Choice of Methodology Impacts Outcome in Indirect Comparisons of Drugs for Idiopathic Pulmonary Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Forced Vital Capacity (FVC)
3.2. Other Endpoints
4. Discussion
Limitations
5. Conclusions
- Whether the SMD is appropriate in this population or whether a bivariate approach could be used [26];
- The functional form of FVC over time to consider the viability of synthesising endpoints across different timepoints;
- Whether the study populations are sufficiently homogeneous to fit a fixed effect model, whether random effects should be preferred, or whether meta-regression would be plausible;
- The efficacy of the combined pirfenidone/nintedanib treatment. As this does not connect to the evidence network, a different methodology such as population matching would be required [27].
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Swigris, J.J.; Stewart, A.L.; Gould, M.K.; Wilson, S.R. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual. Life Outcomes 2005, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Caldwell, D.M.; Ades, A.E.; Higgins, J.P. Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 2005, 331, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Ades, A.E. Evidence synthesis for decision making 1: Introduction. Med. Decis. Making 2013, 33, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.J.; Abrams, K.R. Bayesian methods in meta-analysis and evidence synthesis. Stat. Methods Med. Res. 2001, 10, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Heneghan, C. Interpreting meta-analysis in systematic reviews. Evid. Based Med. 2008, 13, 67–69. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity--Subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Making 2013, 33, 618–640. [Google Scholar] [CrossRef]
- Canestaro, W.J.; Forrester, S.H.; Raghu, G.; Ho, L.; Devine, B.E. Drug treatment of idiopathic pulmonary fibrosis: systematic review and network meta-analysis. Chest 2016, 149, 756–766. [Google Scholar] [CrossRef]
- Fleetwood, K.; McCool, R.; Glanville, J.; Edwards, S.C.; Gsteiger, S.; Daigl, M.; Fisher, M. Systematic review and network meta-analysis of idiopathic pulmonary fibrosis treatments. J. Manag. Care Spec. Pharm. 2017, 23, s5–s16. [Google Scholar] [CrossRef]
