Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play
Abstract
:1. Introduction
2. Materials and Methods
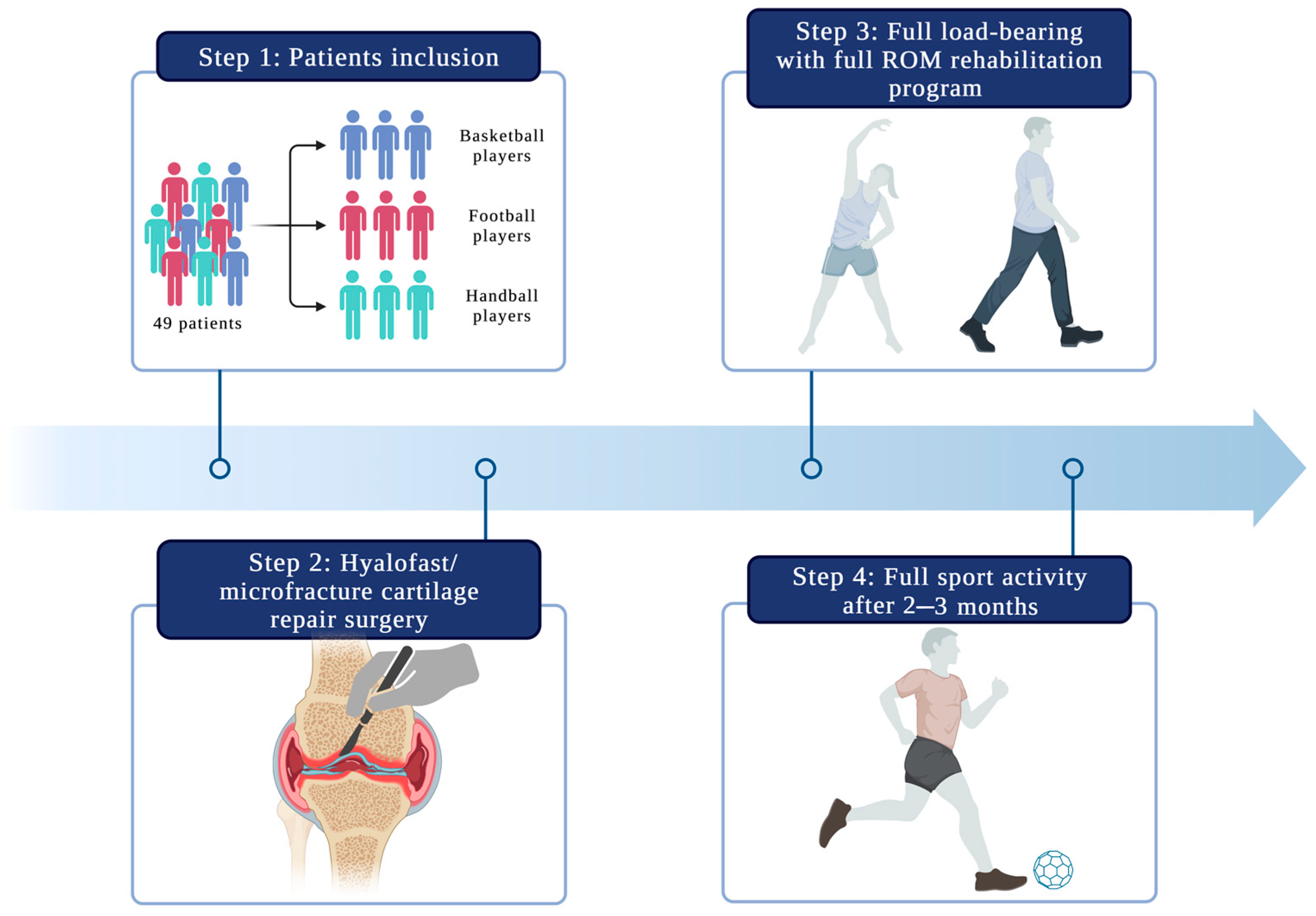
2.1. Patient Selection
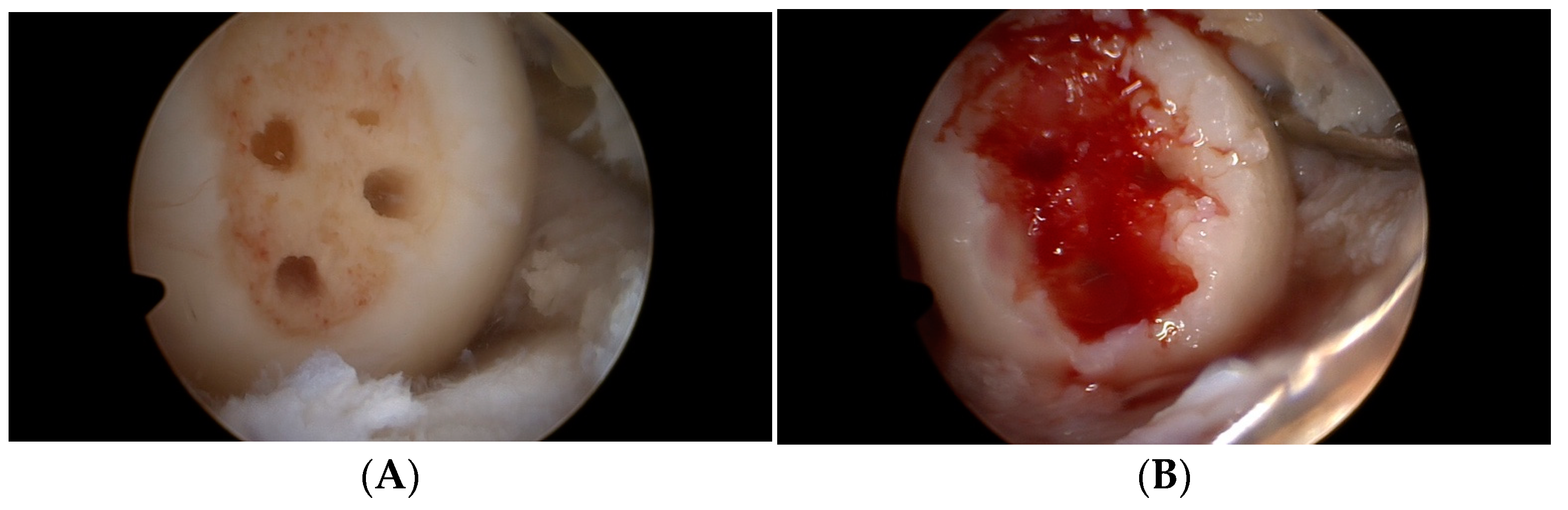
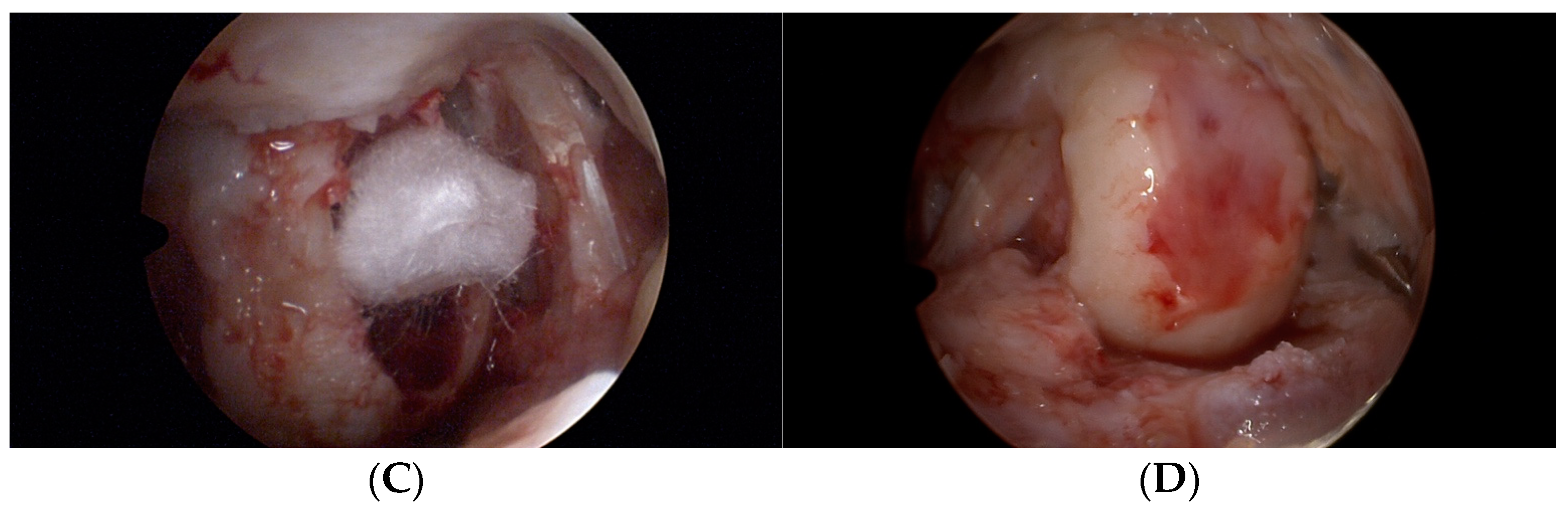
2.2. Operation Procedure
2.3. Follow-Up Evaluation
2.4. Rehabilitation Protocol
2.5. Statistics
3. Results
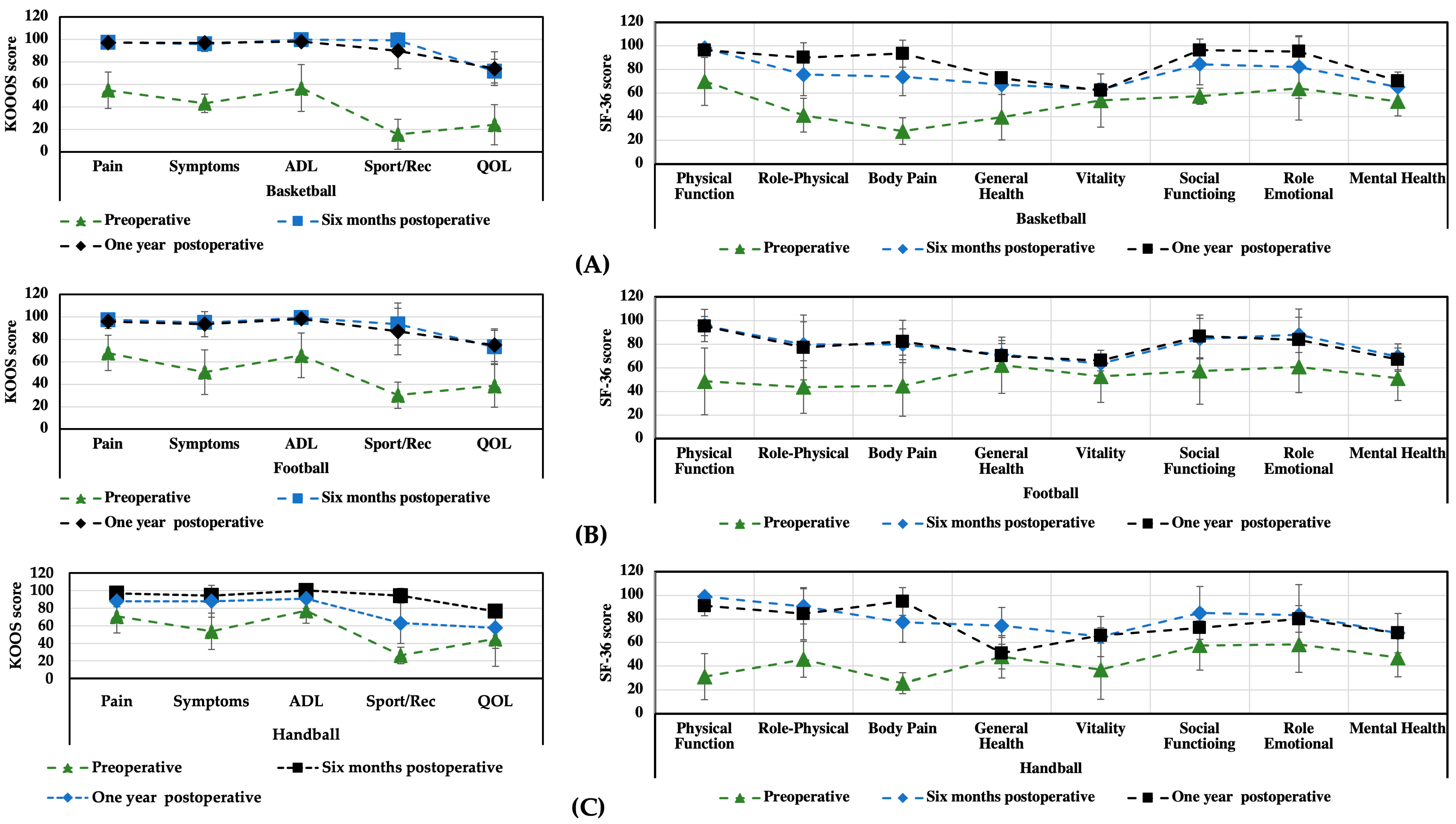
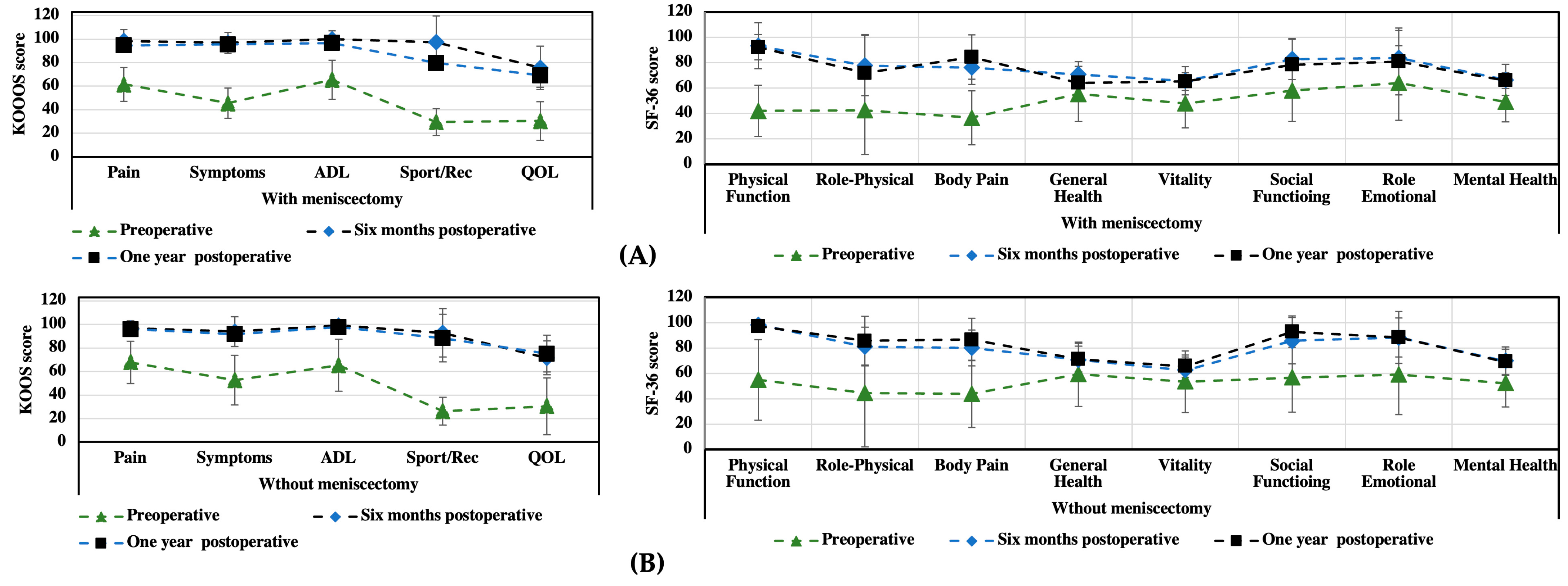
3.1. KOOS and SF-36 Scales
3.2. Functional Assessment
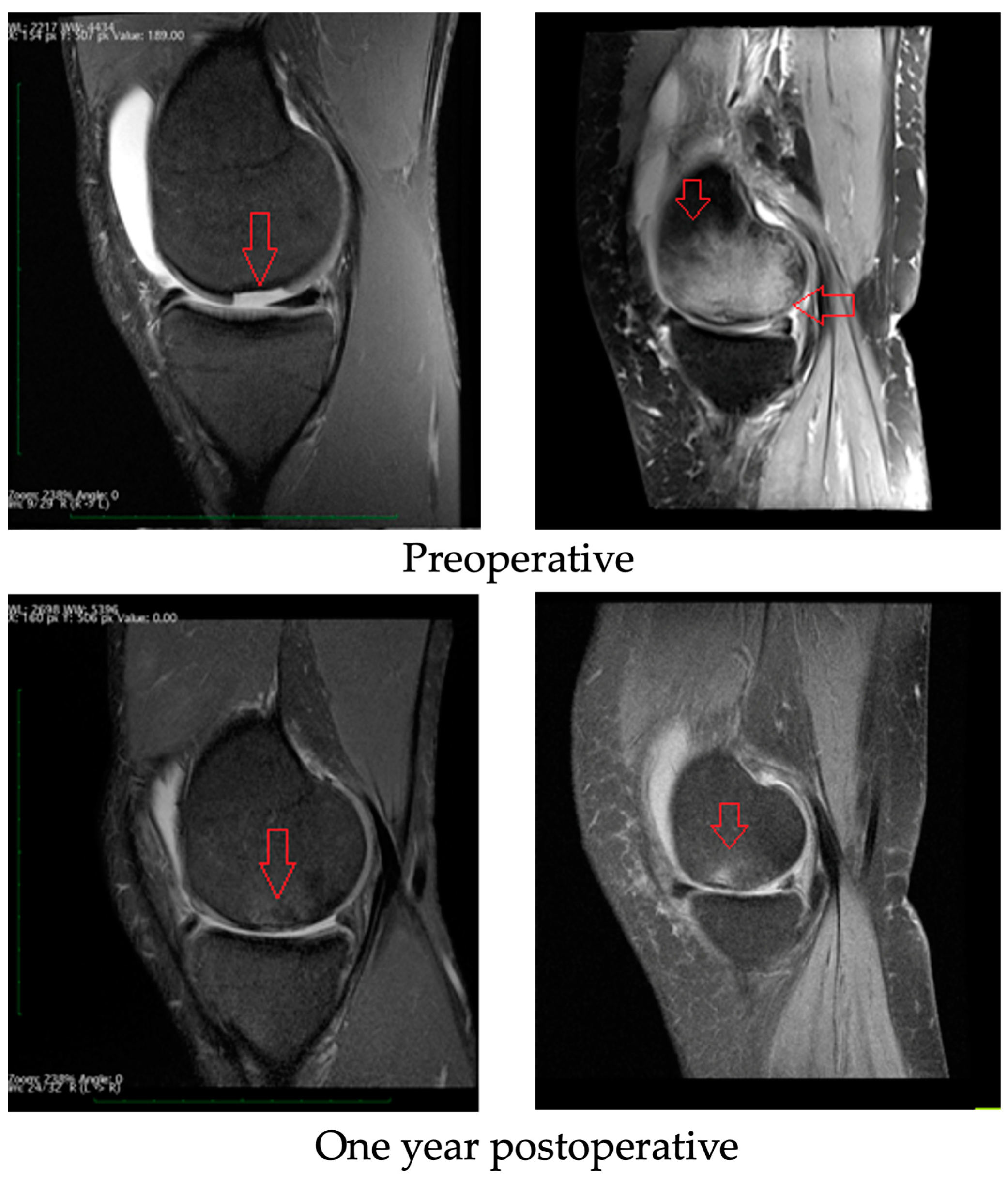
3.3. Radiological Evaluation
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical Technique and Rehabilitation to Treat Chondral Defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sport. Health A Multidiscip. Approach 2016, 8, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Uchio, Y.; Kawasaki, K.; Wakitani, S.; Iwasa, J. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J. Bone Jt. Surg. Br. 2002, 84, 571–578. [Google Scholar] [CrossRef]
- Della Villa, S.; Kon, E.; Filardo, G.; Ricci, M.; Vincentelli, F.; Delcogliano, M.; Marcacci, M. Does Intensive Rehabilitation Permit Early Return to Sport without Compromising the Clinical Outcome after Arthroscopic Autologous Chondrocyte Implantation in Highly Competitive Athletes? Am. J. Sport. Med. 2010, 38, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, J.; Meng, X.; Wang, F. Effects of Electrical Stimulation on Articular Cartilage Regeneration with a Focus on Piezoelectric Biomaterials for Articular Cartilage Tissue Repair and Engineering. Int. J. Mol. Sci. 2023, 24, 1836. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.E.; Helmick, C.G.; Theis, K.A.; Murphy, L.B.; Hootman, J.M.; Brady, T.J.; Cheng, Y.J. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2010–2012. Morb. Mortal. Wkly. Rep. 2013, 62, 869. [Google Scholar]
- Hootman, J.M.; Helmick, C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006, 54, 226–229. [Google Scholar] [CrossRef]
- Kujala, U.M.; Kettunen, J.; Paananen, H.; Aalto, T.; Battié, M.C.; Impivaara, O.; Videman, T.; Sarna, S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995, 38, 539–546. [Google Scholar] [CrossRef]
