Discrimination between Obstructive Coronary Artery Disease and Cardiac Syndrome X in Women with Typical Angina and Positive Exercise Test; Utility of Cardiovascular Risk Calculators
Abstract
:1. Introduction
2. Methods
3. Statistical Analysis
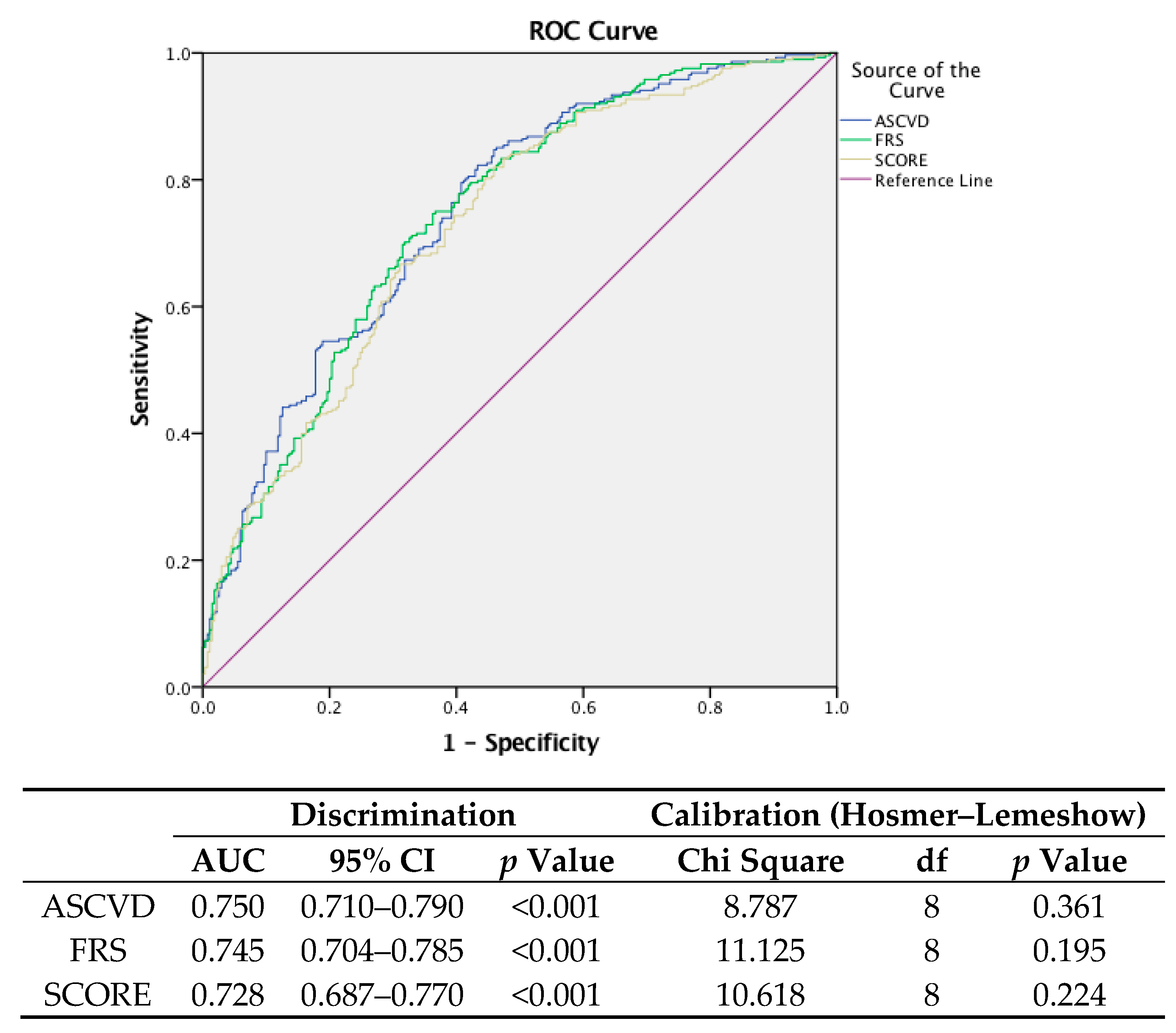
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, S.; Walker, A.; Hügli, O.; Togni, M.; Meier, B. Percutaneous coronary interventions in Europe. Clin. Res. Cardiol. 2007, 96, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, S.; Arora, R.; Khosla, S. Cardiac syndrome X: Current concepts. Int. J. Cardiol. 2010, 142, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Panting, J.R.; Gatehouse, P.D.; Yang, G.-Z.; Grothues, F.; Firmin, D.N.; Collins, P.; Pennell, D.J. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N. Engl. J. Med. 2002, 346, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Schmidt, M.; Schwinger, R. Typical angina pectoris complaints without coronary macroangiopathy. Dtsch. Med. Wochenschr. 2005, 130, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Asbury, E.A.; Collins, P. Cardiac syndrome, X. Int. J. Clin. Pract. 2005, 59, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A. Cardiac syndrome X: A critical overview and future perspectives. Heart 2007, 93, 159–166. [Google Scholar] [CrossRef]
- Vermeltfoort, I.; Raijmakers, P.; Riphagen, I.; Odekerken, D.; Kuijper, A.; Zwijnenburg, A.; Teule, G.J.J. Definitions and incidence of cardiac syndrome X: Review and analysis of clinical data. Clin. Res. Cardiol. 2010, 99, 475–481. [Google Scholar] [CrossRef]
- Glaser, R.; Herrmann, H.C.; Murphy, S.A.; Demopoulos, L.A.; DiBattiste, P.M.; Cannon, C.P.; Braunwald, E. Benefit of an early invasive management strategy in women with acute coronary syndromes. Jama 2002, 288, 3124–3129. [Google Scholar] [CrossRef]
- Hochman, J.S.; Tamis, J.E.; Thompson, T.D.; Weaver, W.D.; White, H.D.; Van de Werf, F.; Aylward, P.; Topol, E.J.; Califf, R.M. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N. Engl. J. Med. 1999, 341, 226–232. [Google Scholar] [CrossRef]
- Johnson, L.W.; Krone, R. Cardiac catheterization 1991: A report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I). Catheter. Cardiovasc. Diagn. 1993, 28, 219–220. [Google Scholar]
- Lichtlen, P.R.; Bargheer, K.; Wenzlaff, P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J. Am. Coll. Cardiol. 1995, 25, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Giannoglou, G.D.; Antoniadis, A.P.; Chatzizisis, Y.S.; Damvopoulou, E.; Parcharidis, G.E.; Louridas, G.E. Sex-related differences in the angiographic results of 14,500 cases referred for suspected coronary artery disease. Coron. Artery Dis. 2008, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kemp, H.G. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am. J. Cardiol. 1973, 32, 375–376. [Google Scholar] [CrossRef]
- Kaski, J.C.; Rosano, G.M.; Collins, P.; Nihoyannopoulos, P.; Maseri, A.; Poole-Wilson, P.A. Cardiac syndrome X: Clinical characteristics and left ventricular function: Long-term follow-up study. J. Am. Coll. Cardiol. 1995, 25, 807–814. [Google Scholar] [CrossRef]