- Loveman, E.; Copley, V.R.; Scott, D.A.; Colquitt, J.L.; Clegg, A.J.; O’Reilly, K.M. Comparing new treatments for idiopathic pulmonary fibrosis--A network meta-analysis. BMC Pulm. Med. 2015, 15, 37. [Google Scholar] [CrossRef]
- Loveman, E.; Copley, V.R.; Colquitt, J.L.; Scott, D.A.; Clegg, A.J.; Jones, J.; O’Reilly, K.M.; Singh, S.; Bausewein, C.; Wells, A. The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: Systematic review, network meta-analysis, and health economic evaluation. BMC Pharmacol. Toxicol. 2014, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Neupane, B.; Zhang, Y.; Garcia, C.C.; Raghu, G.; Richeldi, L.; Brozek, J.; Beyene, J.; Schünemann, H. Treatment of idiopathic pulmonary fibrosis: A network meta-analysis. BMC Med. 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Skandamis, A.; Kani, C.; Markantonis, S.L.; Souliotis, K. Systematic review and network meta-analysis of approved medicines for the treatment of idiopathic pulmonary fibrosis. J. Drug Assess. 2019, 8, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Dai, H.P.; Kang, J.; Chen, B.Y.; Sun, T.Y.; Xu, Z.J. Double-blind randomized trial of pirfenidone in chinese idiopathic pulmonary fibrosis patients. Medicine 2015, 94, e1600. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Taniguchi, H.; Azuma, A.; Inoue, Y.; Kondoh, Y.; Hasegawa, Y.; Bando, M.; Abe, S.; Mochizuki, Y.; Chida, K.; et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Nukiwa, T.; Tsuboi, E.; Suga, M.; Abe, S.; Nakata, K.; Taguchi, Y.; Nagai, S.; Itoh, H.; Ohi, M.; et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Ebina, M.; Kondoh, Y.; Ogura, T.; Azuma, A.; Suga, M.; Taguchi, Y.; Takahashi, H.; Nakata, K.; Sato, A.; et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 821–829. [Google Scholar] [CrossRef]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E., Jr.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): Two randomised trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Richeldi, L.; Costabel, U.; Selman, M.; Kim, D.S.; Hansell, D.M.; Nicholson, A.G.; Brown, K.K.; Flaherty, K.R.; Noble, P.W.; Raghu, G.; et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2011, 365, 1079–1087. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat. Med. 2000, 19, 3127–3131. [Google Scholar] [CrossRef]
- Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed.; Egger, M.; Davey Smith, G.; Altman, D.G. (Eds.) BMJ Publishing Group: London, UK, 2001. [Google Scholar]
- King, T.E., Jr.; Albera, C.; du Bois, R.M.; Bradford, W.; Costabel, U.; Noble, P.W.; Sahn, S.A.; Valeyre, D. The Effect of treatment with pirfenidone on longitudinal change in lung volume in patients with idiopathic pulmonary fibrosis (IPF): A meta-analysis of outcomes in four randomized controlled clinical trials. Am. J. Respir. Crit. Care Med. 2011, 183, A5302. [Google Scholar]
- Nathan, S.D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glaspole, I.; Glassberg, M.K.; Kardatzke, D.R.; Daigl, M.; Kirchgaessler, K.U.; Lancaster, L.H.; et al. Effect of pirfenidone on mortality: Pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir. Med. 2017, 5, 33–41. [Google Scholar] [CrossRef]
- Ades, A.E.; Lu, G.; Dias, S.; Mayo-Wilson, E.; Kounali, D. Simultaneous synthesis of treatment effects and mapping to a common scale: An alternative to standardisation. Res. Synth. Methods 2015, 6, 96–107. [Google Scholar] [CrossRef]
- Phillippo, D.M.; Ades, A.E.; Dias, S.; Palmer, S.; Abrams, K.R.; Welton, N.J. Methods for population-adjusted indirect comparisons in health technology appraisal. Med. Decis. Making 2018, 38, 200–211. [Google Scholar] [CrossRef]
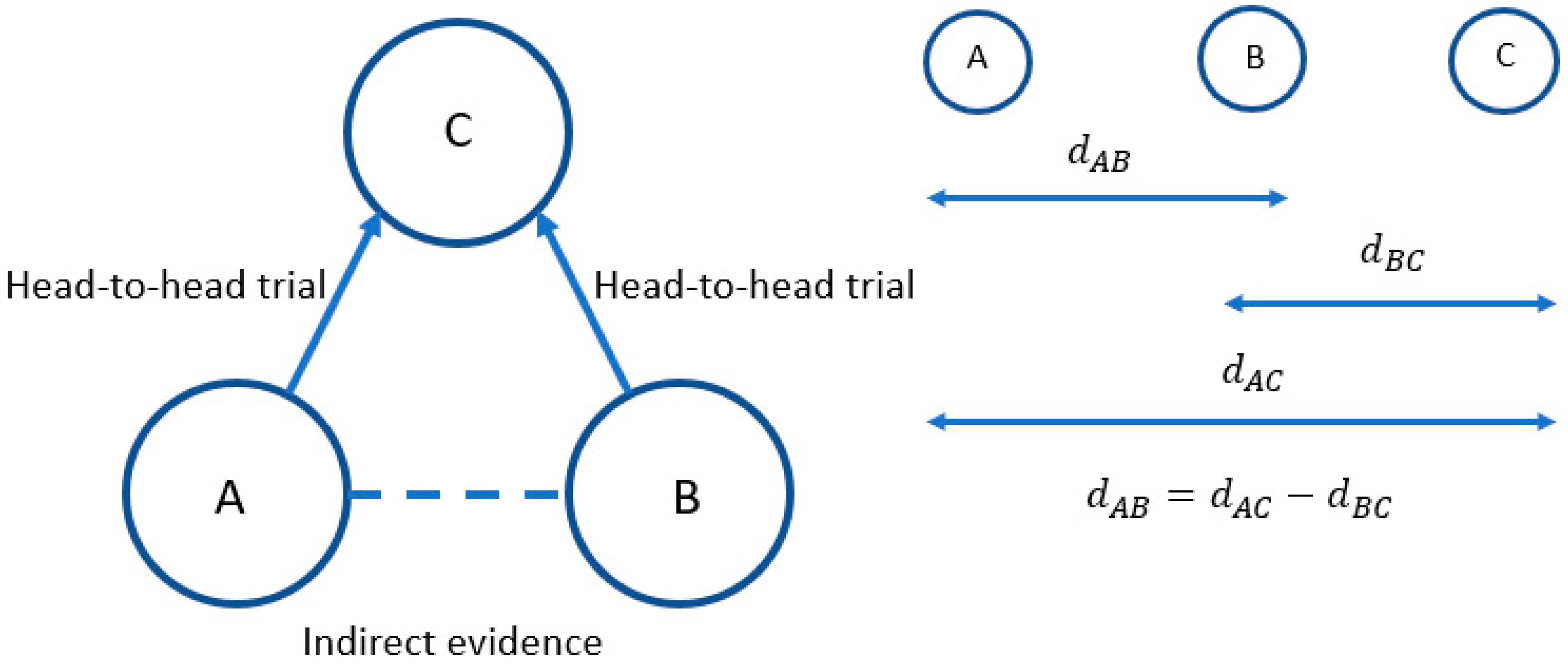
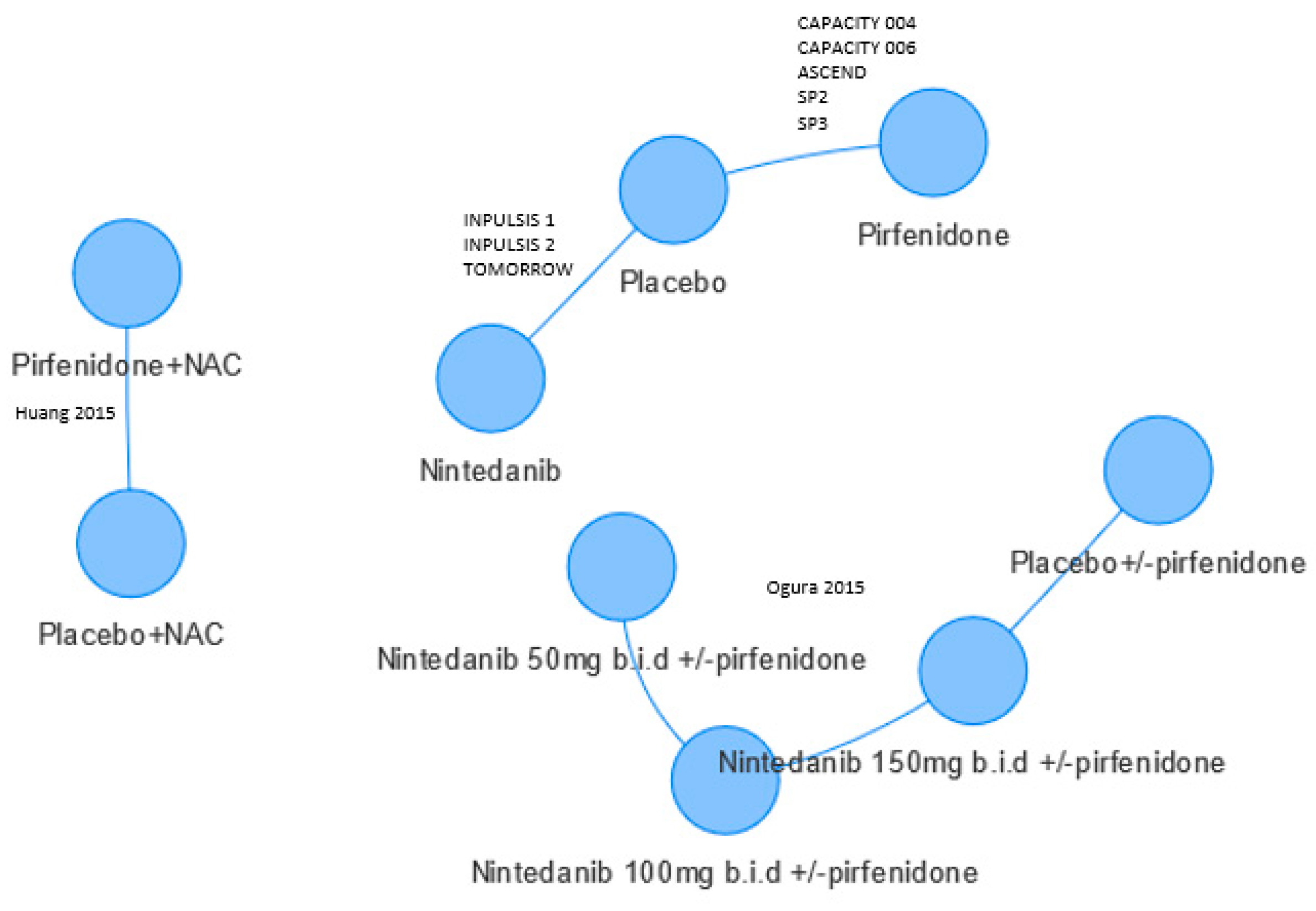
| Relevant RCTs of Nintedanib or Pirfenidone | Inclusion of RCTs (for at Least One Outcome) in the NMAs | |||||
|---|---|---|---|---|---|---|
| Trial Name, Phase, Forced Vital Capacity (FVC) Outcome, and Timepoints | NMA | |||||
| Fleetwood, 2017 [9] Bayesian Markov chain Monte Carlo (MCMC) Methods, Random Effects | Rochwerg, 2016 [12] Bayesian MCMC Methods, Random Effects | Canestaro, 2016 [8] Bayesian MCMC Methods, Fixed Effects | Loveman 2015 [10] Bayesian MCMC Methods, Fixed Effects | Loveman 2014 [11] Bayesian MCMC Methods, Fixed Effects | Skandamis 2018 [13] (poster only) Bayesian MCMC Methods, Random Effects | |
| SP3 [16], Phase II % predicted at 52 weeks a; Litres at 36 weeks | ✓ b | ✓ | ✓ | ✓ | ✓ | ✓ |
| SP2 [17], Phase III % predicted at 52 weeks a; Litres at 52 weeks | ✓ b | ✓ | ✓ | ✓ | ✓ | ✓ |
