The Effect of Moderate-Intensity Physical Exercise on Some Serum Inflammation Markers and the Immune System in Rats Fed Intermittent Fasting with a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flow Cytometry
2.2. Exercise Training
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bishop, D.J.; Botella, J.; Genders, A.J.; Lee, M.J.; Saner, N.J.; Kuang, J.; Yan, X.; Granata, C. High-Intensity Exercise and Mitochondrial Biogenesis: Current Controversies and Future Research Directions. Physiology 2018, 34, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, R.J.H.M.; Eijsvogels, T.M.H.; Catoire, M.; Terink, R.; Ramakers, R.; Bongers, C.C.W.G.; Mensink, M.; Hermus, A.R.M.M.; Thijssen, D.H.J.; Hopman, M.T.E. Cytokine responses to repeated, prolonged walking in lean versus overweight/obese individuals. J. Sci. Med. Sport 2019, 22, 196–200. [Google Scholar] [CrossRef]
- Cullen, T.; Thomas, A.W.; Webb, R.; Hughes, M.G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: Theeffect of exercise intensity and volume. Appl. Physiol. Nutr. Metab. 2016, 41, 803–808. [Google Scholar] [CrossRef]
- Mosby, T.T.; Cosgrove, M.; Sarkardei, S.; Platt, K.L.; Kaina, B. Nutrition in adult and childhood cancer: Role of carcinogens and anti-carcinogens. Anticancer Res. 2012, 32, 4171–4192. [Google Scholar]
- Cani, P.D.; Bibiloni, C.; Knauf, A.; Waget, A.M. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Roohk, D.; Hellerstein, M.K. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J. Appl. Physio. 2007, 103, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Sasson, A.N.; Ananthakrishnan, A.N.; Raman, M. Diet in Treatment of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Hill, C.M.; Bitto, A.; Kaeberlein, M. Antiagingdiets: Separating fact from fiction. Science 2021, 374, eabe7365. [Google Scholar] [CrossRef]
- Elias, S.G.; Peeters, P.H.; Grobbee, D.E.; van Noord, P.A. Transient caloric restriction and cancer risk (The Netherlands). Cancer Causes Control 2007, 18, 1–5. [Google Scholar] [CrossRef]
- Adawi, M.; Watad, A.; Brown, S.; Aazza, K.; Aazza, H.; Zouhir, M.; Sharif, K.; Ghaneyem, K.; Farah, R.; Mahagna, H.; et al. Ramadan fasting exerts immunomodulatory effects: Insights from a systematic review. Front. Immunol. 2017, 27, 1144. [Google Scholar] [CrossRef]
- Trollmo, C.; Verdrengh, M.; Tarkowski, A. Fasting enhances mucosal antigen specific B cell responses in rheumatoid arthritis. Ann. Rheum. Dis. 1997, 56, 130–134. [Google Scholar] [CrossRef]
- Hiramoto, K.; Homma, T.; Jikumaru, M.; Miyashita, H.; Sato, E.F.; Inoue, M. Fasting differen tially modulates theimmunological system: Its mechanism and sex difference. J. Clin. Biochem. Nutr. 2008, 43, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Uden, A.M.; Trang, L.; Venizelos, N.; Palmblad, J. Neutrophil functions and clinical performance after total fasting in patients with rheumatoid arthritis. Ann. Rheum. Dis. 1983, 42, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardio vascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Leandro, C.G.; Castro, R.M.; Nascimento, E.; Pithon-Curi, T.C.; Curi, R. Adaptative mechanisms of the immune system in response to physical training. Rev. Bras. Med. Esporte 2007, 13, 343–348. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise-a systematic review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef]
