Current Trends in Orthognathic Surgery
Abstract
:1. Introduction
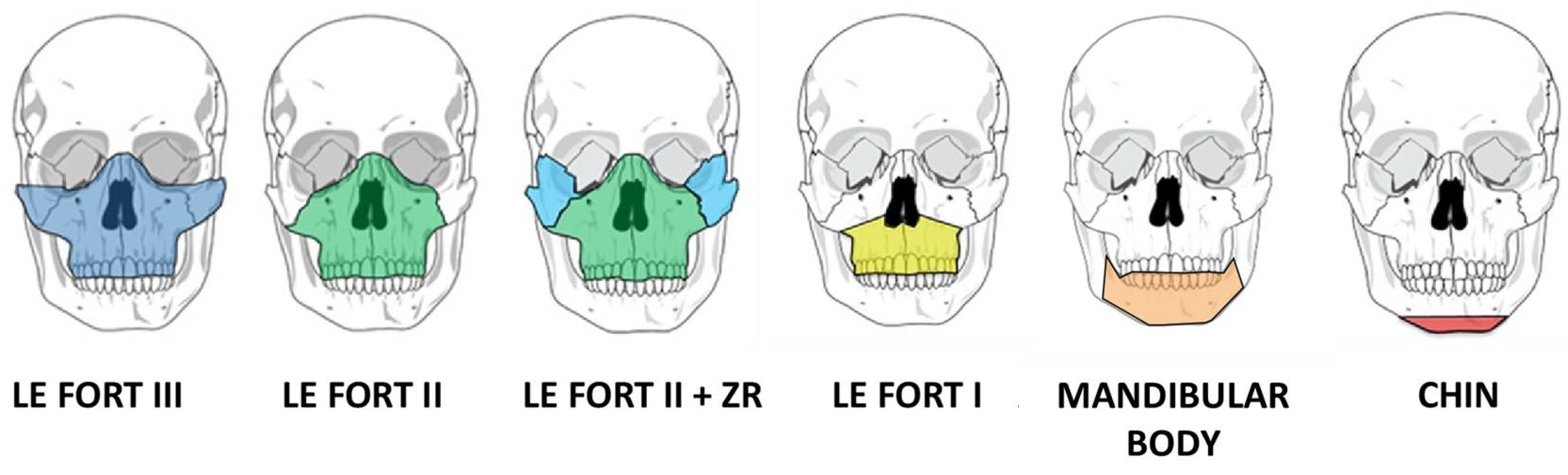
2. Advances in Surgical Planning
3. Surgery First/Surgery Only/Clear Aligner Therapy
3.1. Conventional Orthognathic Surgical Paradigm
3.2. Surgery First Protocol (Figure 5)
- Preoperative Evaluation: Initially, the patient’s occlusion is assessed through the standard model mounting process. This evaluation provided a baseline understanding of the existing dental and skeletal relationships.
- Model Setup: The teeth that are already adapted to the skeletal discrepancy are simulated and reorganized into their predicted locations. Each tooth is individually analyzed, simulated, and separated, mimicking presurgical orthodontic treatment. This step allows for a comprehensive assessment of tooth movements necessary for achieving optimal occlusion.
- Simulation of Orthognathic Surgery: The next phase involves simulating orthognathic surgical movements on the model, similar to the standard approach. The simulated surgical movements demonstrate the potential occlusal outcome after presurgical orthodontics and orthognathic surgery on the model.
- Surgical temporary occlusion: The teeth are restored to their original positions before any presurgical orthodontic treatment on the dental model. By using the original teeth model, can accurately replicate the dental condition that would reflect the outcome of orthognathic surgery without presurgical orthodontics.
- Once the teeth are repositioned to their pre-orthodontic treatment state, one can create intermediate and final splints for orthognathic surgery without presurgical orthodontics.