| Capacity 004 [18], Phase III % predicted at 72 weeks; Litres at 48 and 52 weeks a | ✓ b | ✓c | ✓ c | ✓ | ✓ | ✓ |
| Capacity 006 [18], Phase III % predicted at 72 weeks; Litres at 48 and 52 weeks a | ✓ b | ✓c | ✓ c | ✓ | ✓ | ✓ |
| ASCEND [19], Phase III % predicted at 52 weeks a; Litres at 52 weeks | ✓ b | ✓ | ✓ | ✓ | Not included | ✓ |
| TOMORROW [20], Phase III % predicted at 52 weeks; Litres at 52 weeks | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| INPULSIS 1 [21], Phase II % predicted at 52 weeks; Litres at 52 weeks | ✓ | ✓c | ✓ c | ✓ | Not included | ✓ |
| INPULSIS 2 [21], Phase II % predicted at 52 weeks; Litres at 52 weeks | ✓ | ✓c | ✓ c | ✓ | Not included | ✓ |
| Huang, 2015 [14] Phase II Litres at 48 weeks; % predicted at 48 weeks | Not included | Not included | Not included | Not included | Not included | ✓ |
| Ogura, 2015 [15] Phase II Not reported | Not included | Not included | Not included | Not included | Not included | ✓ |
| Trial Name, Phase | Intervention, n | Comparator, n | Duration of Treatment | Mean Age | % Male | Time Since Diagnosis | Mean % Predicted FVC | Risk of Bias a |
|---|---|---|---|---|---|---|---|---|
| SP3 [16], Phase II | Pirfenidone 1800 mg/day, n = 73 | Placebo, n = 36 | 39 weeks | 64 | 90 | <1 year: 22% | 80 | Unclear |
| SP2 [17], Phase III | Pirfenidone 1800 mg/day, n = 108 | Placebo, n = 104 | 52 weeks | 65 | 78 | <1 year: 37% | 78 | Unclear |
| Capacity 004 [18], Phase III | Pirfenidone 2403 mg/day, n = 174 | Placebo, n = 174 | 72 weeks | 66 | 71 | ≤1 year: 48% | 75 | Low |
| Capacity 006 [18], Phase III | Pirfenidone 2403 mg/day, n = 171 | Placebo, n = 173 | 72 weeks | 67 | 72 | ≤1 year: 59% | 74 | Low |
| ASCEND [19], Phase III | Pirfenidone 2403 mg/day, n = 278 | Placebo, n = 277 | 52 weeks | 68 | 78 | 1.7 years | 68 | Low |
| TOMORROW [20], Phase III | Nintedanib 300 mg/day, n = 85 | Placebo, n = 85 | 52 weeks | 65 | 75 | 1.2 years | 80 | Low |
| INPULSIS 1 [21], Phase II | Nintedanib 300 mg/day, n = 309 | Placebo, n = 204 | 52 weeks | 67 | 81 | 1.7 years | 80 | Low |
| INPULSIS 2 [21], Phase II | Nintedanib 300 mg/day, n = 329 | Placebo, n = 219 | 52 weeks | 67 | 78 | 1.6 years | 79 | Low |