- Tidball, J.G.; Dorshkind, K.; Wehling-Henricks, M. Shared signaling systems in myeloid cell-mediated muscle regeneration. Development 2014, 141, 1184–1196. [Google Scholar] [CrossRef]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef]
- Günbatar, N.; Bayıroğlu, F. The Effect of a Highly Saturated Fat Diet and Intermittent Fasting Diet on Experimental Colon Cancer Development and Some Serum Inflammation Markers in Rats. Van Vet. J. 2015, 26, 123–127. [Google Scholar]
- Kartinah, N.T.; Sianipar, I.R.; Nafi’ah; Rabia. The Effects of Exercise Regimens on Irisin Levels in Obese Rats Model: Comparing High-Intensity Intermittent with Continuous Moderate Intensity Training. Biomed. Res. Int. 2018, 2018, 4708287. [Google Scholar] [CrossRef]
- Rico, H.; Gervas, J.; Hernandez, E.R.; Seco, C.; Villa, L.F.; Revilla, M. Effect of alprazolam supplementation on vertebral and femoral bone mass in rats on strenuous treadmill training exercise. Calcif. Tissue Int. 1999, 65, 139–142. [Google Scholar] [CrossRef]
- Faloia, E.G.; Michetti, M.; de Robertis, M.P.; Luconi, G.; Furlani, M.B. Inflammation as a link between obesity and metabolic syndrome. J. Nut. Metab. 2012, 2012, 476380. [Google Scholar]
- Fairey, A.S.; Courneya, K.S.; Field, C.J.; Mackey, J.R. Physical exercise and immune system function in cancer survivors: A comprehensive review and future directions. Cancer 2002, 94, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A. Exercise and hypoxia: Effects on leukocytes and interleukin-6 shared mechanisms? Med. Sci. Sports Exerc. 2002, 34, 2004–2013. [Google Scholar] [CrossRef]
- Tidball, J.G. Infammatory processes in muscle injury and repair. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wolach, B.; Gavrieli, R.; Ben-Dror, S.G.; Zigel, L.; Eliakim, A.; Falk, B. Transient decrease of neutrophil chemotaxis following aerobic exercise. Med. Sci. Sports Exerc. 2005, 37, 949–954. [Google Scholar]
- Gleeson, M.; Bishop, N.; Walsh, N. Exercise Immunology; Routledge: Abingdon, UK, 2013; pp. 21–28. [Google Scholar]
- Parham, P. The İmmune System, 3rd ed.; Garland Science, Taylor & Francis Group: Abingdon, UK, 2019; pp. 12–18. [Google Scholar]
- Cissé, Y.M.; Borniger, J.C.; Lemanski, E.; Walker, W.H., 2nd; Nelson, R.J. Time-Restricted Feeding Alters the Innate Immune Response to Bacterial Endotoxin. J. Immunol. 2018, 200, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Mc Carthy, D.A.; Dale, M.M. The leucocytosis of exercise. A review and model. Sport Med. 1988, 6, 333–363. [Google Scholar] [CrossRef]
- Brines, R.; Hoffman-Goetz, L.; Pedersen, B.K. Can you exercise to make your immune system fitter? Immunol. Today 1996, 17, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Nieman, D.C. Exercise and immunology: Integration and regulation. Immunol. Today 1998, 19, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Suzuki, N.; Imai, T.; Aizawa, K.; Nanba, H.; Hanaoka, Y.; Kuno, S.; Mesaki, N.; Kono, I.; Akama, T. Monocyte and Tcell responses to exercise training in elderly subjects. J. Strength Cond. Res. 2011, 25, 2565–2572. [Google Scholar] [CrossRef]
- Woods, J.A.; Ceddia, M.A.; Wolters, B.W.; Evans, J.K.; Lu, Q.; Mc Auley, E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech. Ageing Dev. 1999, 109, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Hackney, A.C.; McMurray, R.G.; Randell, S.H.; Muss, H.B.; Deal, M.A.; and Battaglini, C.L. Impact of Acute Intermittent Exercise on Natural Killer Cells in Breast Cancer Survivors. Integr. Cancer Ther. 2015, 14, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Ladha, A.B.; Courneya, K.S.; Bell, G.J.; Field, C.J.; Grundy, P. Effects of acute exercise on neutrophils in pediatric acute lymphoblastic leukemia survivors: A pilot study. J. Pediatr. Hematol. Oncol. 2006, 28, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.A.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef]