- The creation of these intermediate and final splints is based on the results of the simulated model surgery.
- By incorporating the surgical plan derived from the simulation, splints that are aligned with the desired surgical outcome can be fabricated.
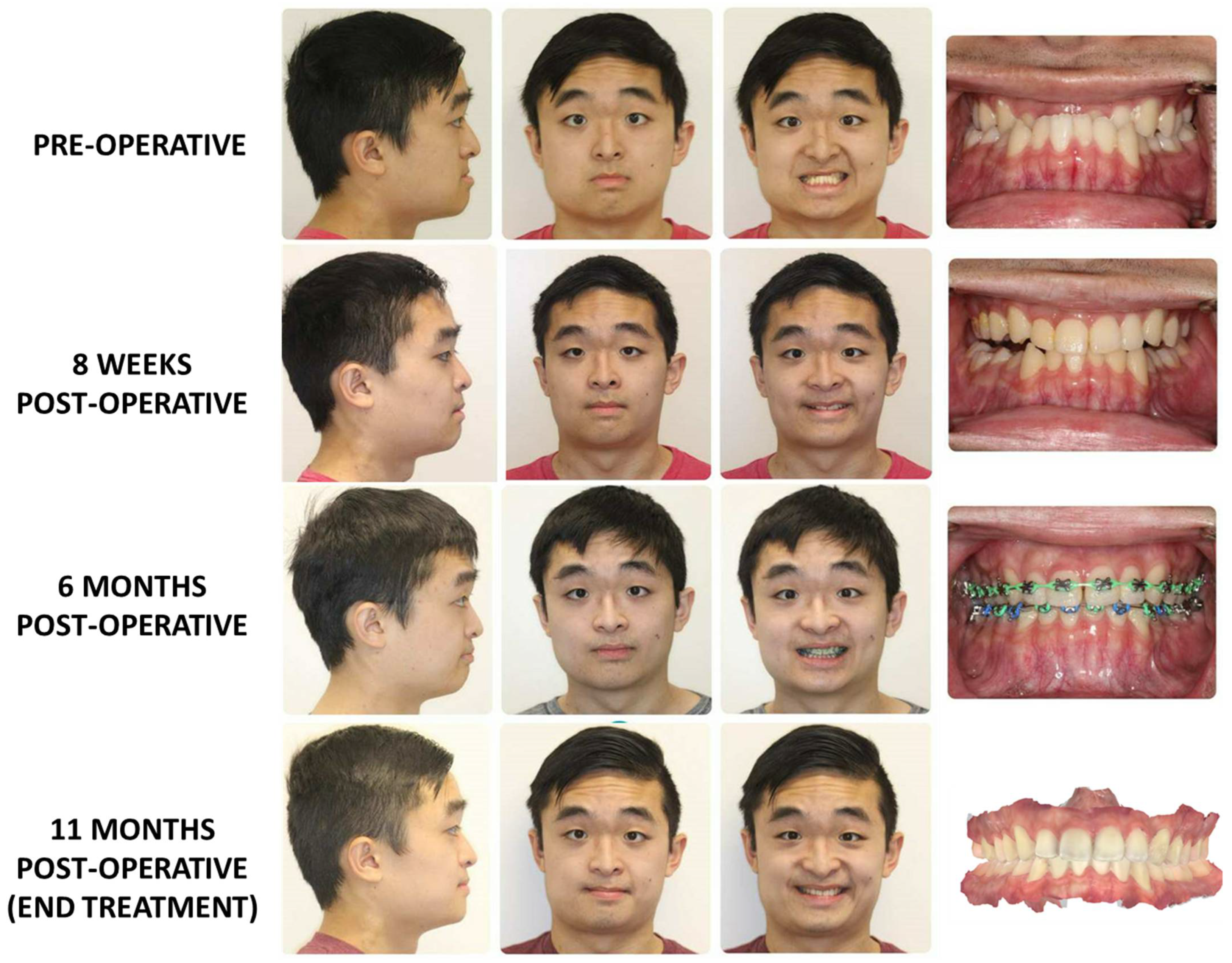
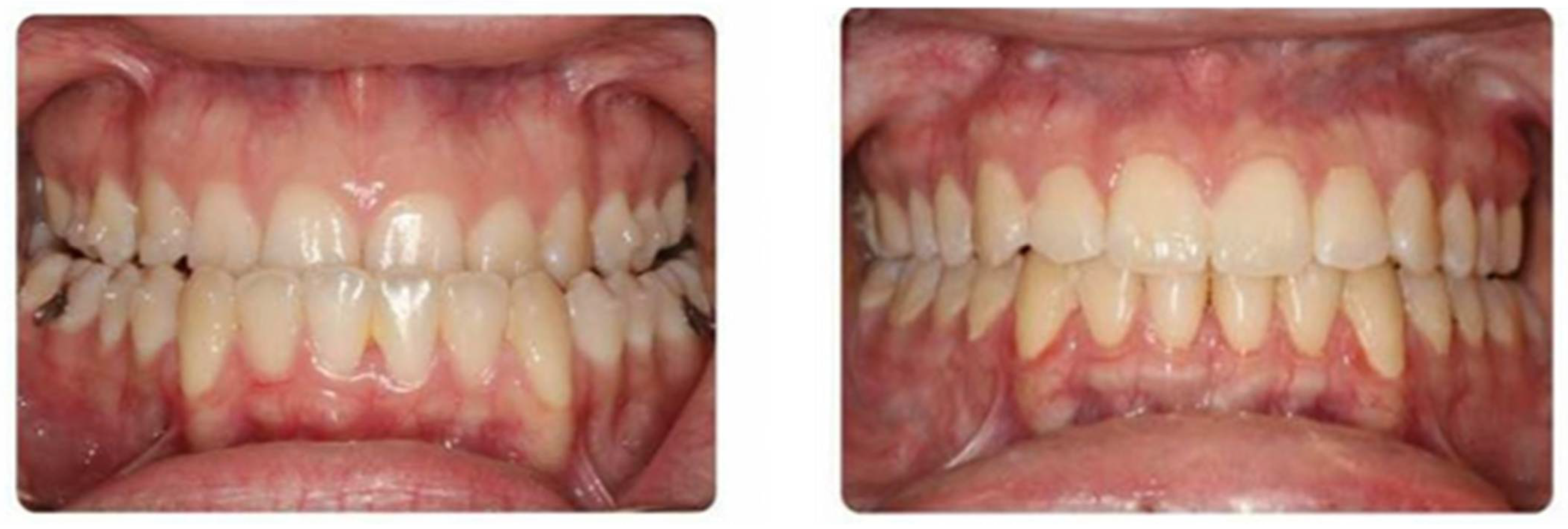
3.3. Surgery-Only Protocol (Figure 6)

3.4. Clear Aligner Therapy (Figure 7)
4. Orthognathic Surgery for Obstructive Sleep Apnea
4.1. Work-Up and Measures of Severity
4.2. Non-Invasive Treatment Options
4.3. Surgical Treatment Options
4.4. Orthognathic Surgery for the Treatment of OSA
- Reduction of intraoperative airway risks using fiberoptic intubation to appropriately assess the airway in the presence of operating surgeon.
- Plan for extubation at the end of the procedure, prior to ICU transfer.
- ICU admission and aggressive hypertension management in the acute post-operative period
- Pre- and post-operative use of nasal CPAP for 2 weeks prior to surgery if respiratory disturbance index is greater than 40 and a low oxyhemoglobin desaturation less than 80.
- Use of humidified oxygen at 35% through face tent if do not meet requirements for nasal CPAP.
- Continuous oximetry until discharge
- Judicious use of IV narcotics: Nurses must monitor patients after the use of any IV narcotics, as well as no patient should get a patient-controlled anesthesia pump.
- Discharge criteria involved adequate oral intake, pain control with oral medication, documented reduction of facial edema, ability to tolerate nasal CPAP if indicated.