- Sullivan, A.K.; Holdright, D.R.; Wright, C.A.; Sparrow, J.L.; Cunningham, D.; Fox, K.M. Chest pain in women: Clinical, investigative, and prognostic features. BMJ 1994, 308, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani-Farahani, A.; Nouri, N.; Seifirad, S.; Fathollahi, M.S.; Hakki, E.; Alidoosti, M.; Davoodi, G.; Masoudkabir, F.; Poorhosseini, H. Comparison of cardiovascular risk factors and biochemical profile in patients with cardiac syndrome X and obstructive coronary artery disease: A propensity score-matched study. ARYA Atheroscler. 2013, 9, 269. [Google Scholar]
- Lier, T. Characteristics of Midlife Women with Coronary Microvascular Dysfunction, Compared with Age-Matched Women with Obstructive Coronary Disease. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Parvin, T.; Rahman, M.; Ferdousi, S.; Shahnaz, A.; Mahal, M.; Ahmed, S.; Ferdous, B.A.; Hussain, D.A.S. Blood Lipid Profile in Acute Coronary Syndrome and Chronic Stable Angina Patients. Bangladesh J. Med. Biochem. 2015, 7, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Pryor, D.B.; Shaw, L.; McCants, C.B.; Lee, K.L.; Mark, D.B.; Harrell, F.E.; Muhlbaier, L.H.; Califf, R.M. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann. Intern. Med. 1993, 118, 81–90. [Google Scholar] [CrossRef]
- Morise, A.P.; Haddad, W.J.; Beckner, D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am. J. Med. 1997, 102, 350–356. [Google Scholar] [CrossRef]
- Diamond, G.A.; Forrester, J.S. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N. Engl. J. Med. 1979, 300, 1350–1358. [Google Scholar] [CrossRef]
- Pryor, D.B.; Harrell, F.E.; Lee, K.L.; Califf, R.M.; Rosati, R.A. Estimating the likelihood of significant coronary artery disease. Am. J. Med. 1983, 75, 771–780. [Google Scholar] [CrossRef]
- Masoudkabir, F.; Vasheghani-Farahani, A.; Kassaian, S.E. A novel scoring system for prediction of cardiac syndrome X in women with typical angina and positive exercise tolerance test: implications for non-invasive imaging. J. Am. Coll. Cardiol. 2015, 65, A1621. [Google Scholar] [CrossRef]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.; Pyörälä, K.; Fitzgerald, A.E.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. Jama 2001, 285, 2864–2870. [Google Scholar] [CrossRef]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N. Guidelines for the management of atrial fibrillationThe Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar]
- Schnabel, R.B.; Sullivan, L.M.; Levy, D.; Pencina, M.J.; Massaro, J.M.; D’Agostino, R.B.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Soliman, E.Z.; Chambless, L.E.; Crow, R.; Ambrose, M.; Alonso, A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am. J. Cardiol. 2011, 107, 85–91. [Google Scholar] [CrossRef]
- Lau, K.-K.; Chan, P.-H.; Yiu, K.-H.; Chan, Y.-H.; Liu, S.; Chan, K.-H.; Yeung, C.-Y.; Li, S.-W.; Tse, H.-F.; Siu, C.-W. Roles of the CHADS2 and CHA2DS2-VASc scores in post-myocardial infarction patients: Risk of new occurrence of atrial fibrillation and ischemic stroke. Cardiol. J. 2014, 21, 474–483. [Google Scholar] [CrossRef]
