Inflammatory Cytokine and Chemokine Patterns in Paediatric Patients with Suspected Serious Bacterial Infection
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, M.; Van den Bruel, A.; Verbakel, J.; Lakhanpaul, M.; Haj-Hassan, T.; Stevens, R.; Moll, H.; Buntinx, F.; Berger, M.; Aertgeerts, B.; et al. Systematic review and validation of prediction rules for identifying children with serious infections in emergency departments and urgent-access primary care. Health Technol. Assess. 2012, 16, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, J.Y.; Van den Bruel, A.; Thompson, M.; Stevens, R.; Aertgeerts, B.; Oostenbrink, R.; Moll, H.A.; Berger, M.Y.; Lakhanpaul, M.; Mant, D.; et al. How well do clinical prediction rules perform in identifying serious infections in acutely ill children across an international network of ambulatory care datasets? BMC Med. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Van den Bruel, A.; Thompson, M.J.; Haj-Hassan, T.; Stevens, R.; Moll, H.; Lakhanpaul, M.; Mant, D. Diagnostic value of laboratory tests in identifying serious infections in febrile children: Systematic review. BMJ 2011, 342, d3082. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis. JAMA 2016, 315, 762. [Google Scholar] [CrossRef] [PubMed]
- Meem, M.; Modak, J.K.; Mortuza, R.; Morshed, M.; Islam, M.S.; Saha, S.K. Biomarkers for diagnosis of neonatal infections: A systematic analysis of their potential as a point-of-care diagnostics. J. Glob. Health 2011, 1, 201–209. [Google Scholar] [PubMed]
- Pierrakos, C.; Vincent, J.-L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.E.; Mave, V.; Gupta, A. Biomarkers for sepsis: A review with special attention to India. Biomed. Res. Int. 2014, 2014, 264351. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, L.G.; Sørup, S.; Aaby, P.; Benn, C.S.; Greisen, G.; Jeppesen, D.L.; Birk, N.M.; Kjærgaard, J.; Nissen, T.N.; Pihl, G.T.; et al. BCG vaccination at birth and early childhood hospitalisation: A randomised clinical multicentre trial. Arch. Dis. Child. 2017, 102, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, K.; Andersen, O.; Kronborg, G.; Tvede, M.; Petersen, J.; Eugen-Olsen, J.; Larsen, K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: A prospective study. Crit. Care 2007, 11, R38. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C.; Williams, G.J.; Jones, M.; Codarini, M.; Macaskill, P.; Hayen, A.; Irwig, L.; Fitzgerald, D.A.; Isaacs, D.; McCaskill, M. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: Prospective cohort study of 15 781 febrile illnesses. BMJ 2010, 340, c1594. [Google Scholar] [CrossRef] [PubMed]