- Latifynia, A.; Vojgani, M.; Gharagozlou, M.J.; Sharifian, R. Neutrophil function (innate immunity) during Ramadan. J. Ayub. Med. Coll. Abbottabad 2009, 21, 111–115. [Google Scholar]
- Nasiri, J.; Khoshdel, A.; Kheiri, S.; Jafari Boroujeni, A. The effect of Ramadan fasting on tuberculin skin test and leukocyte count. J. Fast. Health 2017, 5, 1–5. [Google Scholar]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Faietti, M. Impact of Time-restricted Feeding and Exercise on Immunity in Male C57BL/6 Mice. Electron. Theses Diss. 2020, 4–28. Available online: https://digitalcommons.memphis.edu/etd/2069/ (accessed on 28 April 2020).
- Peake, J.M. Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: Possible mechanisms of action. Exerc. Immunol. Rev. 2020, 8, 49–100. [Google Scholar]
- Tang, J.; Zhu, Q.; Li, Z.; Yang, J.; Lai, Y. Natural Killer Cell-targeted Immuno therapy for Cancer. Curr. Stem. Cell. Res. Ther. 2022, 17, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C. The T cell and NK cell immune response to exercise. Ann. Transpl. 2005, 44, 1044–1148. [Google Scholar]
- Toffoli, E.C.; Sweegers, M.G.; Bontkes, H.J.; Altenburg, T.M.; Verheul, H.M.W.; van der Vliet, H.J.; de Gruijl, T.D.; Buffart, L.M. Effects of physical exercise on natural killer cell activity during (neo) adjuvant chemotherapy: A randomized pilot study. Physiol. Rep. 2021, 9, e14919. [Google Scholar] [CrossRef]
- Scharhag, J.; Meyer, T.; Gabriel, H.H.W.; Schlick, B.; Faude, O.; Kindermann, W. Does prolonged cycling of moderate intensity affect immune cell function? Br. J. Sports Med. 2005, 39, 171–177. [Google Scholar] [CrossRef]
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Markoff, P.A.; Balk-Lamberron, A.J.; Yang, H.; Chriaon, D.B.W.J.; Lee, J.W.; Arabafzis, K. The Effects of Moderate Exercise Training on Natural Killer Cells and Acute Upper Respiratory Tract Infections. Int. J. Sports Med. 1990, 11, 467–473. [Google Scholar] [CrossRef]
- Kendall, A.; Hoffman-Goetz, L.; Houston, M.; MacNeil, B.; Arumugam, Y. Exercise and blood lymphocyte subset responses: Intensity, duration, and subject fitness effects. J. App. Physiol. 1990, 69, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Miller, A.R.; Henson, D.A. Effects of high- vs. moderate-intensity exercise on natural killer cell activity. Med. Sci. Sports Exerc. 1993, 25, 1126–1134. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, M.S.; Pae, M.; Yamamoto, Y.; Eberlé, D.; Shimada, T.; Kamei, N.; Park, H.S.; Sasorith, S.; Woo, J.R.; et al. Adipose naturell killer cell regulate adipose tissue macrophages to promate ınsulin resistance ın obesity. Cell. Metab. 2016, 12, 685–698. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Mori, M.A.; Lord, J.M. Effect of Exercise on Acute Senescent Lymphocyte Counts: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 961–975. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 1993, 25, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Baj, Z.; Kantorski, J.; Majewska, E.; Zeman, K.; Pokoca, L.; Fornalczyk, E.; Tchórzewski, H.; Sulowska, Z.; Lewicki, R. Immunological status of competitive cyclists before and after the training season. Int. J. Sports Med. 1994, 15, 319–324. [Google Scholar] [CrossRef]
