A Novel Supramolecular Assembly Film of Porphyrin Bound DNA: Characterization and Catalytic Behaviors Towards Nitric Oxide
Abstract
:Introduction
Experimental
Chemicals
Preparation of DNA-PADDA composite and cast films
Electrochemical apparatus
Quartz crystal microbalance (QCM) measurements
Results and Discussion
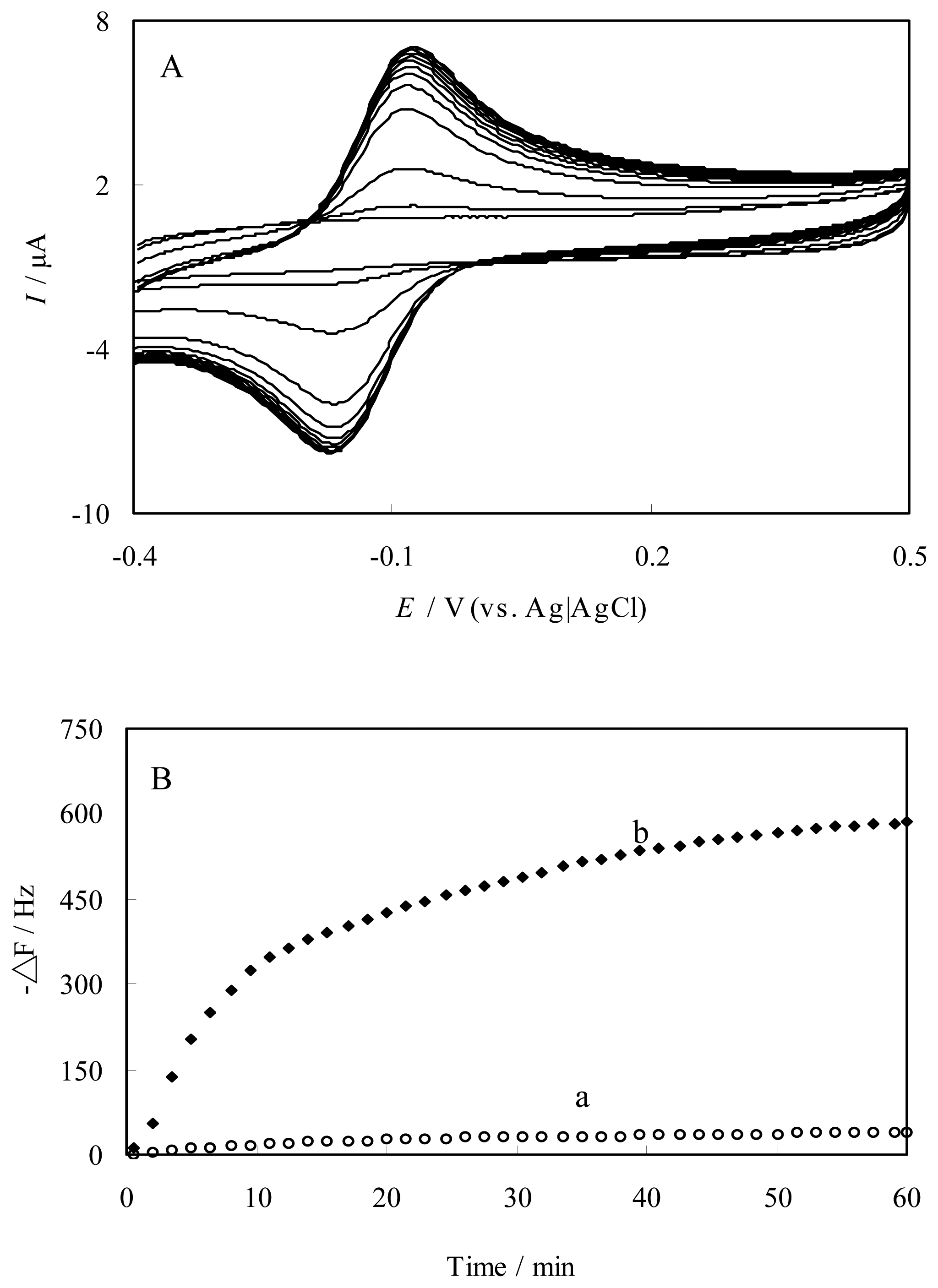
Preparation and characterization of FePyDP film-modified electrode
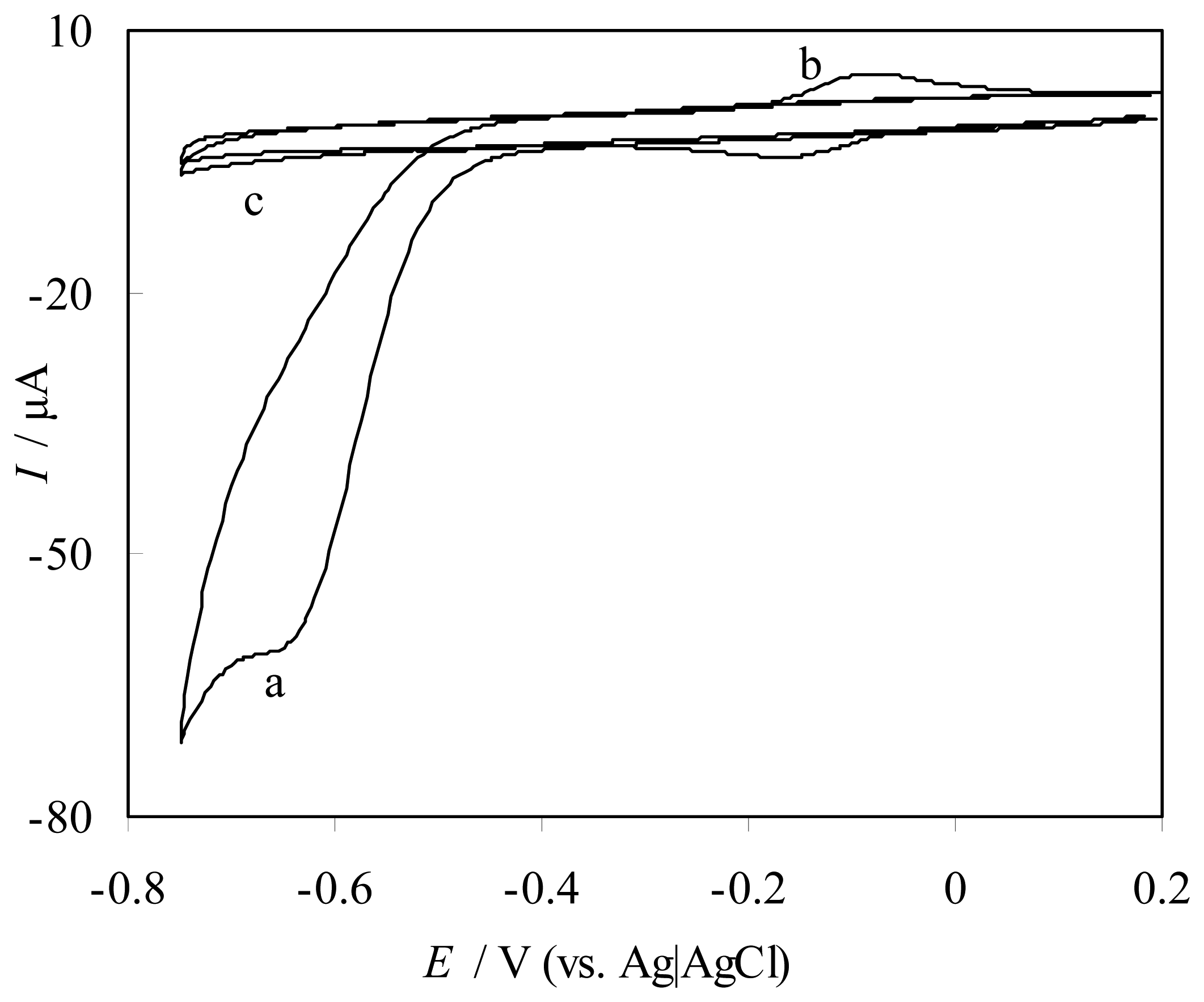
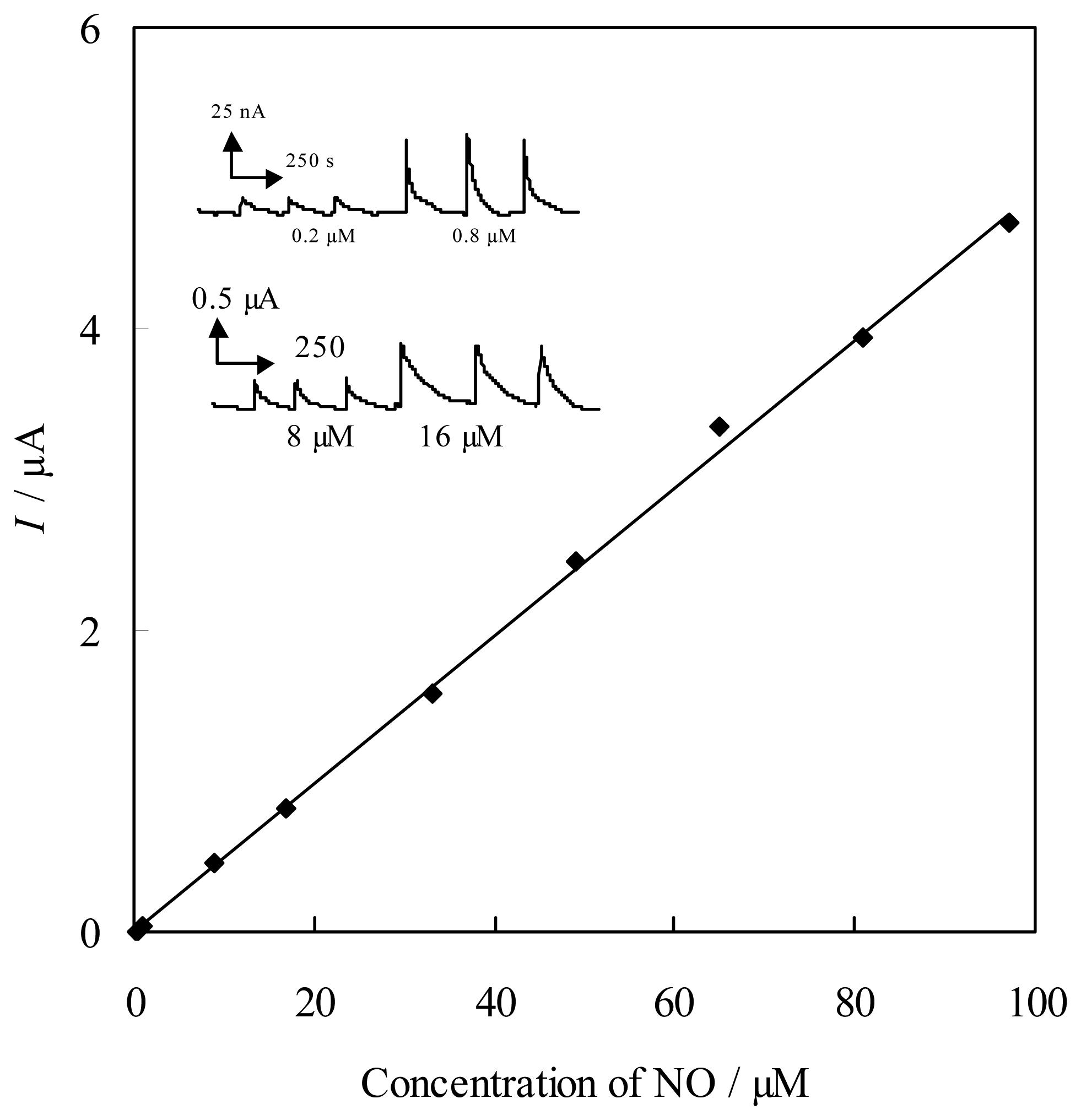
Catalytic reduction of NO at FePyDP film-modified PG electrode
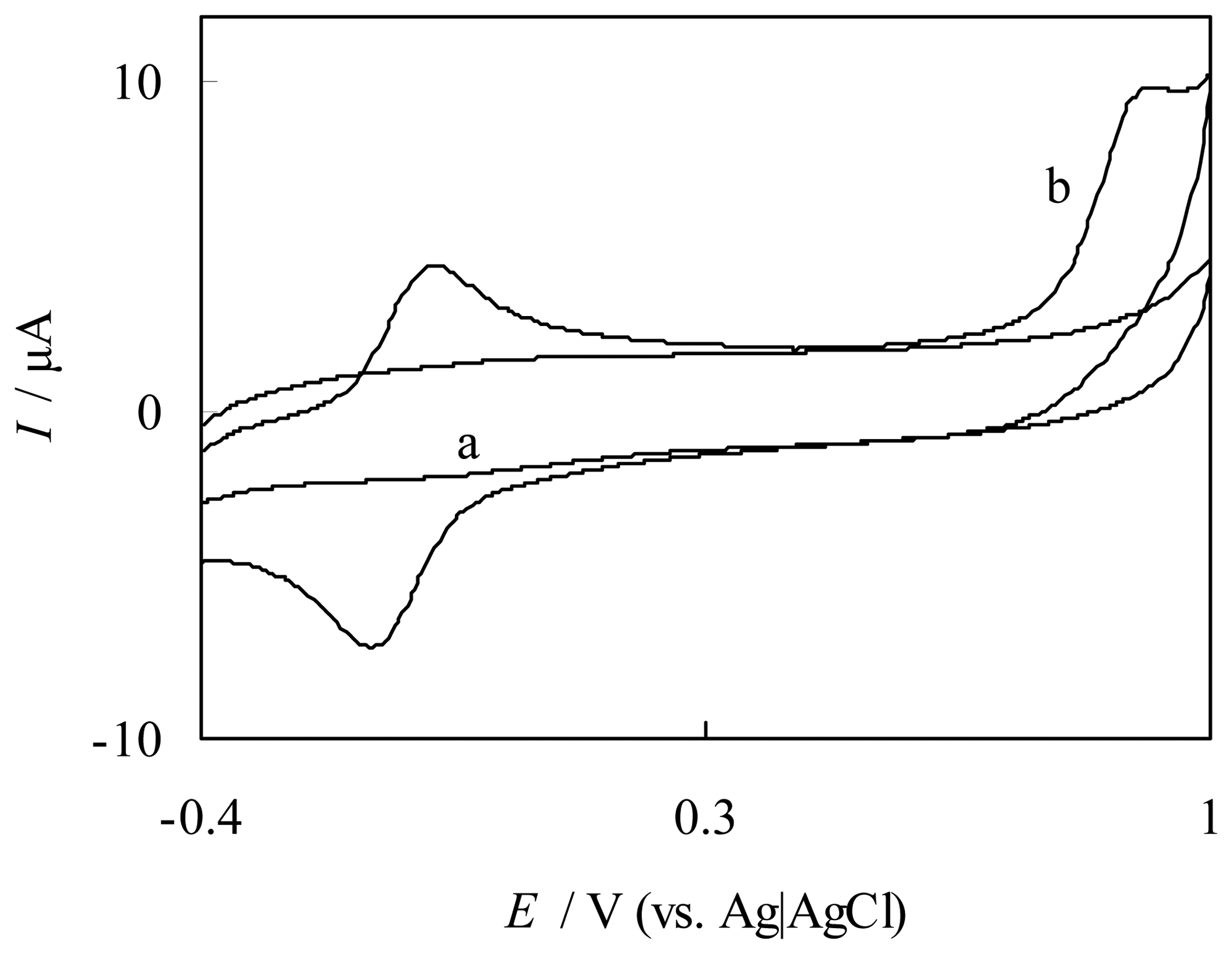
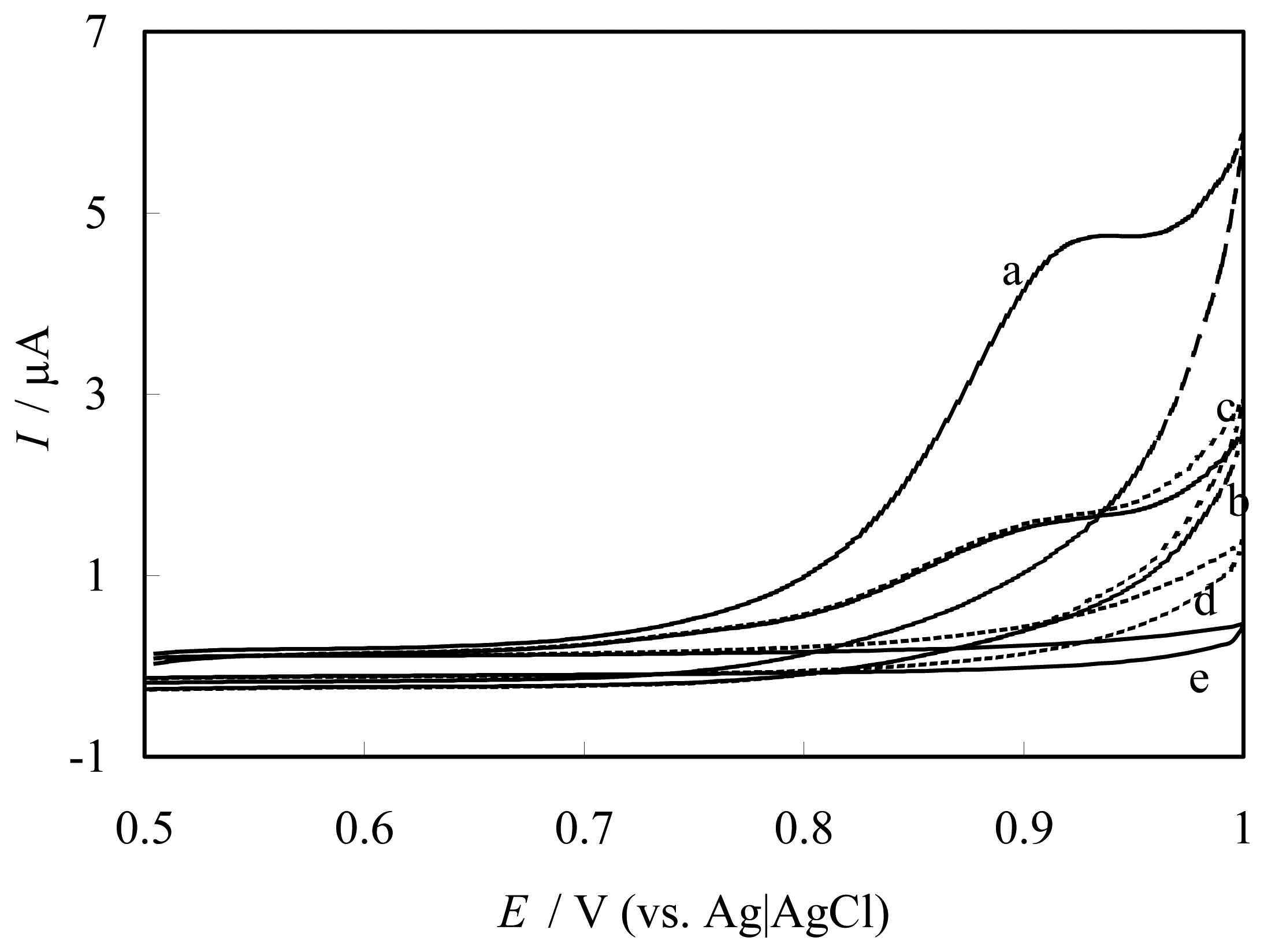
Catalytic oxidation of NO at FePyDP film modified ITO electrode
Conclusions
References
- Malinski, T.; Taha, Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992, 358, 676. [Google Scholar]