- Irwin, A.D.; Marriage, F.; Mankhambo, L.A.; Jeffers, G.; Kolamunnage-Dona, R.; Guiver, M.; Denis, B.; Molyneux, E.M.; Molyneux, M.E.; Day, P.J.; et al. Novel biomarker combination improves the diagnosis of serious bacterial infections in Malawian children. BMC Med. Genom. 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.M.; Jardim-Botelho, A.; Williams, W.; Alexander, N.; Diemert, D.J.; Bethony, J.M. Serum CCL11 (eotaxin-1) and CCL17 (TARC) are serological indicators of multiple helminth infections and are driven by Schistosoma mansoni infection in humans. Trop. Med. Int. Health 2013, 18, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifabadi, A.R.; Hassanshahi, G.; Ghalebi, S.R.; Arababadi, M.K.; Khorramdelazad, H.; Zainodini, N.; Shabani, Z.I.B.A. All eotaxins CCL11, CCL24 and CCL26 are increased but to various extents in pulmonary tuberculosis patients. Clin. Lab. 2014, 60, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Sumino, K.C.; Walter, M.J.; Mikols, C.L.; Thompson, S.A.; Gaudreault-Keener, M.; Arens, M.Q.; Agapov, E.; Hormozdi, D.; Gaynor, A.M.; Holtzman, M.J.; et al. Detection of respiratory viruses and the associated chemokine responses in serious acute respiratory illness. Thorax 2010, 65, 639–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gathwala, G.; Walia, M.; Bala, H.; Singh, S. Recombinant Human Granulocyte Colony-stimulating Factor in Preterm Neonates with Sepsis and Relative Neutropenia: A Randomized, Single-Blind, Non-placebo-controlled Trial. J. Trop. Pediatr. 2012, 58, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, L.; Liu, Y.; Yang, M.; Wang, N.; Wang, K.; Ou, D.; Liu, M.; Chen, G.; Liu, K.; et al. Use of blood urea nitrogen, creatinine, interleukin-6, granulocyte–macrophage colony stimulating factor in combination to predict the severity and outcome of abdominal sepsis in rats. Inflamm. Res. 2012, 61, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Suguna Narasimhulu, S.; Hendricks-Muñoz, K.D.; Borkowsky, W.; Mally, P. Usefulness of Urinary Immune Biomarkers in the Evaluation of Neonatal Sepsis. Clin. Pediatr. 2013, 52, 520–526. [Google Scholar] [CrossRef] [PubMed]
| Title 1 | Severe Bacterial Infection (SBI) Patients (n = 21) | Patients without SBI (n = 30) |
|---|---|---|
| Age, median, months (±SD) | 43.0 ± 63.9 | 30.5 ± 59.62 |
| Sex, percent (n) | 42.9% (9) males 57.1% (12) females | 63.3% (19) males 36.7% (11) females |
| Inclusion day after symptom onset, median (±SD) | 5 ± 2.48 | 3 ± 3.50 |
| C-reactive protein (CRP), median (±SD), mg/L | 131.95 ± 111.94 | 35.65 ± 61.3 |
| Length of hospitalization, median (±SD) | 8 ± 7.81 | 3 ± 2.33 |
| Antibacterial therapy | 100% (21) | 50% (15) |
| Inflammatory Cytokines, Median (min–max) pg/mL | SBI (n = 21) | Patients without SBI (n = 30) | p Value |
|---|---|---|---|
| Soluble apoptosis-stimulating fragment (sFas) | 3356.23 (1606.69–6791.45) | 3740.37 (1925.82–6832.95) | p = 0.153 |
| Soluble vascular cell adhesion molecule (sVCAM1) | 1306.07 (568.63–5042.88) | 1010.95 (392.91–4197.20) | p = 0.243 |
| Total plasminogen activator inhibitor type 1 (tPAI-1) | 147.80 (72.01–353.81) | 136.43 (56.80–327.74) | p = 0.389 |
| Interleukin 8 (IL-8) | 12.6 (1.56–158.57) | 10.2 (4.00–35.70) | p = 0.723 |