- Drawer, S.; Fuller, C.W. Propensity for osteoarthritis and lower limb joint pain in retired professional soccer players. Br. J. Sport. Med. 2001, 35, 402–408. [Google Scholar] [CrossRef]
- Roos, H. ARE THERE LONG-TERM SEQUELAE FROM SOCCER? Clin. Sport. Med. 1998, 17, 819–831. [Google Scholar] [CrossRef]
- Hori, M.; Terada, M.; Suga, T.; Isaka, T. Changes in anterior femoral articular cartilage structure in collegiate rugby athletes with and without a history of traumatic knee joint injury following a five-month competitive season. Sci. Rep. 2021, 11, 15186. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Rejeski, W.; Williamson, J.D.; Morgan, T.; Sevick, M.A.; Loeser, R.F.; Ettinger, W.H.; Messier, S.P. The Arthritis, Diet and Activity Promotion Trial (ADAPT): Design, rationale, and baseline results. Control. Clin. Trials 2003, 24, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of Exercise Is a Major Cause of Chronic Diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.; Smith, T.; Grice, A.; Kingsbury, S.R.; McCrory, P.; Conaghan, P.G. Does sports participation (including level of performance and previous injury) increase risk of osteoarthritis? A systematic review and meta-analysis. Br. J. Sport. Med. 2016, 50, 1459–1466. [Google Scholar] [CrossRef]
- Driban, J.B.; Hootman, J.M.; Sitler, M.R.; Harris, K.; Cattano, N.M. Is Participation in Certain Sports Associated With Knee Osteoarthritis? A Systematic Review. J. Athl. Train. 2017, 52, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Colau, M.M.; Nguyen, C.; Haddad, R.; Delamarche, P.; Paris, G.; Palazzo, C.; Poiraudeau, S.; Rannou, F.; Roren, A. Is Physical Activity, Practiced as Recommended for Health Benefit, a Risk Factor for Osteoarthritis? Ann. Phys. Rehabil. Med. 2016, 59, 196–206. [Google Scholar] [CrossRef]
- Davies, M.; Judge, A.; Stokes, K.; Delmestri, A.; Kemp, S.; Arden, N.K.; Newton, J.L. Is rugby playing load predictive of lower limb osteoarthritis in former international rugby players? Osteoarthr. Cartil. 2016, 24, S533–S534. [Google Scholar] [CrossRef]
- Cavalcanti, F.M.M.D.C.; Doca, D.; Cohen, M.; Ferretti, M. UPDATING ON DIAGNOSIS AND TREATMENT OF CHONDRAL LESION OF THE KNEE. Rev. Bras. Ortop. 2012, 47, 12–20. [Google Scholar] [CrossRef]