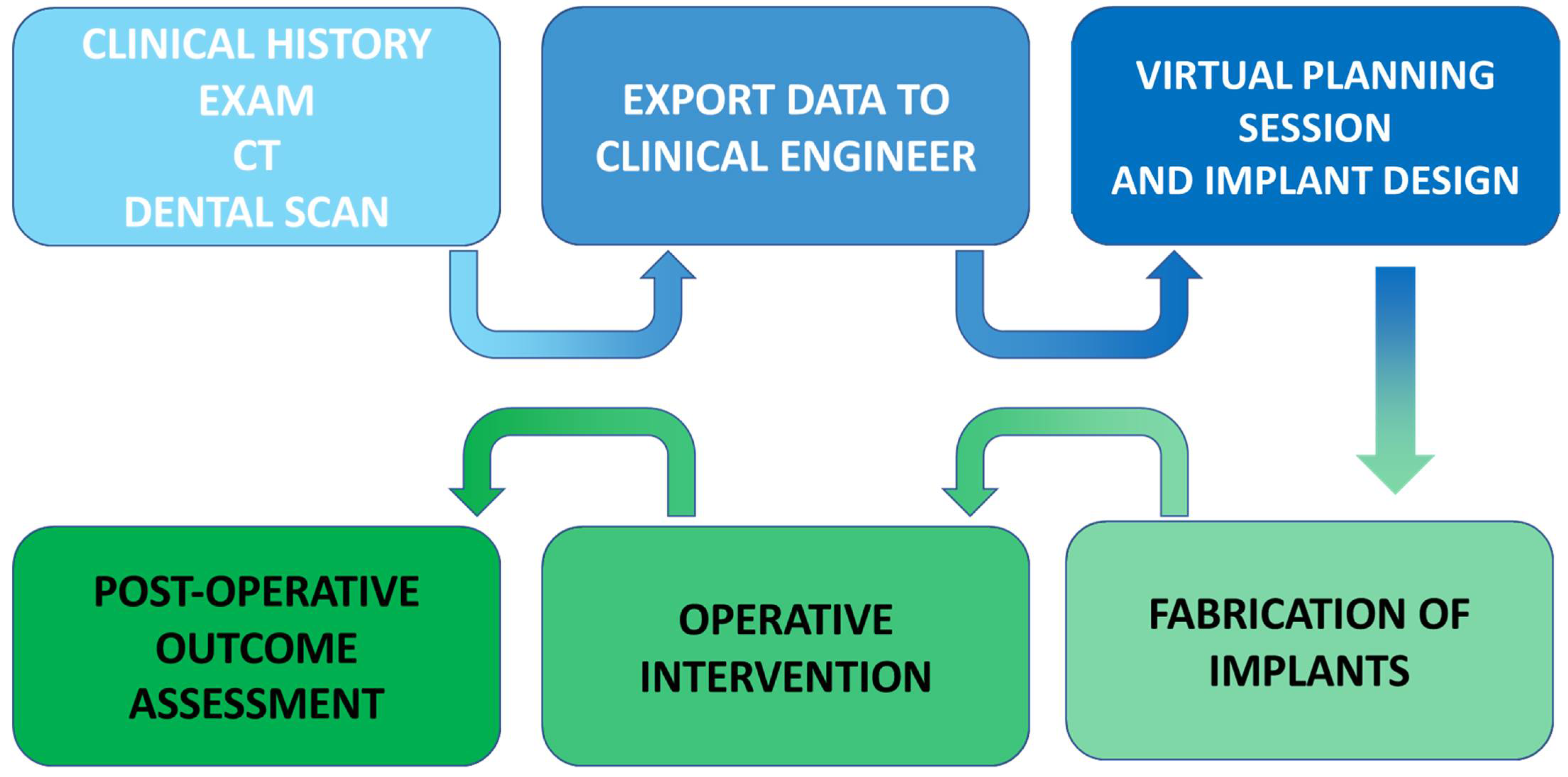
5. In-House Computer-Assisted Surgical Planning
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posnick, J.C. Orthognathic Surgery: Past—Present—Future. J. Oral Maxillofac. Surg. 2021, 79, 1996–1998. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, E.W. Historical development of orthognathic surgery. J. Craniomaxillofac. Surg. 1996, 24, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Naran, S.; Steinbacher, D.M.; Taylor, J.A. Current Concepts in Orthognathic Surgery. Plast. Reconstr. Surg. 2018, 141, 925e–936e. [Google Scholar] [CrossRef] [PubMed]
- Obwegeser, H.L. Orthognathic surgery and a tale of how three procedures came to be: A letter to the next generations of surgeons. Clin. Plast. Surg. 2007, 34, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B. A History of Orthognathic Surgery in North America. J. Oral Maxillofac. Surg. 2018, 76, 2466–2481. [Google Scholar] [CrossRef]
- Patel, P.K.; Novia, M.V. The surgical tools: The LeFort I, bilateral sagittal split osteotomy of the mandible, and the osseous genioplasty. Clin. Plast. Surg. 2007, 34, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Monson, L.A. Bilateral sagittal split osteotomy. Semin. Plast. Surg. 2013, 27, 145–148. [Google Scholar] [CrossRef]
- Buchanan, E.P.; Hyman, C.H. LeFort I Osteotomy. Semin. Plast. Surg. 2013, 27, 149–154. [Google Scholar]
- Konofaos, P.; Wallace, R.D. Distraction Osteogenesis in Craniofacial Surgery: Past, Present, and Future. J. Craniofac. Surg. 2021, 32 (Suppl. S3), 1221–1228. [Google Scholar] [CrossRef]
- Winters, R.; Tatum, S.A. Craniofacial distraction osteogenesis. Facial Plast. Surg. Clin. N. Am. 2014, 22, 653–664. [Google Scholar] [CrossRef]
- Massenburg, B.B.; Susarla, S.M.; Kapadia, H.P.; Hopper, R.A. Subcranial Midface Advancement in Patients with Syndromic Craniosynostosis. Oral Maxillofac. Surg. Clin. N. Am. 2022, 34, 467–475. [Google Scholar] [CrossRef]
- Hopper, R.A.; Ettinger, R.E.; Purnell, C.A.; Dover, M.S.; Pereira, A.R.; Tunçbilek, G. Thirty Years Later: What Has. Craniofacial Distraction Osteogenesis Surgery Replaced? Plast. Reconstr. Surg. 2020, 145, 1073e–1088e. [Google Scholar] [CrossRef] [PubMed]
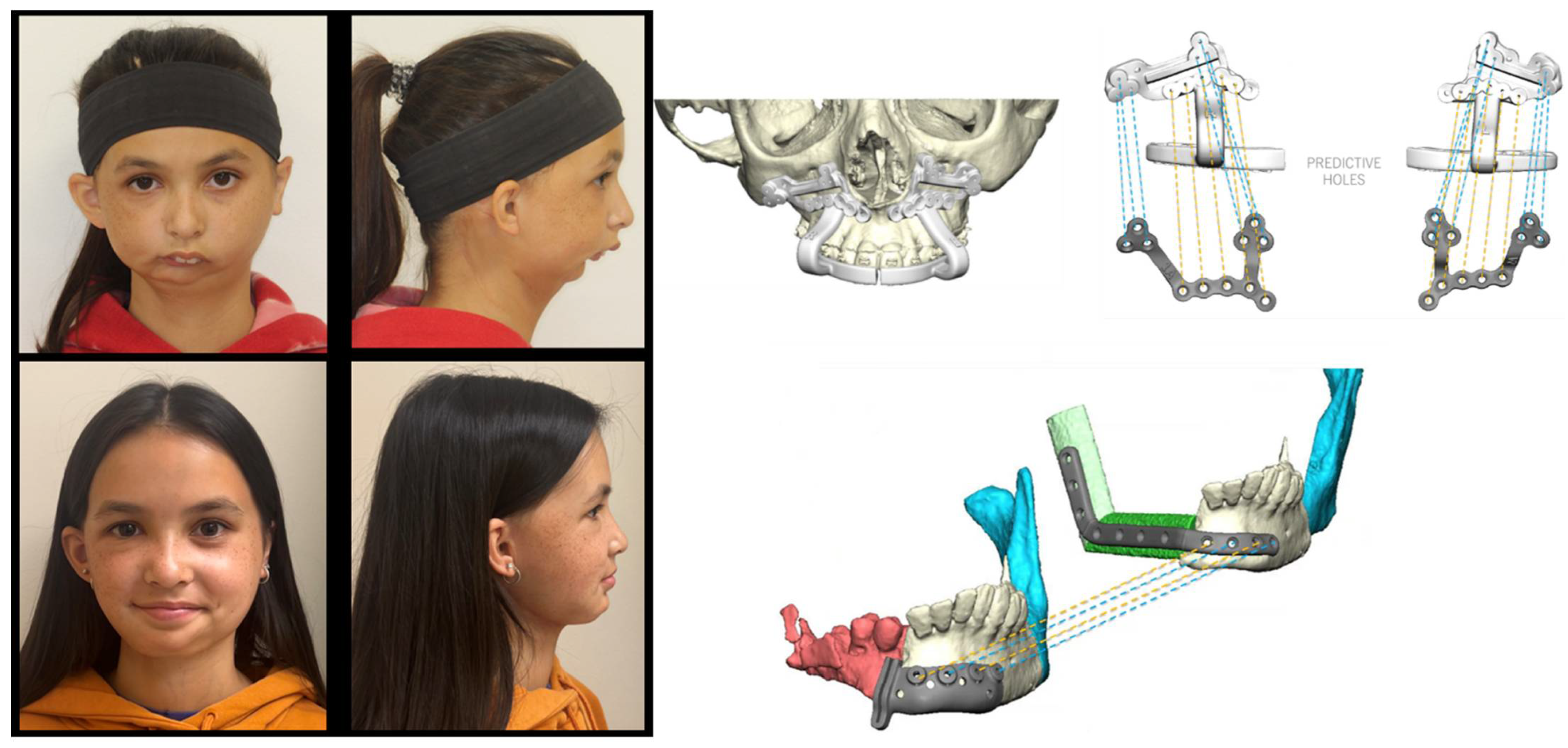
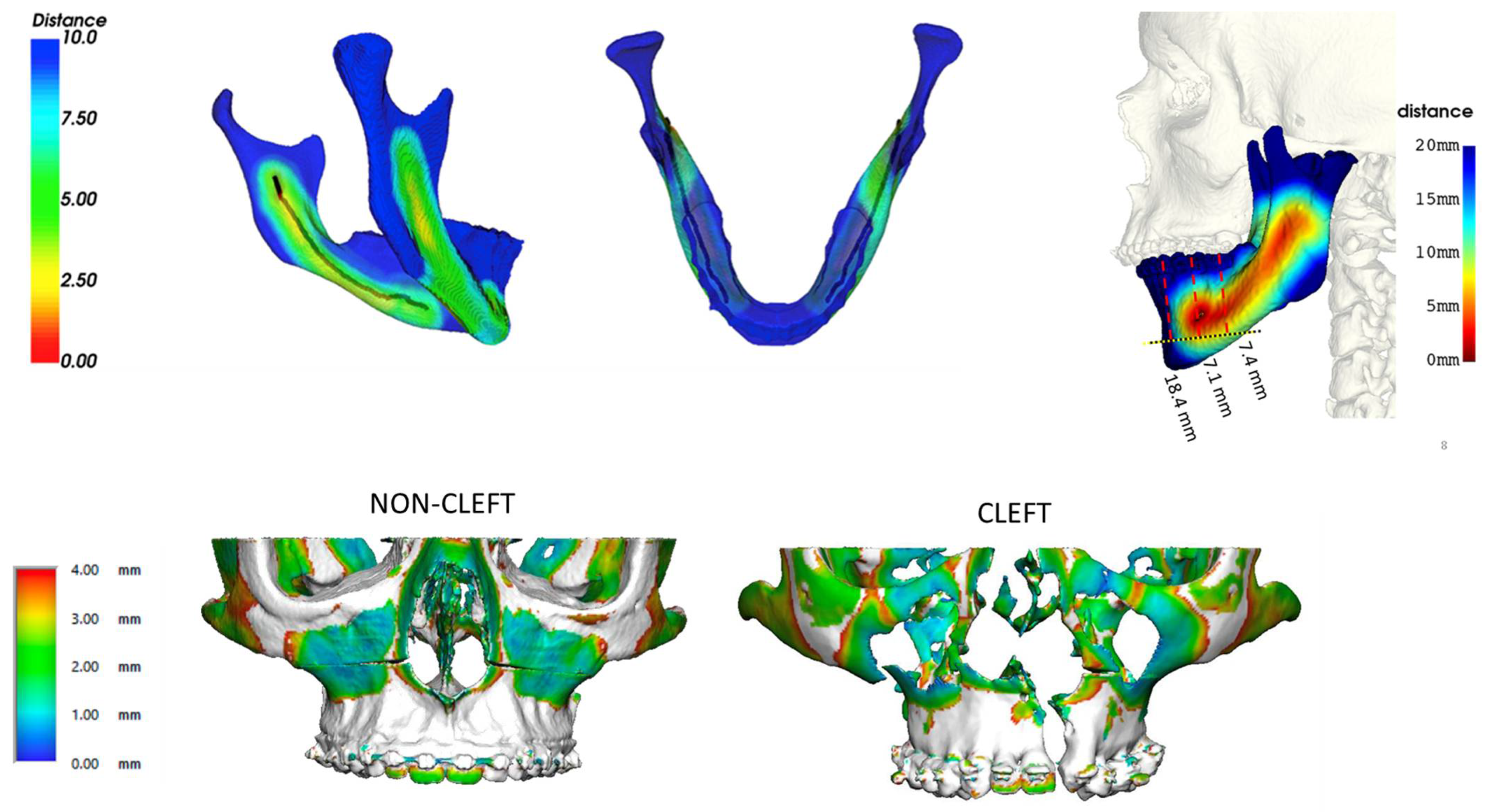