- Nieman, D.C.; Brendle, D.; Henson, D.A.; Suttles, J.; Cook, V.D.; Warren, B.J.; Butter Worth, D.E.; Fagoaga, O.R.; Nehlsen–Cannarella, S.L. Immune function in athletes versus nonathletes. Int. J. Sports Med. 1995, 16, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Campell, P.T.; Wener, M.H.; Sorensen, B.; Wood, B.; Chen-Levy, Z.; Potter, J.D.; Mc Tiernan, A.; Ulrich, C.M. Effect of exercise on in vitro immune function: A 12-month randomized, controlled trial among postmenepausal women. J. Appl. Physiol. 2008, 104, 1648–1655. [Google Scholar] [CrossRef]
- Sitlinger, A.; Brander, D.M.; and Bartlett, D.B. Impact of exercise on the immune system and out comes in hematologic malignancies. Blood Adv. 2020, 4, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the myth of exercise-induced immunesuppression: Redefining the impact of exercise on immunological health across the life span. Front. Immunolgy. 2018, 9, 648. [Google Scholar] [CrossRef]
- Natale, V.M.; Brenner, I.K.; Moldoveanu, A.I.; Vasiliou, P.; Shek, P.; Shephard, R.J. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med. J. 2003, 121, 9–14. [Google Scholar] [CrossRef]
- Clemmons, D.R. Role of IGF-binding proteins in regulating IGF responses to changes in metabolism. J. Mol. Endocrinol. 2018, 61, 139–169. [Google Scholar] [CrossRef]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2002, 92, 1472–1489. [Google Scholar] [CrossRef]
- Bjersing, J.L.; Larsson, A.; Palstam, A.; Ernberg, M.; Bileviciute-Ljungar, I.; Löfgren, M.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Benefits of resistance exercise in lean women with fibromyalgia: Involvement of IGF-1 and leptin. BMC Musculoskelet Disord. 2017, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Valkeinen, H.; Hakkinen, K.; Pakarinen, A.; Hannonen, P.; Hakkinen, A.; Airaksinen, O.; Niemitukia, L.; Kraemer, W.J.; Alen, M. Muscle hypertrophy, strength development, and serum hormones during strength trainingin elderly women with fibromyalgia. Scand. J. Rheumatol. 2005, 34, 309–314. [Google Scholar] [CrossRef]
- Herbert, P.; Hayes, L.D.; Sculthorpe, N.; Grace, F.M. High-intensity interval training (HIIT) increases insulin-like growth factor-I (IGF-I) in sedentary aging men but not masters’ athletes: An observational study. Aging Male 2017, 20, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.J.; de Mello, M.A.; Caetano, F.H.; Sibuya, C.Y.; Anaruma, C.A.; Rogatto, G.P.; Pauli, J.R.; Luciano, E. Effects of swimming training on bone mass and the GH/IGF-1 axis in diabetic rats. Growth Horm. IGF Rec. 2006, 16, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Özbeyli, D.; Sarı, G.; Özkan, N.; Karademir, B.; Yüksel, M.; Çilingir Kaya, Ö.T.; Kasımay Çakır, Ö. Protective effect of different exercise modalities in an alzheımer’s disease-like model. Behav. Brain Res. 2017, 328, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.E.; Kari, F.W.; French, J.; Leininger, J.R.; Travlos, G.; Wilson, R.; Barrett, J.C. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997, 57, 4667–4672. [Google Scholar]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; and Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Anwar, A.; Zahid, A.A.; Scheidegger, K.J.; Brink, M.; Delafontaine, P. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vasculars mooth muscle. Circulation 2002, 105, 1220–1225. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptionalregulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Maynard, C.L. Contrasting roles for all-trans retinoic acid in TGF-[beta]-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med. 2009, 206, 343–357. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 23, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Gholamnezhad, Z.; Boskabady, M.H.; Hosseini, M.; Sankian, M.; KhajaviRad, A. Evaluation of immune response after moderate and over training exercise in wistar rat. J. Immunol. 2004, 172, 7713–7720. [Google Scholar]
- Ostrowski, K.; Rohde, T.; Asp, P.; Schjerling, P.; Pedersen, B.K. Pro-and anti-inflammatory cytokine balance in strenuous exercise in human. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Santos, C.; Gerosa-Neto, J.; Inoue, D.S.; Panissa, V.L.; Gobbo, L.A.; Zagatto, A.M.; Campos, E.Z.; Lira, F.S. Similar anti-inflammatory acute responses from moderate intensity continuous and high-intensity intermittent exercise. J. Sports Sci. Med. 2015, 14, 849–856. [Google Scholar]
- Elosua, R.; Bartali, B.; Ordovas, J.M.; Corsi, A.M.; Lauretani, F.; Ferrucci, L. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 760–767. [Google Scholar] [CrossRef]
- Helmark, I.C.; Mikkelsen, U.R.; Jens Børglum, J.; Rothe, A.; Petersen, M.C.H.; Andersen, O.; Langberg, H.; Kjaer, M. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: A randomized controlled trial. Arthritis Res. Ther. 2010, 12, R126. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Yshii, L.M.; Viel, T.A.; Buck, H.S.; Mattson, M.P.; Scavone, C.; Kawamoto, E.M. Intermittent fasting attenuates lipopolysaccharide induced neuro inflammation and memory İmpairment. J. Neuroinflamm. 2014, 11, 85. [Google Scholar] [CrossRef]
- Sharman, M.J.; Volek, J.S. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in over weight men. Clin. Sci. 2004, 107, 365–369. [Google Scholar] [CrossRef]
- Ugochukwu, N.H.; Figgers, C.L. Caloric restriction inhibit sup-regulation of inflammatory cytokines and TNF-alpha, and activates IL-10 and haptoglobin in the plasma of streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2007, 18, 120–126. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Stachura, J.; Konturek, P.C. IGF-1 stimulates production of interleukin-10 and inhibits development of caerulein-induced pancreatitis. J. Physiol. Pharmacol. 2003, 54, 575–590. [Google Scholar] [PubMed]
- Higashi, Y.; Sukhanov, S.; Shai, S.Y.; Danchuk, S.; Tang, R.; Snarski, P.; Li, Z.; Lobelle-Rich, P.; Wang, M.; Wang, D.; et al. Insulin-Like Growth Factor-1 Receptor Deficiency in Macrophages Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Apolipoprotein E-Deficient Mice. Circulation 2016, 133, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Harpal, S.; Taranjeet, K.; Shaffi, M.; Gurcharan, K. Intermittent fasting combined with supplementation with A yurvedic herbs reduces anxiety in middle aged female rats by anti-inflammatory path ways. Biogerontol 2017, 18, 601–614. [Google Scholar]
- Strle, K.; Zhou, J.H.; Shen, W.H.; Broussard, S.R.; Johnson, R.W.; Freund, G.G.; Dantzer, R.; Kelley, K.W. Interleukin-10 in the brain. Crit. Rev. Immunol. 2001, 21, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and TNP receptor super family: Structure-function relationship. Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Strangfeld, A.; Listing, J.; Herzer, P.; Liebhaber, A.; Rockwitz, K.; Richter, C.; Zink, A. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 2009, 301, 737–744. [Google Scholar] [CrossRef]