- Bedioui, F.; Trevin, S.; Albin, V.; Villegas, M.G.G.; Devynck, J. Design and characterization of chemically modified electrodes with iron(III) porphyrinic-based polymers: Study of their reactivity toward nitrites and nitric oxide in aqueous solution. Anal. Chim. Acta 1997, 341, 177. [Google Scholar]
- Vilakazi, S.L.; Nyokong, T. Electrocatalytic properties of vitamin B12 towards oxidation and reduction of nitric oxide. Electrochim. Acta 2000, 46, 453. [Google Scholar]
- Diab, N.; Schuhmann, W. Electropolymerized manganese porphyrin/polypyrrole films as catalytic surfaces for the oxidation of nitric oxide. Electrochim. Acta 2001, 47, 265. [Google Scholar]
- Ciszewski, A.; Kubaszewski, E.; Lozynski, M. The role of nickel as central metal in conductive polymeric porphyrin film for electrocatalytic oxidation of nitric oxide. Electroanalysis 1996, 8, 293. [Google Scholar]
- Hayon, J.; Ozer, D.; Rishpon, J.; Bettelheim, A. Spectroscopic and electrochemical response to nitrogen monoxide of a cationic iron porphyrin immobilized in nafion-coated electrodes or membranes. J. Chem. Soc. Chem. Commun. 1994, 619. [Google Scholar]
- Kashevskii, A.V.; Safronov, A. Y.; Ikeda, O. Behaviors of H2TPP and CoTPPCl in Nafion film and the catalytic activity for nitric oxide oxidation. J. Electroanal. Chem. 2001, 510, 86. [Google Scholar]
- Kashevskii, A.V.; Lei, J.; Safronov, A.Y.; Ikeda, O. Electrocatalytic properties of meso- tetraphenylporphyrin cobalt for nitric oxide oxidation in methanolic solution and in Nafion® film. J. Electroanal. Chem. 2002, 531, 71. [Google Scholar]