| Interleukin 10 (IL-10) | 30.10 (16.22–7127.79) | 40.35 (9.71–3365.79) | p = 0.841 |
| Interferon gamma (INF-gamma) | 16.9 (0.13–172.36) | 13.6 (0.34–838.20) | p = 0.688 |
| Tumor necrosis factor alpha (TNF-alfa) | 13.97 (0.67–100.41) | 13.99 (6.42–35.24) | p = 0.566 |
| Eotaxin | 50.23 (14.80–107.76) | 73.61 (13.50–107.76) | p = 0.035 |
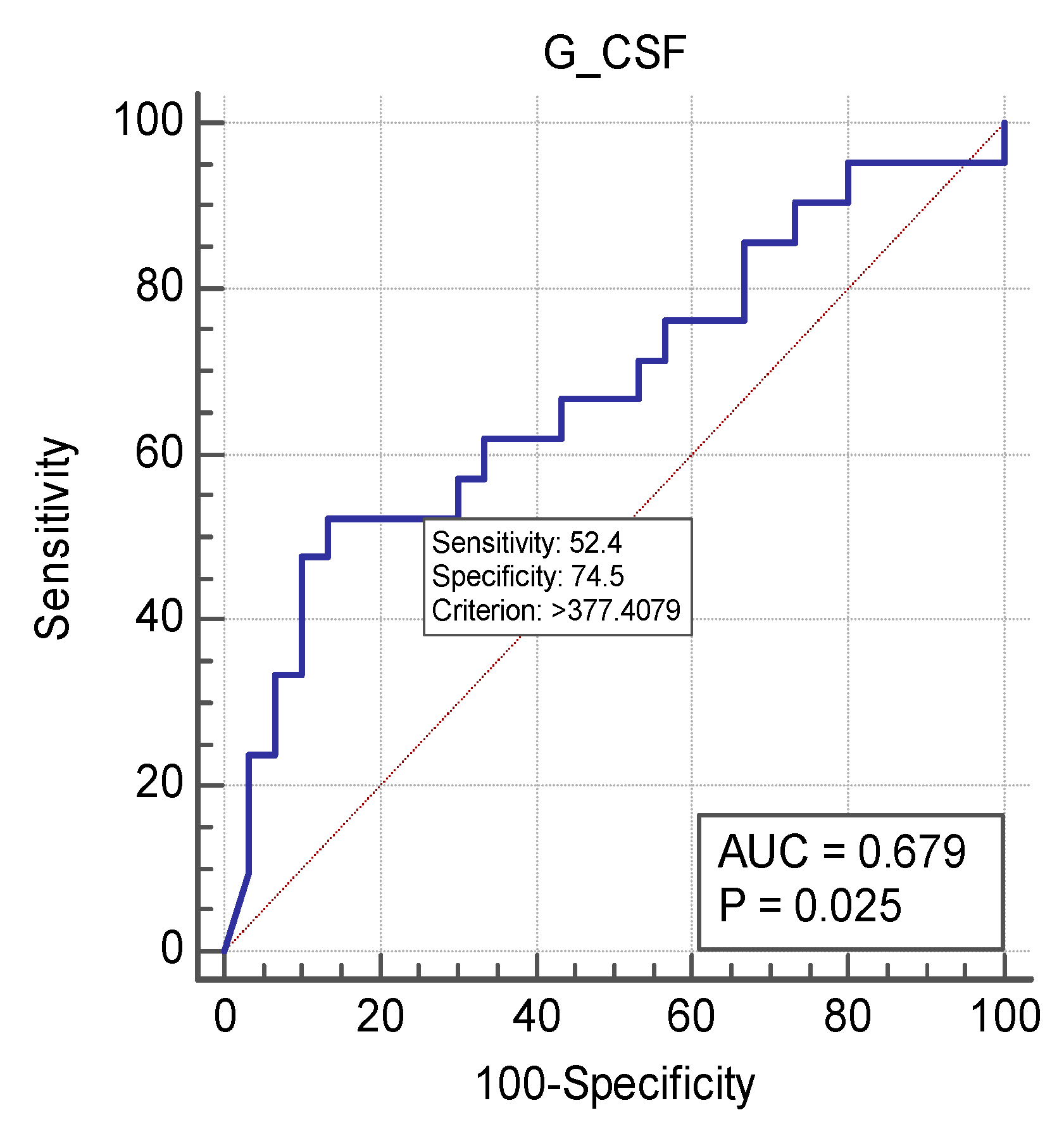
| Granulocyte colony-stimulating factor (G-CSF) | 504.69 (18.88–10,000) | 187.27 (29.19–10,000) | p = 0.031 |
| Interleukin-1 receptor antagonist (IL1ra) | 4.94 (3.20–158.60) | 16.1 (2.00–125.17) | p = 0.601 |
| Interferon-inducible protein-10 (IP10) | 977.78 (218.74–10,000) | 1070.93 (100.60–10,000) | p = 0.836 |
| Monocyte chemoattractant protein-1 (MCP1) | 319.05 (126.35–4788.12) | 411.28 (39.15–5763.23) | p = 0.168 |
| Inflammatory Cytokines, Median (min-max) pg/mL | Time of Inclusion | After 24 h | p Value |
|---|---|---|---|
| Soluble apoptosis-stimulating fragment (sFas) | 3356.23 (1606.69–6791.45) | 3530.31 (1925.82–7553.48) | p = 0.041 |
| Soluble vascular cell adhesion molecule (sVCAM1) | 1306.07 (568.63–5042.88) | 919.10 (628.18–4859.74) | p = 0.078 |
| Total plasminogen activator inhibitor type 1 (tPAI-1) | 147.80 (72.01–353.81) | 154.99 (92.44–286.00) | p = 0.383 |
| Interleukin 8 (IL-8) | 12.6 (1.56–158.57) | 8.78 (2.89–105.36) | p = 0.0383 |
| Interleukin 10 (IL-10) | 30.10 (16.22–7127.79) | 26.12 (6.47–3385.94) | p = 0.027 |
| Interferon gamma (INF-gamma) | 16.9 (0.13–172.36) | 5.18 (0.13–120.94) | p = 0.016 |
| Tumor necrosis factor alpha (TNF-alfa) | 13.97 (0.67–100.41) | 12.42 (4.74–73.12) | p = 1.000 |
| Eotaxin | 50.23 (14.80–107.76) | 64.02 (20.49–122.77) | p = 0.027 |
| Granulocyte colony-stimulating factor (G-CSF) | 504.69 (18.88–10,000) | 129.75 (29.19–2074.56) | p < 0.001 |
| Interleukin-1 receptor antagonist (IL1ra) | 4.94 (3.20–158.60) | 3.20 (1.66–77.85) | p = 0.146 |
| Interferon-inducible protein-10 (IP10) | 977.78 (218.74–10,000) | 576.579 (161.67–10,000) | p = 0.012 |
| Monocyte chemoattractant protein-1 (MCP1) | 319.05 (126.35–4788.12) | 309.16 (65.71–4327.81) | p = 0.383 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautiainen, L.; Pavare, J.; Grope, I.; Tretjakovs, P.; Gardovska, D. Inflammatory Cytokine and Chemokine Patterns in Paediatric Patients with Suspected Serious Bacterial Infection. Medicina 2019, 55, 4. https://doi.org/10.3390/medicina55010004
Rautiainen L, Pavare J, Grope I, Tretjakovs P, Gardovska D. Inflammatory Cytokine and Chemokine Patterns in Paediatric Patients with Suspected Serious Bacterial Infection. Medicina. 2019; 55(1):4. https://doi.org/10.3390/medicina55010004
Chicago/Turabian StyleRautiainen, Linda, Jana Pavare, Ilze Grope, Peteris Tretjakovs, and Dace Gardovska. 2019. "Inflammatory Cytokine and Chemokine Patterns in Paediatric Patients with Suspected Serious Bacterial Infection" Medicina 55, no. 1: 4. https://doi.org/10.3390/medicina55010004