- Hudzik, B.; Szkodziński, J.; Hawranek, M.; Lekston, A.; Poloński, L.; Gąsior, M. CHA2DS2-VASc score is useful in predicting poor 12-month outcomes following myocardial infarction in diabetic patients without atrial fibrillation. Acta Diabetol. 2016, 53, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kim, W.; Hwang, S.H.; Kang, W.Y.; Cho, S.C.; Kim, W.; Jeong, M.H.; Korean Working Group in Myocardial Infarction Registry Investigators. The CHA 2 DS 2 VASc score can be used to stratify the prognosis of acute myocardial infarction patients irrespective of presence of atrial fibrillation. J. Cardiol. 2015, 65, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.-K.; Lo, H.-M.; Chiu, C.-Z.; Shyu, K.-G. Use of CHADS 2 and CHA 2 DS 2-VASc Scores to Predict Subsequent Myocardial Infarction, Stroke, and Death in Patients with Acute Coronary Syndrome: Data from Taiwan Acute Coronary Syndrome Full Spectrum Registry. PLoS ONE. 2014, 9, e111167. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Battler, A.; Brindis, R.G.; Cox, J.L.; Ellis, S.G.; Every, N.R.; Flaherty, J.T.; Harrington, R.A.; Krumholz, H.M.; Simoons, M.L.; et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. J. Am. Coll. Cardiol. 2001, 38, 2114–2130. [Google Scholar] [PubMed]
- Gazzaruso, C.; Garzaniti, A.; Giordanetti, S.; Falcone, C.; Fratino, P. Silent coronary artery disease in type 2 diabetes mellitus: The role of Lipoprotein (a), homocysteine and apo (a) polymorphism. Cardiovasc. Diabetol. 2002, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; King, S.B.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 60, e44–e164. [Google Scholar] [PubMed]
- Wong, Y.; Rodwell, A.; Dawkins, S.; Livesey, S.A.; Simpson, I.A. Sex differences in investigation results and treatment in subjects referred for investigation of chest pain. Heart 2001, 85, 149–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Bailey, S.R.; Bonow, R.O.; Chambers, C.E.; Chan, P.S.; Dehmer, G.J.; Kirtane, A.J.; Wann, L.S.; Ward, R.P. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012, 59, 1995–2027. [Google Scholar]
- Ridker, P.M.; Cook, N.R. Statins: New American guidelines for prevention of cardiovascular disease. Lancet 2013, 382, 1762–1765. [Google Scholar] [CrossRef]
- DeFilippis, A.P.; Young, R.; Carrubba, C.J.; McEvoy, J.W.; Budoff, M.J.; Blumenthal, R.S.; Kronmal, R.A.; McClelland, R.L.; Nasir, K.; Blaha, M.J. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann. Intern. Med. 2015, 162, 266–275. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N.R. Comparing Cardiovascular Risk Prediction ScoresComparing Cardiovascular Risk Prediction Scores. Ann. Intern. Med. 2015, 162, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Beanlands, R.S.; Chow, B.J.; Dick, A.; Friedrich, M.G.; Gulenchyn, K.Y.; Kiess, M.; Leong-Poi, H.; Miller, R.M.; Nichol, G.; Freeman, M.; et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease--executive summary. Can. J. Cardiol. 2007, 23, 107–119. [Google Scholar] [PubMed]