- Wauters, M.; Considine, R.V.; Van Gaal, L.F. Human leptin: From an adipocyte hormone to an endocrine mediator. Eur. J. Endocrinol. 2000, 143, 293–311. [Google Scholar] [CrossRef]
- Mito, N.; Yoshino, H.; Hosoda, T.; Sato, K. Analysis of theeffect of leptin on immune function in vivo using diet- induced obese mice. J. Endocrinol. 2004, 180, 167–173. [Google Scholar] [CrossRef]
- Dimitrov, S.; Hulteng, E.; Hong, S. Inflammation and Exercise Inhibition of Monocytic Intracelluler Tnf Production by Acute Exercise Vıa B2-Adrenergic Activation. Brain Behav. Immun. 2017, 61, 60–68. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Carè, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Guletta, E. Effects of acute physical exercise on oxidative stres and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef]
- Colbert, L.H.; Visser, M.; Simonsick, E.M.; Tracy, R.P.; Newman, A.B.; Kritchevsky, S.B.; Pahor, M.; Taaffe, D.R.; Brach, J.; Rubin, S.; et al. Physical activity, exercise, and inflammatory markers in olderadults: Findings from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2004, 52, 1098–1104. [Google Scholar] [CrossRef]
- Jiménez-Maldonado, A.; Montero, S.; Lemus, M.; Cerna-Cortés, J.; Rodríguez-Hernández, A.; Mendoza, M.A.; Melnikov, V.; Gamboa-Domínguez, A.; Muñiz, J.; Virgen-Ortiz, A.; et al. Moderate and high intensity chronic exercise reduces plasma tumor necrosis factor alpha and increases the Langerhans islet are a in healthy rats. J. Musculoskelet Neuronal. Interact. 2019, 19, 354–361. [Google Scholar]
- Park, S.; Park, N.Y.; Valacchi, G.; Lim, Y. Calorie restriction with a high-Fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediat. Inflamm. 2012, 2012, 984643. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [PubMed]
- te Velde, A.A.; Huijbens, R.J.; Heije, K.; de Vries, J.E.; Figdor, C.G. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood 1990, 76, 1392–1397. [Google Scholar] [CrossRef]
- Zamani, A.; Salehi, I.; and Alahgholi-Hajibehzad, M. Moderate Exercise Enhances the Production of Interferon-γ and Interleukin-12 in Peripheral Blood Mononuclear Cells. Immune Netw. 2017, 17, 186–191. [Google Scholar] [CrossRef]
- Conroy, S.M.; Courneya, K.S.; Brenner, D.R.; Shaw, E.; O’Reilly, R.; Yasui, Y.; Woolcott, C.G.; Friedenreich, C.M. Impact of aerobicexercise on levels of IL-4and IL-10: Results from two randomized intervention trials. Cancer Med. 2016, 5, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Jiffri, O.H.; Ashmawy, E.M.; Gaowgzeh, R.A. Treadmill walking exercise modulates bone mineral status and inflammatorycytokines in obese asthmatic patients with long term intake of corticosteroids. Afr. Health Sci. 2016, 16, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, 437–450. [Google Scholar] [CrossRef]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy—Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef]
- Kohut, M.L.; Boehm, G.W.; Moynihan, J.A. Moderate exercise is associated with enhanced antigen-specific cytokine, but not IgM antibody production in agedmice. Mech. Ageing Dev. 2001, 122, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, B.F.C.; Zanluqui, N.G.; de AtaidesRaquel, H.; Lovo-Martins, M.I.; Tatakihara, V.L.H.; de OliveiraBelém, M.; Michelini, L.C.; de Almeida Araújo, E.J.; Pinge-Filho, P.; Martins-Pinge, M.C. Moderate Treadmill Exercise Training Improves Cardiovascular and Nitrergic Response and Resistance to Trypanosomacruzi Infection in Mice. Front. Physiol. 2017, 18, 315. [Google Scholar] [CrossRef]