- Donaldson, C.D.; Manisali, M.; Naini, F.B. Three-dimensional virtual surgical planning (3D-VSP) in orthognathic surgery: Advantages, disadvantages and pitfalls. J. Orthod. 2021, 48, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, C.L.; Xu, W.; Zimmerman, C.E.; Vu, G.H.; Humphries, L.S.; Swanson, J.W.; Bartlett, S.P.; Taylor, J.A. Trends in Utilization of Virtual Surgical Planning in Pediatric Craniofacial Surgery. J. Craniofac Surg. 2020, 31, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Farrell, B.B.; Franco, P.B.; Tucker, M.R. Virtual surgical planning in orthognathic surgery. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, D.; Michelinakis, G.; Kamposiora, P.; Papavasiliou, G. The current state of computer assisted orthognathic surgery: A narrative review. J. Dent. 2022, 119, 104052. [Google Scholar] [CrossRef]
- Roy, T.; Steinbacher, D.M. Virtual Planning and 3D Printing in Contemporary Orthognathic Surgery. Semin. Plast. Surg. 2022, 36, 169–182. [Google Scholar] [CrossRef]
- Li, D.T.S.; Leung, Y.Y. Patient-Specific Implants in Orthognathic Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2023, 35, 61–69. [Google Scholar] [CrossRef]
- Chen, J.; Abousy, M.; Girard, A.; Duclos, O.; Patel, V.; Jenny, H.; Redett, R.; Yang, R. The Impact of Virtual Surgical Planning on Orthognathic Surgery: Contributions from Two Specialties. J. Craniofac. Surg. 2022, 33, 1418–1423. [Google Scholar] [CrossRef]
- Farrell, B.B. Evolving Management of Dentofacial Deformities with Digital Planning and Patient-Specific Fixation. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2020, 28, 59–71. [Google Scholar] [CrossRef]
- Diaconu, A.; Holte, M.B.; Berg-Beckhoff, G.; Pinholt, E.M. Three-Dimensional Accuracy and Stability of Personalized Implants in Orthognathic Surgery: A Systematic Review and a Meta-Analysis. J. Pers. Med. 2023, 13, 125. [Google Scholar] [CrossRef]
- Huamán, E.T.; Juvet, L.M.; Nastri, A.; Denman, W.T.; Kaban, L.B.; Dodson, T.B. Changing patterns of hospital length of stay after orthognathic surgery. J. Oral Maxillofac. Surg. 2008, 66, 492–497. [Google Scholar] [CrossRef]
- Stanbouly, D.; Tummala, H.; Shleiwet, N.H.; Zeng, Q.; Selvi, F.; Chuang, S.K.; Kinard, B. What factors influence the cost of orthognathic surgery among patients in the US? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 23–32. [Google Scholar] [CrossRef]
- Bowe, C.M.; Gurney, B.; Sloane, J.; Johnson, P.; Newlands, C. Operative time, length of stay and reoperation rates for orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2021, 59, 163–167. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Parsaei, Y.; Peck, C.J.; Henry, A.; Lopez, J.; Steinbacher, D.M. Major Complications and Associated Risk Factors for Osseous Genioplasty With Bimaxillary Orthognathic Surgery: An American College of Surgeons-National Surgical Quality Improvement Program Analysis. J. Craniofac. Surg. 2022, 33, 632–635. [Google Scholar] [CrossRef]
- Choi, J.W.; Bradley, J.P. Surgery First Orthognathic Approach without Presurgical Orthodontic Treatment: Questions and Answers. J. Craniofac. Surg. 2017, 28, 1330–1333. [Google Scholar] [CrossRef]