- Di Carli, M.F.; Hachamovitch, R. New technology for noninvasive evaluation of coronary artery disease. Circulation 2007, 115, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Schuijf, J.D.; Bax, J.J.; Shaw, L.J.; de Roos, A.; Lamb, H.J.; van der Wall, E.E.; Wijns, W. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am. Heart J. 2006, 151, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.J.; Abraham, A.; Wells, G.A.; Chen, L.; Ruddy, T.D.; Yam, Y.; Govas, N.; Galbraith, P.D.; Dennie, C.; Beanlands, R.S. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ. Cardiovasc. Imaging 2009, 2, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 2010, 56, 1864–1894. [Google Scholar] [PubMed]
- Kones, R. Primary prevention of coronary heart disease: Integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des. Dev. Ther. 2011, 5, 325–380. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Pike, M.M.; Decker, P.A.; Larson, N.B.; Sauver, J.L.S.; Takahashi, P.Y.; Roger, V.L.; Rocca, W.A.; Miller, V.M.; Olson, J.E.; Pathak, J.; et al. Improvement in Cardiovascular Risk Prediction with Electronic Health Records. J. Cardiovasc. Transl. Res. 2016, 9, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, P.; Gupta, D.; Chatterjee, S.; Huston, J.; Ryan, J.J. A review of the role of electronic health record in genomic research. J. Cardiovasc. Transl. Res. 2014, 7, 692–700. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Brindle, P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: Cohort study using QResearch database. BMJ 2010, 341, c6624. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J. Cardiovascular Disease Risk Scores and Novel Risk Factors in Relation to Race and Gender. 2016. Graduate Theses and Dissertations. Available online: http://scholarcommons.usf.edu/etd/6434 (accessed on 14 June 2016).
- Ridker, P.M.; Buring, J.E.; Rifai, N.; Cook, N.R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. Jama 2007, 297, 611–619. [Google Scholar] [CrossRef] [PubMed]
| CAD (288) (51.5%) | CSX (271) (48.5%) | Total (559) (100%) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age (Years) | 59.01 | (0.45) | 53.14 | (0.48) | 56.17 | (0.35) | <0.001 |
| DM, n (%) | 110 | (38.2%) | 57 | (21.0%) | 167 | (29.9%) | <0.001 |
| HTN, n (%) | 202 | (70.1%) | 129 | (47.8%) | 331 | (59.3%) | <0.001 |
| CS, n (%) | 208 | (72.7%) | 162 | (59.8%) | 370 | (66.2%) | <0.001 |
| FH, n (%) | 80 | (28.2%) | 64 | (23.7%) | 144 | (26.0%) | NS |
| BMI | 28.89 | (0.26) | 29.05 | (0.28) | 28.97 | (0.19) | NS |
| EF (%) | 58.69 | (0.37) | 59.19 | (0.33) | 58.93 | (0.25) | NS |
| SBP (mmHg) | 153.65 | (1.35) | 144.49 | (1.25) | 149.20 | (0.94) | <0.001 |
| DBP (mmHg) | 80.24 | (0.58) | 78.00 | (0.58) | 79.15 | (0.41) | 0.006 |
| T.Cho (mg/dL) | 208.74 | (2.82) | 195.04 | (2.17) | 202.10 | (1.93) | <0.001 |
| TG (mg/dL) | 181.03 | (5.26) | 157.30 | (4.93) | 169.52 | (3.64) | <0.001 |
| LDL (mg/dL) | 127.85 | (2.57) | 118.23 | (2.17) | 123.19 | (1.70) | 0.005 |