- Abedelmalek, S.; Souissi, N.; Takayuki, A.; Hadouk, S.; and Tabka, Z. Effect of Acute Maximal Exercise on Circulating Levels of Interleukin-12 during Ramadan Fasting. Asian J. Sports Med. 2011, 2, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.S.; Khan, N.; Boddupalli, C.S.; Hasnain, S.E.; Mukhopadhyay, S. Interleukin-10 (IL-10) mediated suppression of IL-12 production in RAW 264.7 cells also involves c-rel transcription factor. Immuology 2005, 114, 313–321. [Google Scholar]


| Antibody | Cell Type | Fluorophore | Clone | Isotype |
|---|---|---|---|---|
| Anti-rat CD4 antibody | T cell | FITC | RM4-5 | Mouse IgG2a K |
| Anti-rat CD56 antibody | NK cell | PE | NCAM-1 | Mouse IgG1 K |
| Anti-rat CD19 antibody | B cell | APC | SJ25C1 | Mouse IgG1 K |
| Group | Median (Min–Max) | p | |
|---|---|---|---|
| WBC/mm3 | CG | 7.41 (6.16–9.35) ab | 0.003 * |
| CG + IF | 5.26 (0.00–8.28) b | ||
| CG + E | 7.85 (6.26–10.65) ab | ||
| CG + IF + E | 6.92 (4.50–9.05) ab | ||
| HFD | 8.36 (5.40–13.27) ab | ||
| HFD + IF | 9.67 (6.86–13.01) a | ||
| HFD + E | 11.04 (8.35–14.45) a | ||
| HFD + IF + E | 10.18 (6.85–11.82) a | ||
| CG | 1.23 (0.98–1.45) c | 0.001 * | |
| CG + IF | 1.16 (0.42–2.15) c | ||
| CG + E | 1.40 (0.95–1.96) c | ||
| Lymphocyte/mm3 | CG + IF + E | 1.04 (0.60–1.48) c | |
| HFD | 0.18 (0.10–0.41) d | ||
| HFD + IF | 2.07 (1.48–2.93) ab | ||
| HFD + E | 2.24 (1.39–2.78) a | ||
| HFD + IF + E | 2.32 (1.63–2.72) a | ||
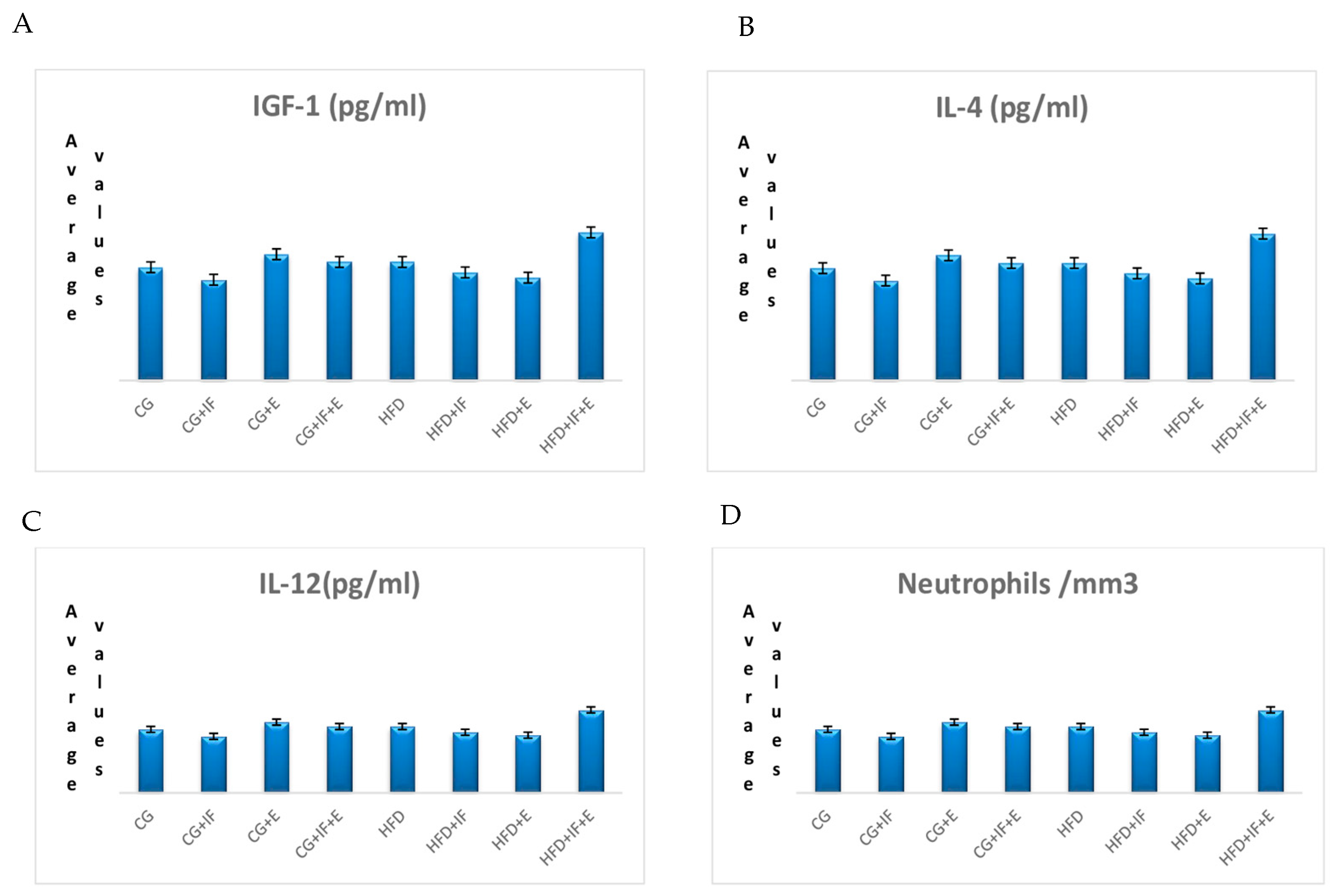
| Neutrophils/mm3 | CG | 3.60 (2.64–5.58) | 0.115 |
| CG + IF | 2.81 (0.42–4.30) | ||
| CG + E | 3.77 (2.19–5.50) | ||
| CG + IF + E | 3.64 (2.21–4.86) | ||
| HFD | 3.98 (2.08–6.09) | ||
| HFD + IF | 4.34 (2.97–6.13) | ||
| HFD + E | 5.37 (4.09–7.62) | ||
| HFD + IF + E | 4.39 (2.53–5.42) | ||
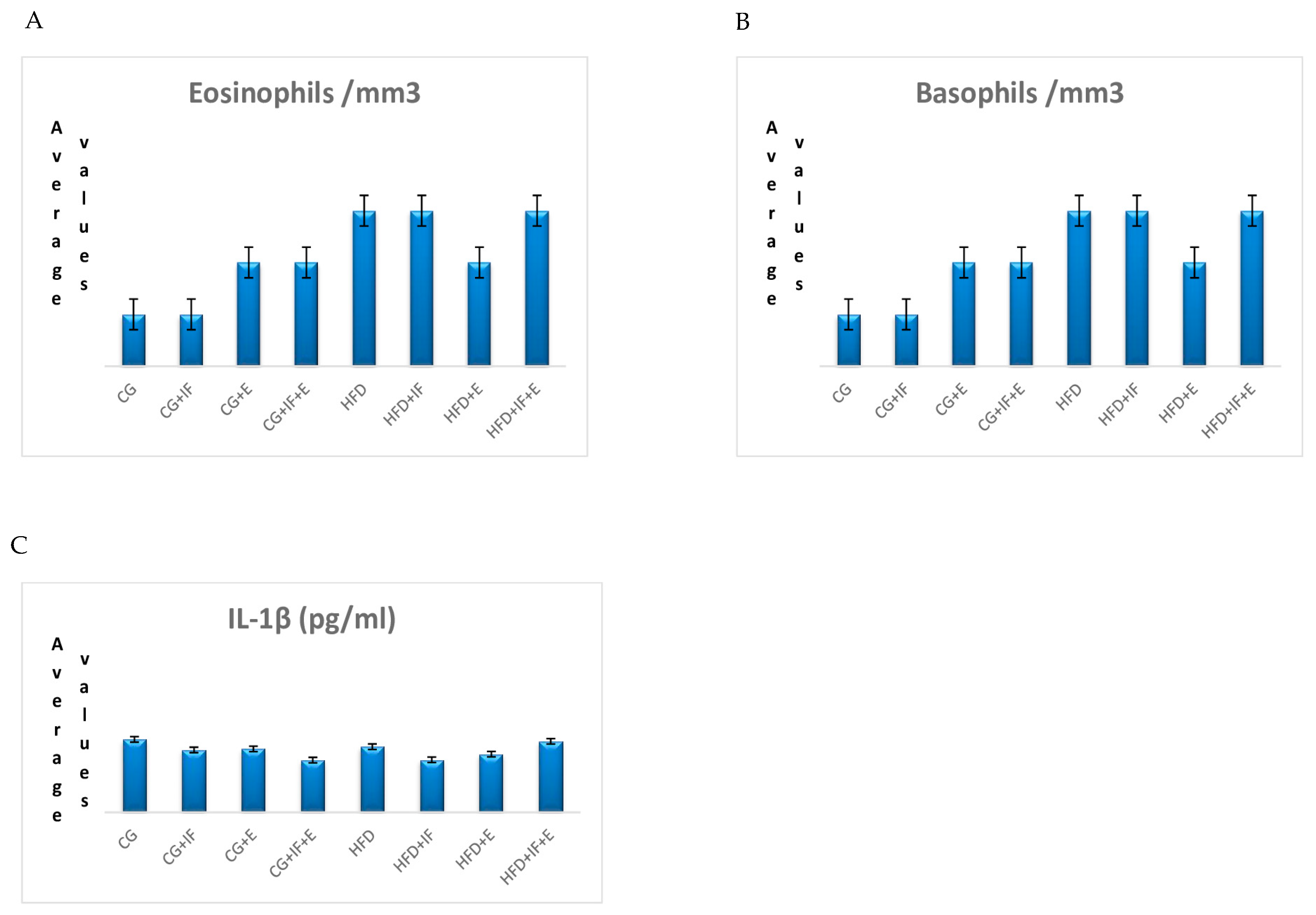
| Basophils/mm3 | CG | 0.01 (0.01–0.02) | 0.723 |
| CG + IF | 0.01 (0.00–0.04) | ||
| CG + E | 0.02 (0.01–0.07) | ||
| CG + IF + E | 0.02 (0.00–0.05) | ||
| HFD | 0.03 (0.00–0.08) | ||
| HFD + IF | 0.03 (0.02–0.05) | ||
| HFD + E | 0.02 (0.01–0.06) | ||
| HFD + IF + E | 0.03 (0.01–0.07) | ||
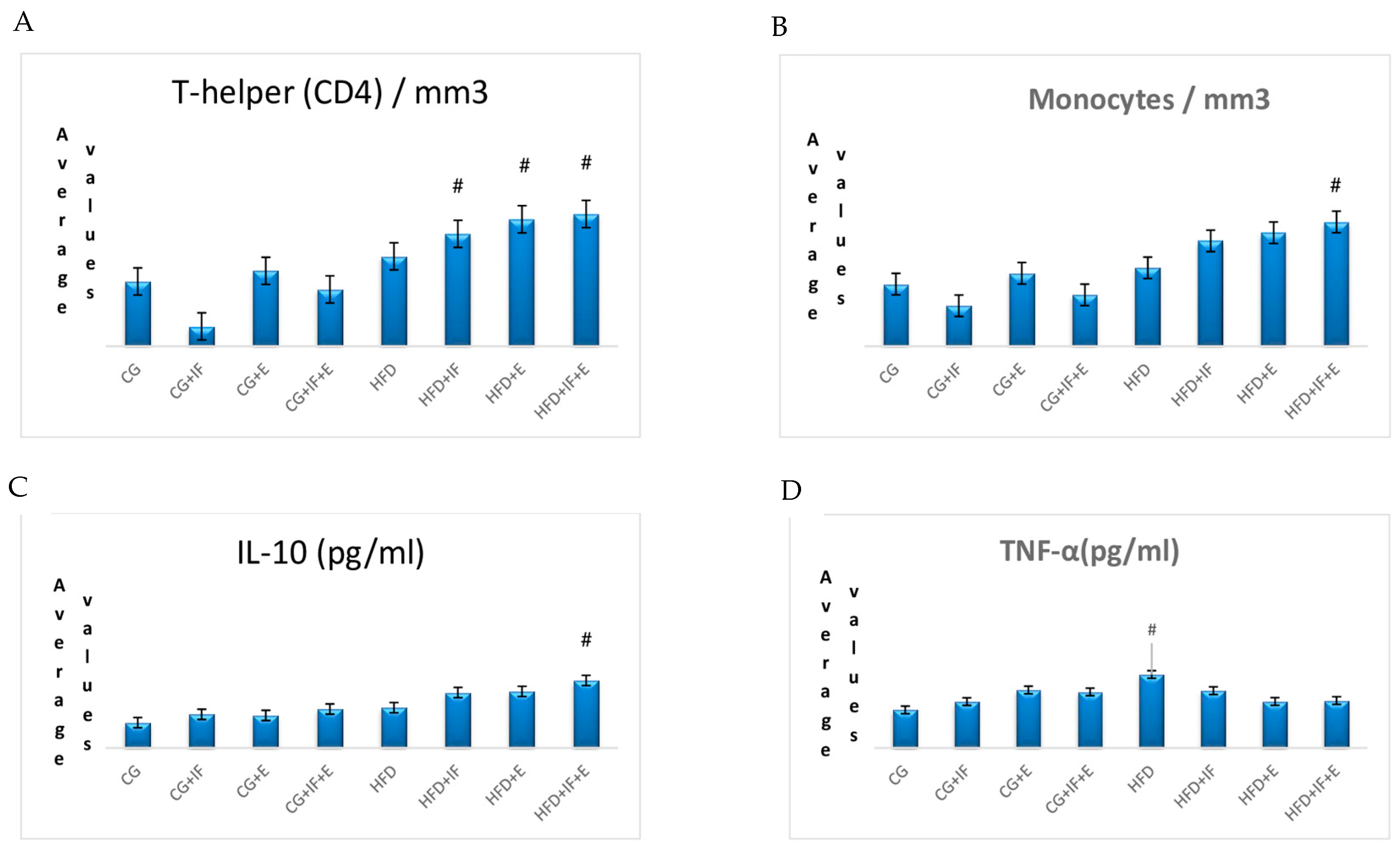
| T-helper(CD4)/mm3 | CG | 0.49 (0.40–0.59) bcd | 0.001 * |
| CG + IF | 0.15 (0.03–0.26) d | ||
| CG + E | 0.57 (0.36–0.84) abcd | ||
| CG + IF + E | 0.43 (0.23–0.64) cd | ||
| HFD | 0.68 (0.28–1.30) abc | ||
| HFD + IF | 0.85 (0.44–1.27) ab | ||
| HFD + E | 0.96 (0.59–1.16) a | ||
| HFD + IF + E | 1.00 (0.70–1.17) a |
| Group | Median (Min–Max) | p | |
|---|---|---|---|
| CG | 0.16 (0.13–0.20) bc | 0.001 * | |
| CG + IF | 0.16 (0.05–0.30) c | ||
| CG + E | 0.27 (0.16–0.42) abc | ||
| B-Lymphocyte /mm3 | CG + IF + E | 0.14 (0.08–0.20) c | |
| HFD | 0.18 (0.10–0.41) abc | ||
| HFD + IF | 0.28 (0.10–0.30) abc | ||
| HFD + E | 0.30 (0.20–0.41) ab | ||
| HFD + IF + E | 0.32 (0.22–0.38) a | ||
| CG | 0.23 (0.19–0.30) bcd | 0.001 * | |
| CG + IF | 0.15 (0.03–0.26) d | ||
| CG + E | 0.27 (0.16–0.42) cd | ||
| Monocytes/mm3 | CG + IF + E | 0.19 (0.11–0.32) abcd | |
| HFD | 0.29 (0.14–0.57) abcd | ||
| HFD + IF | 0.39 (0.28–0.64) abc | ||
| HFD + E | 0.42 (0.27–0.60) ab | ||
| HFD + IF + E | 0.46 (0.28–0.59) a | ||
| CG | 0.02 ± 0.03 | 0.169 | |
| CG + IF | 0.01 ± 0.05 | ||