- Talesa, G.R.; Manfreda, F.; Ceccarini, P.; Pace, V.; Antinolfi, P.; Rinonapoli, G.; Caraffa, A. The treatment of knee cartilaginous injuries: State of the art. Acta Bio Med. Atenei Parmensis. 2022, 93, 11740. [Google Scholar]
- Swan, E.R.; Lynch, T.B.; Sheean, A.J. Treatment of Cartilage Defects of the Knee in Military Tactical Athletes: An Overview of Management and Clinical Outcomes. J. Knee Surg. 2022, 35, 1165–1174. [Google Scholar] [CrossRef]
- Yang, B.W.; Brusalis, C.M.; Fabricant, P.D.; Greditzer, H.G., IV. Articular Cartilage Repair in the Knee: Postoperative Imaging. J. Knee Surg. 2021, 34, 2–10. [Google Scholar] [CrossRef]
- Dekker, T.J.; Aman, Z.S.; DePhillipo, N.N.; Dickens, J.F.; Anz, A.W.; LaPrade, R.F. Chondral Lesions of the Knee: An Evidence-Based Approach. J. Bone Jt. Surg. Am. 2021, 103, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Rosneck, J.; A Higuera, C.; Tadross, N.; Krebs, V.; Barsoum, W.K. Managing knee osteoarthritis before and after arthroplasty. Clevel. Clin. J. Med. 2007, 74, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Epanomeritakis, I.E.; Lee, E.; Lu, V.; Khan, W. The Use of Autologous Chondrocyte and Mesenchymal Stem Cell Implants for the Treatment of Focal Chondral Defects in Human Knee Joints—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 4065. [Google Scholar] [CrossRef]
- Cerynik, D.L.; Lewullis, G.E.; Joves, B.C.; Palmer, M.P.; Tom, J.A. Outcomes of microfracture in professional basketball players. Knee Surg. Sport. Traumatol. Arthrosc. 2009, 17, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Namdari, S.; Baldwin, K.; Anakwenze, O.; Park, M.-J.; Huffman, G.R.; Sennett, B.J. Results and Performance after Microfracture in National Basketball Association Athletes. Am. J. Sport. Med. 2009, 37, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Gudas, R.; Gudaitė, A.; Mickevičius, T.; Masiulis, N.; Simonaitytė, R.; Čekanauskas, E.; Skurvydas, A. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: A prospective study with a 3-year follow-up. Arthroscopy 2013, 29, 89–97. [Google Scholar] [CrossRef]