| HDL (mg/dL) | 47.92 | (0.59) | 48.91 | (0.71) | 48.40 | (0.46) | NS |
| Cr (g/dL) | 0.92 | (0.01) | 0.86 | (0.01) | 0.89 | (0.01) | NS |
| FBS (mg/dL) | 127.24 | (3.40) | 112.25 | (2.47) | 119.98 | (2.14) | <0.001 |
| CAD | CSX | Total | |||||
|---|---|---|---|---|---|---|---|
| ASCVD Risk (New Pooled Cohort Equation) | Less than 2.5% | 13.8% | 86.2% | 65 | (11.6%) | ||
| (5.42%–22.18%) | (77.82%–94.58%) | (8.95%–14.25%) | |||||
| 2.5% to 7.5% | 35.5% | 64.5% | 166 | (29.7%) | |||
| (28.22%–42.78%) | (57.22%–71.78%) | (25.91%–33.49%) | |||||
| 7.5% or More | 67.1% | 32.9% | 328 | (58.7%) | |||
| (62.02%–72.18%) | (27.82%–37.98%) | (54.62%–62.78%) | |||||
| Framingham Risk Score | Less than 7.5% | 11.3% | 88.7% | 81 | (14.4%) | ||
| (4.26%–18.24%) | (81.76%–95.64%) | (11.40%–17.20%) | |||||
| 7.5% to 15% | 37.6% | 62.4% | 133 | (23.8%) | |||
| (29.27%–45.73%) | (54.17%–70.63%) | (20.27%–27.33%) | |||||
| 15% or More | 66.4% | 33.6% | 345 | (61.8%) | |||
| (61.42%–71.38%) | (28.62%–38.58%) | (57.77%–65.83%) | |||||
| Euro Score | Less than 0.5% | 19.5% | 80.5% | 77 | (13.8%) | ||
| (10.65%–28.35%) | (71.65%–89.35%) | (10.94%–16.66%) | |||||
| 0.5% to 1.5% | 32.8% | 67.2% | 128 | (22.9%) | |||
| (24.67%–40.93%) | (59.07%–75.33%) | (19.42%–26.38%) | |||||
| More than 1.5% | 65.3% | 34.7% | 354 | (63.3%) | |||
| (60.34%–70.26%) | (29.74%–39.66%) | (59.61%–67.59%) | |||||
| Total | 288 | (51.5%) | 271 | (48.5%) | 559 (100%) | ||
| (47.36%–55.64%) | (44.36%–52.64%) | ||||||
| Association with CAD | Odds Ratio (95% Confidence Interval) | p-Value | ||
|---|---|---|---|---|
| ASCVD | <2.5% | Reference category | ref | |
| 2.5–7.5% | 3.694 | (2.495–5.470) | <0.001 | |
| ≥7.5% | 14.514 | (6.687–31.503) | <0.001 | |
| FRS | <7.5% | Reference category | ref | |
| 7.5–15% | 3.277 | (2.162–4.967) | <0.001 | |
| ≥15% | 17.767 | (8.277–38.137) | <0.001 | |
| SCORE | <0.5% | Reference category | ref | |
| 0.5–1.5% | 3.846 | (2.504–5.905) | <0.001 | |
| ≥1.5% | 8.451 | (4.551 -15.695) | <0.001 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadat, M.; Masoudkabir, F.; Afarideh, M.; Ghodsi, S.; Vasheghani-Farahani, A. Discrimination between Obstructive Coronary Artery Disease and Cardiac Syndrome X in Women with Typical Angina and Positive Exercise Test; Utility of Cardiovascular Risk Calculators. Medicina 2019, 55, 12. https://doi.org/10.3390/medicina55010012
Saadat M, Masoudkabir F, Afarideh M, Ghodsi S, Vasheghani-Farahani A. Discrimination between Obstructive Coronary Artery Disease and Cardiac Syndrome X in Women with Typical Angina and Positive Exercise Test; Utility of Cardiovascular Risk Calculators. Medicina. 2019; 55(1):12. https://doi.org/10.3390/medicina55010012
Chicago/Turabian StyleSaadat, Mohammad, Farzad Masoudkabir, Mohsen Afarideh, Saeed Ghodsi, and Ali Vasheghani-Farahani. 2019. "Discrimination between Obstructive Coronary Artery Disease and Cardiac Syndrome X in Women with Typical Angina and Positive Exercise Test; Utility of Cardiovascular Risk Calculators" Medicina 55, no. 1: 12. https://doi.org/10.3390/medicina55010012