- Jeong, W.S.; Choi, J.W.; Kim, D.Y.; Lee, J.Y.; Kwon, S.M. Can a surgery-first orthognathic approach reduce the total treatment time? Int. J. Oral Maxillofac. Surg. 2017, 46, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiao, Y.D.; Liang, Y.J.; Wang, X.; Li, J.Y.; Liao, G.Q. Does the Surgery-First Approach Produce Better Outcomes in Orthognathic Surgery? A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Park, H.; In-Hwan Kim, B.S.; Kim, N.; Kwon, S.M.; Lee, J.Y. Surgery-First Orthognathic Approach to Correct Facial Asymmetry: Artificial Intelligence-Based Cephalometric Analysis. Plast. Reconstr. Surg. 2022, 149, 496e–499e. [Google Scholar] [CrossRef] [PubMed]
- Tremont, T.J.; Posnick, J.C. Selected Orthodontic Principles for Management of Cranio-Maxillofacial Deformities. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.A.; Lee, K.C.; Chuang, S.K. The Surgery-first Approach to Orthognathic Surgery. J. Craniofac. Surg. 2021, 32, e153–e156. [Google Scholar] [CrossRef]
- Ebker, T.; Korn, P.; Heiland, M.; Bumann, A. Comprehensive virtual orthognathic planning concept in surgery-first patients. Br. J. Oral Maxillofac. Surg. 2022, 60, 1092–1096. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, H.; Kwon, S.M.; Lee, J.Y. Surgery-first orthognathic approach for the correction of facial asymmetry. J. Craniomaxillofac. Surg. 2021, 49, 435–442. [Google Scholar] [CrossRef] [PubMed]
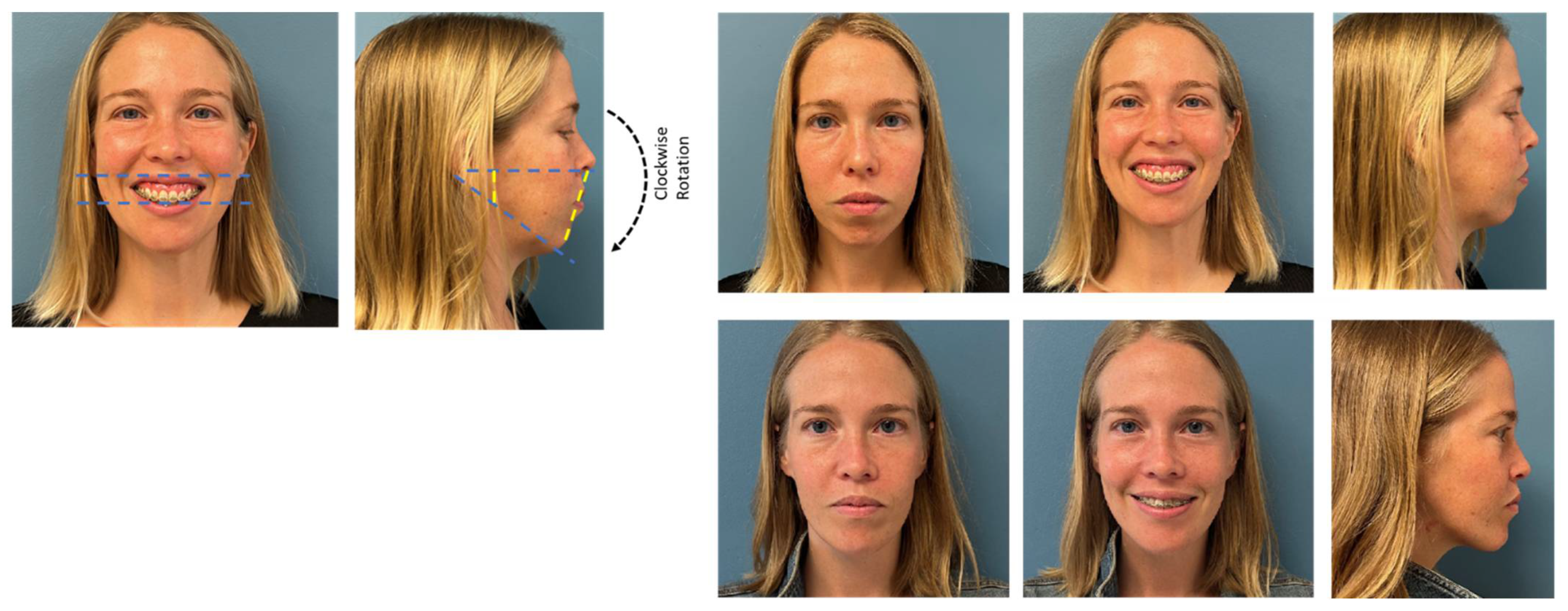
- Wannalerkngam, C.; Sinha, S.P.; Tran-Duy, T.D.; Wen-Ching Ko, E.; Chen, Y.R.; Huang, C.S. Does Clockwise Rotation of Maxillomandibular Complex Using Surgery-First Approach to Correct Mandibular Prognathism Improve Facial Appearance? J. Oral Maxillofac. Surg. 2023, 81, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Border, M.; Strait, R.; Vega, L. Clear Aligner Orthognathic Splints (CAOS) and Custom Maxillary Fixation Plates for Surgery-First or Surgery-Only Cases. J. Oral Maxillofac. Surg. 2021, 79, e6–e11. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.J.; Chen, P.R.; Tsai, T.Y.; Lin, S.; Chou, P.Y.; Lo, C.M.; Chen, Y.R. Comparative assessment of orthodontic and aesthetic outcomes after orthognathic surgery with clear aligner or fixed appliance therapy. Plast. Reconstr. Surg. 2023. [CrossRef]
- Parsaei, Y.; Uribe, F.; Steinbacher, D.M. Clear aligner therapy and orthognathic surgery. J. Clin. Orthod. 2022, 56, 692–707. [Google Scholar] [PubMed]
- Kankam, H.; Madari, S.; Sawh-Martinez, R.; Bruckman, K.C.; Steinbacher, D.M. Comparing Outcomes in Orthognathic Surgery Using Clear Aligners versus Conventional Fixed Appliances. J. Craniofac Surg. 2019, 30, 1488–1491. [Google Scholar] [CrossRef]