| CG + E | 0.04 ± 0.15 | ||
| Eosinophils/mm3 | CG + IF + E | 0.02 ± 0.04 | |
| HFD | 0.02 ± 0.01 | ||
| HFD + IF | 0.03 ± 0.21 | ||
| HFD + E | 0.00 ± 0.05 | ||
| HFD + IF + E | 0.00 ± 0.00 | ||
| CG | 0.23 ± 0.03 b | 0.001 * | |
| CG + IF | 0.18 ± 0.12 b | ||
| CG + E | 0.25 ± 0.06 b | ||
| Naturel killer/mm3 | CG + IF + E | 0.20 ± 0.027 b | |
| HFD | 7.02 ± 3.45 a | ||
| HFD + IF | 0.38 ± 0.10 b | ||
| HFD + E | 0.45 ± 0.09 b | ||
| HFD + IF + E | 0.44 ± 0.07 b | ||
| CG | 270.50 ± 7.92 c | 0.001 * | |
| HFD | 385.92 ± 5.27 a | ||
| Live weight (g) | HFD + IF | 297.71 ± 5.71 b | |
| HFD +E | 299.90 ± 5.80 b | ||
| HFD + IF +E | 275.33 ± 7.98 c |
| Group | p | ||
|---|---|---|---|
| IGF-1 (pg/mL) | CG | 115.598 ± 33.205 | 0.126 |
| CG + IF | 128.901 ± 37.909 | ||
| CG + E | 80.644 ± 38.623 | ||
| CG + IF + E | 115.962 ± 37.044 | ||
| HFD | 76.563 ± 33.614 | ||
| HFD + IF | 104.141 ± 21.587 | ||
| HFD + E | 97.290 ± 57.474 | ||
| HFD + IF + E | 170.503 ± 70.075 | ||
| TNF-α (pg/mL) | CG | 14.633 ± 4.966 c | 0.012 * |
| CG + IF | 17.817 ± 4.042 bc | ||
| CG + E | 22.250 (12.900–24.300) bc | ||
| CG + IF + E | 21.533 ± 9.369 b | ||
| HFD | 28.200 ± 4.525 a | ||
| HFD + IF | 22.017 ± 3.036 b | ||
| HFD + E | 17.867 ± 4.622 bc | ||
| HFD + IF + E | 18.217 ± 1.747 bc | ||
| IL-4 (pg/mL) | CG | 31.769 ± 18.280 | 0.473 |
| CG + IF | 22.165 ± 9.819 | ||
| CG + E | 23.392 ± 11.657 | ||
| CG + IF + E | 37.923 ± 13.686 | ||
| HFD | 29.578 ± 8.051 | ||
| HFD + IF | 25.854 ± 12.010 | ||
| HFD + E | 24.892 ± 9.565 | ||
| HFD + IF + E | 25.467 ± 7.925 | ||
| IL-12 (pg/mL) | CG | 2.181 ± 1.781 | 0.447 |
| CG + IF | 1.948 ± 0.230 | ||
| CG + E | 2.432 ± 1.394 | ||
| CG + IF + E | 2.282 ± 0.737 | ||
| HFD | 2.282 ± 1.048 | ||
| HFD + IF | 2.086 ± 1.323 | ||
| HFD + E | 1.989 ± 0.786 | ||
| HFD + IF + E | 2.853 ± 1.696 | ||
| IL-10 (pg/mL) | CG | 151.307 ± 19.408 e | 0.001 * |
| CG + IF | 200.495 ± 19.416 d | ||
| CG + E | 194.117 ± 27.545 d | ||
| CG + IF + E | 231.958 ± 37.695 c | ||
| HFD | 240.428 ± 13.687 c | ||
| HFD + IF | 330.343 ± 21.373 b | ||
| HFD + E | 336.845 ± 18.980 b | ||
| HFD + IF + E | 402.237 ± 17.589 a | ||
| IL-1β (pg/mL) | CG | 573.107 ± 77.924 | 0.211 |
| CG + IF | 490.470 ± 90.677 | ||
| CG + E | 498.572 ± 213.88 | ||
| CG + IF + E | 410.527 ± 149.49 | ||
| HFD | 515.736 ± 100.42 | ||
| HFD + IF | 412.719 ± 137.27 | ||
| HFD + E | 456.762 ± 147.04 | ||
| HFD + IF + E | 557.773 ± 69.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günbatar, N.; Bulduk, B.; Bezgin, S.; Oto, G.; Bayıroğlu, F.; Bulduk, M. The Effect of Moderate-Intensity Physical Exercise on Some Serum Inflammation Markers and the Immune System in Rats Fed Intermittent Fasting with a High-Fat Diet. Medicina 2023, 59, 1687. https://doi.org/10.3390/medicina59091687
Günbatar N, Bulduk B, Bezgin S, Oto G, Bayıroğlu F, Bulduk M. The Effect of Moderate-Intensity Physical Exercise on Some Serum Inflammation Markers and the Immune System in Rats Fed Intermittent Fasting with a High-Fat Diet. Medicina. 2023; 59(9):1687. https://doi.org/10.3390/medicina59091687
Chicago/Turabian StyleGünbatar, Nizamettin, Bahattin Bulduk, Selver Bezgin, Gökhan Oto, Fahri Bayıroğlu, and Mehmet Bulduk. 2023. "The Effect of Moderate-Intensity Physical Exercise on Some Serum Inflammation Markers and the Immune System in Rats Fed Intermittent Fasting with a High-Fat Diet" Medicina 59, no. 9: 1687. https://doi.org/10.3390/medicina59091687