- Bugbee, W.D.; Pallante-Kichura, A.L.; Görtz, S.; Amiel, D.; Sah, R. Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications. J. Orthop. Res. 2016, 34, 31–38. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Munir, N.; McDonald, A.; Callanan, A. Integrational Technologies for the Development of Three-Dimensional Scaffolds as Platforms in Cartilage Tissue Engineering. ACS Omega 2020, 5, 12623–12636. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, H.; Miao, Y.; Xiong, S.; Hu, Y. Recent strategies of collagen-based biomaterials for cartilage repair: From structure cognition to function endowment. J. Leather Sci. Eng. 2022, 4, 11. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Z.; Zhou, Y.; Li, L.; Wang, Y.; Wang, Z.; Chen, Y.; Zhang, P. Biomimetic porous collagen/hydroxyapatite scaffold for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45271. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical Efficacy of the Microfracture Technique for Articular Cartilage Repair in the Knee: An Evidence-Based Systematic Analysis. Am. J. Sport. Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J.; Warren, R.F.; Wickiewicz, T.L.; Marx, R.G. High-impact athletics after knee articular cartilage repair: A prospective evaluation of the microfracture technique. Am. J. Sport. Med. 2006, 34, 1413–1418. [Google Scholar] [CrossRef]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.C.C.; Lie, D.T.T. Clinical Outcomes of an All-Arthroscopic Technique for Single-Stage Autologous Matrix-Induced Chondrogenesis in the Treatment of Articular Cartilage Lesions of the Knee. Arthrosc. Sport. Med. Rehabil. 2020, 2, e353–e359. [Google Scholar] [CrossRef] [PubMed]
- Benthien, J.P.; Behrens, P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop. Belg. 2010, 76, 260. [Google Scholar] [PubMed]
- Zanasi, S.; Ventura, C.; Stefano, Z.P. One Step Tissue Engineering for Cartilage Reconstruction in Severe Osteoarthritis of the Knee and Ankle: A Comprehensive Review of the Technique Resorting to Isolated BMAC or ADSCS and their Last Combination. NanoWorld J. 2017, 3, 59–65. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Roffi, A.; Andriolo, L.; Marcacci, M. New trends for knee cartilage regeneration: From cell-free scaffolds to mesenchymal stem cells. Curr. Rev. Musculoskelet. Med. 2012, 5, 236–243. [Google Scholar] [CrossRef]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral Lesions of the Knee: A New One-Step Repair Technique with Bone-Marrow-Derived Cells. J. Bone Jt. Surg. 2010, 92 (Suppl. 2), 2–11. [Google Scholar] [CrossRef]
- Aytekin, K.; Zeki Esenyel, C. Cite This Article as: Aytekin K, Esenyel CZ. Comparing BST-CarGel® with Hyalofast for the Treatment of Hyaline Cartilage Defects. Eur. Arch. Med. Res. 2021, 37, 217–239. [Google Scholar] [CrossRef]
- Slynarski, K.; Deszczynski, J.; Jopowicz, R.; Krzesniak, A. Use of hyaluronan scaffold in combination with fresh bone marrow transplantation or with microfractures in treatment of cartilage defects of the knee joint. Osteoarthr. Cartil. 2014, 22, S152. [Google Scholar] [CrossRef]
- Disler, D.G.; McCauley, T.R.; Kelman, C.G.; Fuchs, M.D.; Ratner, L.M.; Wirth, C.R.; Hospodar, P.P. Fat-suppressed three-dimensional spoiled gradient-echo MR imaging of hyaline cartilage defects in the knee: Comparison with standard MR imaging and arthroscopy. AJR Am. J. Roentgenol. 1996, 167, 127–132. [Google Scholar] [CrossRef]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J. Patient Satisfaction after Total Knee Arthroplasty: Who is Satisfied and Who is Not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef]
- Bajuri, M.Y.; Sabri, S.; Mazli, N.; Sarifulnizam, F.A.; Apandi, H.M. Osteochondral Injury of the Talus Treated With Cell-Free Hyaluronic Acid-Based Scaffold (Hyalofast®)—A Reliable Solution. Cureus 2021, 13, e17928. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.K.; Ackland, T.; Ebert, J.R. Clinical Rehabilitation Guidelines for Matrix-Induced Autologous Chondrocyte Implantation on the Tibiofemoral Joint. J. Orthop. Sport. Phys. Ther. 2014, 44, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, P.; Miniaci, A.; Arnoczky, S.P.; Fowler, P.J.; Boughner, D.R. The Effect of Cast Immobilization on Meniscal Healing. Am. J. Sport. Med. 1995, 23, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Srinivasan, A.; Deschner, J.; Gassner, R.; Baliko, F.; Piesco, N.; Salter, R.; Agarwal, S. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J. Orthop. Res. 2005, 23, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Origuchi, T.; Okita, M.; Nakano, J.; Kato, K.; Yoshimura, T.; Izumi, S.-I.; Komori, T.; Nakamura, H.; Ida, H.; et al. Immobilization-Induced Cartilage Degeneration Mediated Through Expression of Hypoxia-Inducible Factor-1α, Vascular Endothelial Growth Factor, and Chondromodulin-I. Connect. Tissue Res. 2021, 50, 37–45. [Google Scholar] [CrossRef]
- Hurley, E.T.; Davey, M.S.; Jamal, M.S.; Manjunath, A.K.; Alaia, M.J.; Strauss, E.J. Return-to-Play and Rehabilitation Protocols following Cartilage Restoration Procedures of the Knee: A Systematic Review. Cartilage 2021, 13, 907S–914S. [Google Scholar] [CrossRef]
- Wang, H.-C.; Lin, T.-H.; Chang, N.-J.; Hsu, H.-C.; Yeh, M.-L. Continuous Passive Motion Promotes and Maintains Chondrogenesis in Autologous Endothelial Progenitor Cell-Loaded Porous PLGA Scaffolds during Osteochondral Defect Repair in a Rabbit Model. Int. J. Mol. Sci. 2019, 20, 259. [Google Scholar] [CrossRef]
- Ağır, I.; Tunçer, N.; Küçükdurmaz, F.; Gümüstaş, S.; Akgül, E.D.; Akpinar, F. Functional Comparison of Immediate and Late Weight Bearing after Ankle Bimalleolar Fracture Surgery. Open Orthop. J. 2015, 9, 188–190. [Google Scholar] [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a Self-Administered Outcome Measure. J. Orthop. Sport. Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 Health Survey Update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- E Brown, W.; Potter, H.G.; Marx, R.G.; Wickiewicz, T.L.; Warren, R.F. Magnetic Resonance Imaging Appearance of Cartilage Repair in the Knee. Clin. Orthop. Relat. Res. 2004, 422, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Quiceno, G.A.J.; Riveros, P.A.S.; Perea, G.A.O.; Vergara, M.G.; Muñoz, L.F.R.; Perez, R.D.A.; Piovesan, N.O.; Salamanca, J.A.M. Satisfactory clinical outcomes with autologous matrix-induced chondrogenesis in the treatment of grade IV chondral injuries of the knee. J. ISAKOS 2022. [Google Scholar] [CrossRef]