- Kankam, H.K.N.; Gupta, H.; Sawh-Martinez, R.; Steinbacher, D.M. Segmental Multiple-Jaw Surgery without Orthodontia: Clear Aligners Alone. Plast. Reconstr. Surg. 2018, 142, 181–184. [Google Scholar] [CrossRef]
- Barrera, J.E.; Powell, N.B.; Riley, R.W. Facial skeletal surgery in the management of adult obstructive sleep apnea syndrome. Clin. Plast. Surg. 2007, 34, 565–573. [Google Scholar] [CrossRef]
- Quah, B.; Sng, T.J.H.; Yong, C.W.; Wen Wong, R.C. Orthognathic Surgery for Obstructive Sleep Apnea. Oral Maxillofac. Surg. Clin. N. Am. 2023, 35, 49–59. [Google Scholar] [CrossRef]
- Jandali, D.; Barrera, J.E. Recent advances in orthognathic surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Capasso, R. Skeletal Surgery for Obstructive Sleep Apnea. Otolaryngol. Clin. N. Am. 2020, 53, 459–468. [Google Scholar] [CrossRef]
- Awad, M.; Gouveia, C.; Zaghi, S.; Camacho, M.; Liu, S.Y. Changing practice: Trends in skeletal surgery for obstructive sleep apnea. J. Craniomaxillofac. Surg. 2019, 47, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Awad, M.; Riley, R.W. Maxillomandibular Advancement: Contemporary Approach at Stanford. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2019, 27, 29–36. [Google Scholar] [CrossRef]
- Susarla, S.M.; Thomas, R.J.; Abramson, Z.R.; Kaban, L.B. Biomechanics of the upper airway: Changing concepts in the pathogenesis of obstructive sleep apnea. Int. J. Oral Maxillofac. Surg. 2010, 39, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
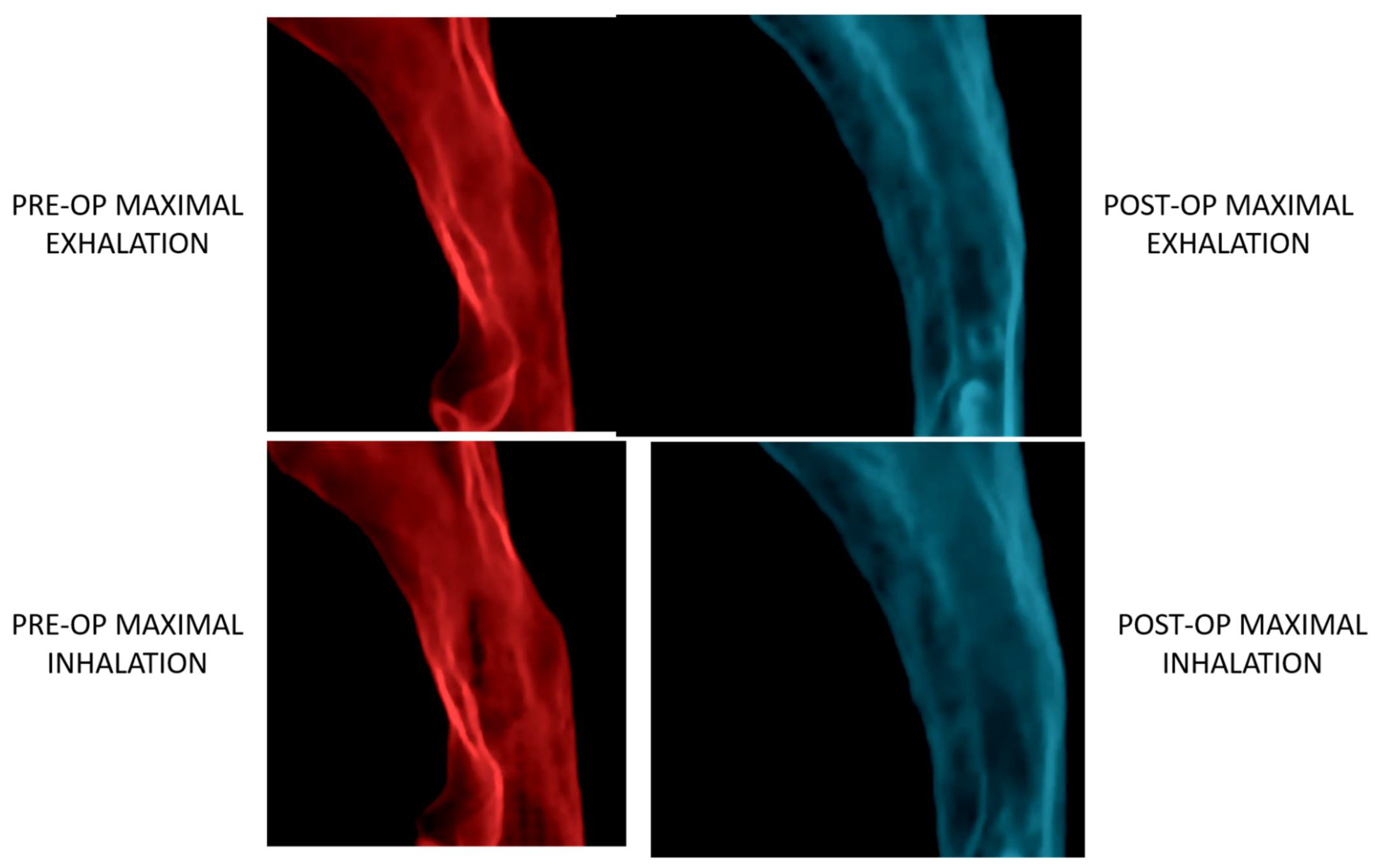
- Steegman, R.; Hogeveen, F.; Schoeman, A.; Ren, Y. Cone beam computed tomography volumetric airway changes after orthognathic surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 2023, 52, 60–71. [Google Scholar] [CrossRef]
- Fleck, R.J.; Ishman, S.L.; Shott, S.R.; Gutmark, E.J.; McConnell, K.B.; Mahmoud, M.; Mylavarapu, G.; Subramaniam, D.R.; Szczesniak, R.; Amin, R.S. Dynamic Volume Computed Tomography Imaging of the Upper Airway in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 13, 189–196. [Google Scholar] [CrossRef]
- Chousangsuntorn, K.; Bhongmakapat, T.; Apirakkittikul, N.; Sungkarat, W.; Supakul, N.; Laothamatas, J. Computed Tomography Characterization and Comparison with Polysomnography for Obstructive Sleep Apnea Evaluation. J. Oral Maxillofac. Surg. 2018, 76, 854–872. [Google Scholar] [CrossRef]
- Volner, K.; Chao, S.; Camacho, M. Dynamic sleep MRI in obstructive sleep apnea: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2022, 279, 595–607. [Google Scholar] [CrossRef]