| Huang 2015 [14], Phase II | Pirfenidone 1800 mg/day + NAC, n = 38 | Placebo + NAC, n = 38 | 48 weeks | 60 | 93 | Not reported | 77 | Unclear |
| Ogura 2015 [15], Phase II | Nintedanib b 100 mg/day, n = 6; 200 mg/day, n = 8; 300 mg/day, n = 24 | Placebo b, n = 12 | up to 28 days | 65 | 70 | Not reported | 74 | Unclear |
| Outcome | NMA | |||||
|---|---|---|---|---|---|---|
| Fleetwood, 2017 [9] Bayesian MCMC Methods, Random Effects | Rochwerg, 2016 [12] Bayesian MCMC Methods, Random Effects | Canestaro, 2016 [8] Bayesian MCMC Methods, Fixed Effects | Loveman 2015 [10] Bayesian MCMC Methods, Fixed Effects | Loveman 2014 [11] Bayesian MCMC Methods, Fixed Effects | Skandamis 2018 [13] (poster only) Bayesian MCMC Methods, Random Effects | |
| Change in % predicted FVC | WMD −0.23 (−2.13, 1.66) | Not estimated | Not estimated | OR 0.67 (0.51, 0.88) a | OR 0.56 (0.31, 1.03) | Not estimated |
| Change in FVC Litres | WMD −0.01 (−0.15, 0.13) | Not estimated | Not estimated | Not estimated | ||
| >10% decline in FVC | OR 1.11 (0.60, 2.0) | Not estimated | OR 1.16 (0.83, 1.67) | OR 1.21 (0.86, 1.72) | Not estimated | OR 1.10 (0.49, 2.22) |
| Mortality | OR 1.35 (0.51, 3.70) | OR 1.05 (0.45, 2.78) | OR 1.02 (0.55, 1.89) | OR 1.39 (0.7, 2.82) | Not estimated | OR 1.08 (0.52, 2.63) |
| Respiratory mortality | Not estimated | Not estimated | 1.09 (0.49, 2.38) | OR 2.1 (0.77, 6.17) | Not estimated | Not estimated |
| Serious adverse events | Not estimated | OR 1.04 (0.51, 2.24) | Not estimated | Not estimated | Not estimated | OR 0.98 (0.62, 1.61) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, D.A.; Loveman, E.; Colquitt, J.L.; O’Reilly, K. Choice of Methodology Impacts Outcome in Indirect Comparisons of Drugs for Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 443. https://doi.org/10.3390/medicina55080443
Scott DA, Loveman E, Colquitt JL, O’Reilly K. Choice of Methodology Impacts Outcome in Indirect Comparisons of Drugs for Idiopathic Pulmonary Fibrosis. Medicina. 2019; 55(8):443. https://doi.org/10.3390/medicina55080443
Chicago/Turabian StyleScott, David A., Emma Loveman, Jill L. Colquitt, and Katherine O’Reilly. 2019. "Choice of Methodology Impacts Outcome in Indirect Comparisons of Drugs for Idiopathic Pulmonary Fibrosis" Medicina 55, no. 8: 443. https://doi.org/10.3390/medicina55080443