- Gobbi, A.; Scotti, C.; Karnatzikos, G.; Mudhigere, A.; Castro, M.; Peretti, G.M. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 25, 2494–2501. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Whyte, G.P. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid–Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am. J. Sport. Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.I.; Tho, S.J.W.; Tho, K.S. Biological resurfacing of grade IV articular cartilage ulcers in knee joint with Hyalofast. J. Orthop. Surg. 2020, 28, 2309499020905158. [Google Scholar] [CrossRef]
- Brophy, R.H.; Wojahn, R.D.; Lamplot, J.D. Cartilage Restoration Techniques for the Patellofemoral Joint. J. Am. Acad. Orthop. Surg. 2017, 25, 321–329. [Google Scholar] [CrossRef]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef]
- Solheim, E.; Hegna, J.; Strand, T.; Harlem, T.; Inderhaug, E. Randomized Study of Long-term (15-17 Years) Outcome After Microfracture Versus Mosaicplasty in Knee Articular Cartilage Defects. Am. J. Sport. Med. 2018, 46, 826–831. [Google Scholar] [CrossRef]
- Yen, Y.-M.; Cascio, B.; O'Brien, L.; Stalzer, S.; Millett, P.J.; Steadman, J.R. Treatment of Osteoarthritis of the Knee with Microfracture and Rehabilitation. Med. Sci. Sport. Exerc. 2008, 40, 200–205. [Google Scholar] [CrossRef]
- Mithoefer, K.; Williams, R.J.; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The Microfracture Technique for the Treatment of Articular Cartilage Lesions in the Knee. A prospective cohort study. J. Bone Jt. Surg. 2005, 87, 1911–1920. [Google Scholar] [CrossRef]
- Hambly, K.; Bobic, V.; Wondrasch, B.; Van Assche, D.; Marlovits, S. Autologous Chondrocyte Implantation Postoperative Care and Rehabilitation: Science and practice. Am. J. Sport. Med. 2006, 34, 1020–1038. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Hambly, K.; Della Villa, S.; Silvers, H.; Mandelbaum, B.R. Return to Sports Participation after Articular Cartilage Repair in the Knee. Am. J. Sport. Med. 2009, 37 (Suppl. 1), 167S–176S. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribol. 2022, 33, 31–38. [Google Scholar]

| Knee Joint (n = 49) | |
|---|---|
| Age, mean ± SD | 30 ± 6.7 |
| Sporting activity | |
| Professional | 49 |
| Gender, F/M | 15/34 |
| Cause of damage | |