- Chen, H.; Aarab, G.; de Ruiter, M.H.; de Lange, J.; Lobbezoo, F.; van der Stelt, P.F. Three-dimensional imaging of the upper airway anatomy in obstructive sleep apnea: A systematic review. Sleep Med. 2016, 21, 19–27. [Google Scholar] [CrossRef]
- Abramson, Z.; Susarla, S.M.; Lawler, M.; Bouchard, C.; Troulis, M.; Kaban, L.B. Three-dimensional computed tomographic airway analysis of patients with obstructive sleep apnea treated by maxillomandibular advancement. J. Oral Maxillofac. Surg. 2011, 69, 677–686. [Google Scholar] [CrossRef]
- Liu, M.T.; Kurnik, N.M.; Mercan, E.; Susarla, S.M.; Purnell, C.A.; Hopper, R.A. Magnitude of Horizontal Advancement is Associated with Apnea Hypopnea Index Improvement and Counter-Clockwise Maxillary Rotation After Subcranial Distraction for Syndromic Synostosis. J. Oral Maxillofac. Surg. 2021, 79, e1–e1133. [Google Scholar] [CrossRef]
- Zaghi, S.; Holty, J.E.; Certal, V.; Abdullatif, J.; Guilleminault, C.; Powell, N.B.; Riley, R.W.; Camacho, M. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea: A Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Christino, M.; Vinha, P.P.; Faria, A.C.; Garcia, D.M.; de Mello-Filho, F.V. Impact of counterclockwise rotation of the occlusal plane on the mandibular advancement, pharynx morphology, and polysomnography results in maxillomandibular advancement surgery for the treatment of obstructive sleep apnea patients. Sleep Breath 2021, 25, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, T.B.; Laulund, A.S.; Ingerslev, J.; Homøe, P.; Pinholt, E.M. Improved apnea-hypopnea index and lowest oxygen saturation after maxillomandibular advancement with or without counterclockwise rotation in patients with obstructive sleep apnea: A meta-analysis. J. Oral Maxillofac. Surg. 2015, 73, 719–726. [Google Scholar] [CrossRef]
- Giralt-Hernando, M.; Valls-Ontañón, A.; Guijarro-Martínez, R.; Masià-Gridilla, J.; Hernández-Alfaro, F. Impact of surgical maxillomandibular advancement upon pharyngeal airway volume and the apnoea-hypopnoea index in the treatment of obstructive sleep apnoea: Systematic review and meta-analysis. BMJ Open Respir. Res. 2019, 6, e000402. [Google Scholar] [CrossRef] [PubMed]
- John, C.R.; Gandhi, S.; Sakharia, A.R.; James, T.T. Maxillomandibular advancement is a successful treatment for obstructive sleep apnoea: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1561–1571. [Google Scholar] [CrossRef]
- Rojo-Sanchis, C.; Almerich-Silla, J.M.; Paredes-Gallardo, V.; Montiel-Company, J.M.; Bellot-Arcís, C. Impact of Bimaxillary Advancement Surgery on the Upper Airway and on Obstructive Sleep Apnea Syndrome: A Meta-Analysis. Sci. Rep. 2018, 8, 5756. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Jeong, W.S.; Kang, M.K.; Lee, J.Y.; Chung, Y.S. Counterclockwise Rotational Orthognathic Surgery for the Obstructive Sleep Apnea in Class II Dentofacial Deformity: Polysomnography and 3D Computed Tomographic Analysis. Ann. Plast. Surg. 2021, 86, 640–646. [Google Scholar] [CrossRef]
