of Microcystis aeruginosa. Bot. Bull. Academ. Sinica 2000, 41, 197–202. [Google Scholar]
- Puddick, J. Spectroscopic Investigations of oligopeptides from aquatic cyanobacteria: Characterisation of new oligopeptides, development of microcystin quantification tools and investigations into microcystin production. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand, 2013. [Google Scholar]
- Diehnelt, C.W.; Dugan, N.R.; Peterman, S.M.; Budde, W.L. Identification of microcystin toxins from a strain of Microcystis aeruginosa by liquid chromatography introduction into a hybrid linear ion trap-fourier transform ion cyclotron resonance mass spectrometer. Anal. Chem. 2006, 78, 501–512. [Google Scholar] [CrossRef]
- Miles, C.O.; Sandvik, M.; Haande, S.; Nonga, H.; Ballot, A. First use of LC-MS analysis with thiol derivatization to differentiate [Dhb7]- from [Mdha7]-microcystins: Analysis of cyanobacterial blooms, Planktothrix cultures and European crayfish from Lake Steinsfjorden, Norway. Environ. Sci. Technol. 2013, 47, 4080–4087. [Google Scholar] [CrossRef]
- Miles, C.O.; Sandvik, M.; Nonga, H.E.; Rundberget, T.; Wilkins, A.L.; Rise, F.; Ballot, A. Thiol derivatization for LC-MS identification of microcystins in complex matrices. Environ. Toxicol. 2012, 46, 8937–8944. [Google Scholar]
- Itakura, K.; Uchida, K.; Kawakishi, S. A novel tryptophan dioxygenation by superoxide. Tetrahedron Lett. 1992, 33, 2567–2570. [Google Scholar] [CrossRef]
- Quintanilla-Licea, R.; Colunga-Valladares, J.; Caballero-Quintero, A.; Rodríguez-Padilla, C.; Tamez-Guerra, R.; Gómez-Flores, R.; Waksman, N. NMR detection of isomers arising from restricted rotation of the C-N amide bond of N-formyl-o-toluidine and N,N'-bis-formyl-o-tolidine. Molecules 2002, 7, 662–673. [Google Scholar] [CrossRef]
- Botes, D.P.; Tuinman, A.A.; Wessels, P.L.; Viljoen, C.C.; Kruger, H.; Williams, D.H.; Santikarn, S.; Smith, R.J.; Hammond, S.J. The structure of cyanoginosin-LA, a cyclic heptapeptide toxin from the cyanobacterium Microcystis aeruginosa. Org. Bioorg. Chem. 1984, 10, 2311–2318. [Google Scholar]
- Harada, K.; Ogawa, K.; Matsuura, K.; Murata, H.; Suzuki, M.; Watanabe, M.F.; Itezono, Y.; Nakayama, N. Structural determination of geometrical isomers of microcystins LR and RR from cyanobacteria by two-dimensional NMR spectroscopic techniques. Chem. Res. Toxicol. 1990, 3, 473–481. [Google Scholar] [CrossRef]
- Harada, K.-I.; Ogawa, K.; Matsuura, K.; Nagai, H.; Murata, H.; Suzuki, M.; Itezono, Y.; Nakayama, N.; Shirai, M.; Nakano, M. Isolation of two toxic heptapeptide microcystins from an axenic strain of Microcystis aeruginosa, K-139. Toxicon 1991, 29, 479–489. [Google Scholar] [CrossRef]
- Christiansen, G.; Yoshida, W.Y.; Blom, J.F.; Portmann, C.; Gademann, K.; Hemscheidt, T.; Kurmayer, R. Isolation and structure determination of two microcystins and sequence comparison of the McyABC adenylation domains in Planktothrix species. J. Nat. Prod. 2008, 71, 1881–1886. [Google Scholar] [CrossRef]
- Park, H.; Namikoshi, M.; Brittain, S.M.; Carmichael, W.W.; Murphy, T. [D-Leu1] microcystin-LR, a new microcystin isolated from waterbloom in a Canadian prairie lake. Toxicon 2001, 39, 855–862. [Google Scholar] [CrossRef]
- Hummert, C.; Dahlmann, J.; Reinhardt, K.; Dang, H.; Dang, D.; Luckas, B. Liquid chromatography-mass spectrometry identification of microcystins in Microcystis aeruginosa strain from lake Thanh Cong, Hanoi, Vietnam. Chromatographia 2001, 54, 569–575. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R. MALDI-TOF mass spectrometry of cyanobacteria: A global approach to the discovery of novel secondary metabolites. Chem. N. Z. 2008, 72, 68–71. [Google Scholar]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar] [CrossRef]
- Ostakhov, S.S.; Kazakov, V.P.; Osina, I.O. A new mechanism of the photochemical oxidation of tryptophan sensitised with the uranyl ion. Mendeleev Commun. 2009, 19, 113–114. [Google Scholar] [CrossRef]
- Casbeer, E.M.; Sharma, V.K.; Zajickova, Z.; Dionysiou, D.D. Kinetics and mechanism of oxidation of tryptophan by ferrate (VI). Environ. Sci. Technol. 2013, 47, 4572–4580. [Google Scholar] [CrossRef]
- Rueckert, A.; Wood, S.A.; Cary, S.C. Development and field assessment of a quantitative PCR for the detection and enumeration of the noxious bloom-former Anabaena planktonica. Limnol. Oceanogr. Methods 2007, 5, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Cawthron Institute micro-algae culture collection. Available online: http://cultures.cawthron.org.nz (accessed on 6 June 2013).
- Bolch, C.; Blackburn, S. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Puddick, J.; Prinsep, M.R.; Wood, S.A.; Miles, C.O.; Rise, F.; Cary, S.C.; Hamilton, D.P.; Wilkins, A.L. Structural Characterization of New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues. Mar. Drugs 2013, 11, 3025-3045. https://doi.org/10.3390/md11083025
Puddick J, Prinsep MR, Wood SA, Miles CO, Rise F, Cary SC, Hamilton DP, Wilkins AL. Structural Characterization of New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues. Marine Drugs. 2013; 11(8):3025-3045. https://doi.org/10.3390/md11083025
Chicago/Turabian StylePuddick, Jonathan, Michèle R. Prinsep, Susanna A. Wood, Christopher O. Miles, Frode Rise, Stephen Craig Cary, David P. Hamilton, and Alistair L. Wilkins. 2013. "Structural Characterization of New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues" Marine Drugs 11, no. 8: 3025-3045. https://doi.org/10.3390/md11083025
APA StylePuddick, J., Prinsep, M. R., Wood, S. A., Miles, C. O., Rise, F., Cary, S. C., Hamilton, D. P., & Wilkins, A. L. (2013). Structural Characterization of New Microcystins Containing Tryptophan and Oxidized Tryptophan Residues. Marine Drugs, 11(8), 3025-3045. https://doi.org/10.3390/md11083025