| Traumatic | 32 |
| Non-traumatic | 6 |
| OCD | 11 |
| Location of the lesion | |
| LFC | 29 |
| MFC | 20 |
| Size of lesion (cm2), mean ± SD | 2.96 ± 1.05 |
| Previous surgeries | 4 ACL reconstructions |
| Accompanying procedures | |
| Meniscectomy | 19 |
| Preoperative | Six Months Postoperative | One Year Postoperative | (before/after Six Months) | |
|---|---|---|---|---|
| KOOS | Mean ± SD | Mean ± SD | Mean ± SD | p |
| Patients (n) | 49 | 49 | 43 | |
| Pain intensity | 55 ± 18.2 | 96 ± 4.2 | 99.5 ± 2.3 | <0.05 |
| Symptoms | 46 ± 16.6 | 93 ± 6.5 | 98.2 ± 1.8 | <0.05 |
| Activities of daily living | 57 ± 25.5 | 99 ± 2.6 | 100 ± 0.0 | <0.05 |
| Sport/Rec | 14 ± 11.1 | 95 ± 7.7 | 99.8 ± 1.8 | <0.05 |
| Quality of life | 30 ± 18 | 76 ± 4.7 | 88 ± 8.8 | <0.05 |
| Preoperative | Six Months Postoperative | One Year Postoperative | ||
|---|---|---|---|---|
| SF-36 | Mean ± SD | Mean ± SD | Mean ± SD | (before/after Six Months) |
| Patients (n) | 49 | 49 | 43 | p |
| Physical function | 34 ± 18.5 | 95.6 ± 2.0 | 97 ± 2.5 | <0.05 |
| Role physical | 63 ± 46.5 | 76 ± 15.0 | 96 ± 3.7 | 0.345 |
| Bodily pain | 36 ± 18.7 | 78 ± 15,6 | 98 ± 2.3 | <0.05 |
| General health | 68 ± 17.1 | 70 ± 16.1 | 83 ± 15.8 | 0.401 |
| Vitality | 53 ± 20.6 | 64 ± 8.0 | 87 ± 7.1 | 0.067 |
| Social function | 64 ± 29.6 | 87 ± 13.6 | 90 ± 9.5 | 0.068 |
| Role emotional | 73 ± 42.0 | 83 ± 22.9 | 90 ± 13.3 | 0.285 |
| Mental health | 52 ± 9.8 | 68 ± 11 | 70 ± 10.8 | 0.464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacprzak, B.; Rosińska, K.; Siuba-Jarosz, N. Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play. Medicina 2023, 59, 804. https://doi.org/10.3390/medicina59040804
Kacprzak B, Rosińska K, Siuba-Jarosz N. Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play. Medicina. 2023; 59(4):804. https://doi.org/10.3390/medicina59040804
Chicago/Turabian StyleKacprzak, Bartłomiej, Karolina Rosińska, and Natalia Siuba-Jarosz. 2023. "Hyalofast Cartilage Repair Surgery with a Full Load-Bearing Rehabilitation Program One Day after Operation Reduces the Time for Professional Athletes to Return to Play" Medicina 59, no. 4: 804. https://doi.org/10.3390/medicina59040804