- Curran, J.; Shimizu, M.; Tassi, A. Evaluation of Facial Profile Esthetics after Maxillomandibular Advancement Surgery for the Treatment of Obstructive Sleep Apnea. J. Oral Maxillofac. Surg. 2022, 80, 174–184. [Google Scholar] [CrossRef]
- De Riu, G.; Vaira, L.A.; Ligas, E.; Vaittinen, V.; Spano, G.; Salzano, G.; Piombino, P. New protocol for in-house management of computer assisted orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2020, 58, e265–e271. [Google Scholar] [CrossRef]
- Mascarenhas, W.; Makhoul, N. Efficient in-house 3D printing of an orthognathic splint for single-jaw cases. Int. J. Oral Maxillofac. Surg. 2021, 50, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.H.; Liu, Q.; Chen, A.; Kuang, T.; Yuan, P.; Gateno, J.; Kim, D.; Barber, J.C.; Xiong, K.G.; Yu, P.; et al. Clinical feasibility of deep learning-based automatic head CBCT image segmentation and landmark detection in computer-aided surgical simulation for orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2023, 52, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Lampen, N.; Kim, D.; Fang, X.; Xu, X.; Kuang, T.; Deng, H.H.; Barber, J.C.; Gateno, J.; Xia, J.; Yan, P. Deep learning for biomechanical modeling of facial tissue deformation in orthognathic surgical planning. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Deng, H.; Lian, C.; Kuang, T.; Liu, Q.; Ma, L.; Lang, Y.; Chen, X.; Kim, D.; Gateno, J.; et al. Unsupervised learning of reference bony shapes for orthognathic surgical planning with a surface deformation network. Med. Phys. 2021, 48, 7735–7746. [Google Scholar] [CrossRef]
- Ettinger, R.E.; Mercan, E.; Podolsky, D.; Susarla, S.M. Defining the Safe Zone for the Low Medial Horizontal Cut in the Sagittal Split Osteotomy. J. Oral Maxillofac. Surg. 2022, 80, 822–826. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammit, D.; Ettinger, R.E.; Sanati-Mehrizy, P.; Susarla, S.M. Current Trends in Orthognathic Surgery. Medicina 2023, 59, 2100. https://doi.org/10.3390/medicina59122100
Zammit D, Ettinger RE, Sanati-Mehrizy P, Susarla SM. Current Trends in Orthognathic Surgery. Medicina. 2023; 59(12):2100. https://doi.org/10.3390/medicina59122100
Chicago/Turabian StyleZammit, Domenick, Russell E. Ettinger, Paymon Sanati-Mehrizy, and Srinivas M. Susarla. 2023. "Current Trends in Orthognathic Surgery" Medicina 59, no. 12: 2100. https://doi.org/10.3390/medicina59122100