- Giese, B.; Biland, A. Recent developments of charge injection and charge transfer in DNA. Chem. Commun. 2002, 667. [Google Scholar]
- Nakayama, H.; Ohno, H.; Okahata, Y. Intramolecular electron conduction along DNA strands and their temperature dependency in a DNA-aligned cast film. Chem. Commun. 2001, 2300. [Google Scholar]
- Pasternack, R.F.; Gibbs, E.J.; Villafranca, J.J. Interactions of porphyrins with nucleic acids. Biochemistry 1983, 22, 2406. [Google Scholar]
- Makundan, N.E.; Petho, G.; Dixon, D.W.; Marzilli, L.G. DNA-tentacle porphyrin interactions: AT over selectivity exhibited by an outside binding self-stacking porphyrin. Inorg. Chem. 1995, 34, 3677. [Google Scholar]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal.Chem. 2002, 74, 2781. [Google Scholar]
- Lei, J.P.; Ju, H.X.; Ikeda, O. Supramolecular assembly of porphyrin bound DNA and its catalytic behavior for nitric oxide reduction. Electrochimica Acta 2004, 49, 2453. [Google Scholar]
- Trofimova, N.S.; Safronov, A.Y.; Ikeda, O. Electrochemical and spectral studies on the reductive nitrosylation of water-soluble iron porphyrin. Inorg. Chem. 2003, 42, 1945. [Google Scholar]
- Lei, J.P.; Trofimova, N.S.; Ikeda, O. Selective oxidation of nitric oxide against nitrite by oxo-iron(IV) porphyrin at an ITO electrode. Chem. Lett. 2003, 32, 610. [Google Scholar]
- Lei, J.P.; Ju, H.X.; Ikeda, O. Catalytic oxidation of nitric oxide and nitrite mediated by water-soluble high-valent iron porphyrins at an ITO elevtrode. J. Electroanal. Chem. 2004, 567, 331. [Google Scholar]
- Pasternack, R.F.; Lee, H.; Makel, P.; Spencer, C. Solution properties of tetrakis-(4-N-methyl) -pyridylporphineiron(III). J. Inorg. Nucl. Chem. 1977, 39, 1865. [Google Scholar]
- Bedioui, F.; Bouhier, Y.; Sorel, C.; Devynck, J.; Coche-Guerente, L.; Deronzier, A.; Moutet, J.C. Incorporation of anionic metalloporphryins into poly(pyrrole-alkylammonium) film: Characterization of the reactivity of the iron(III) porphyrinic-based polymer. Electrochim. Acta 1993, 38, 2485. [Google Scholar]
- Lide, D. R. (Ed.) CRC Handbook of Chemistry and Physics, 76 th ed.; CRC Press: Boca Raton, FL, 1995; section 6–3.
- Borissevitch, I.E.; Gandini, S.C.M. Photophysical studies of excited-state characteristics of meso-tetrakis(4-N-methyl-pyridiniumyl) porphyrin bound to DNA. J. Photochem. Photobiol. B: Biol. 1998, 43, 112. [Google Scholar]
- Hoshino, M.; Maeda, M.; Konishi, R.; Seki, H.; Ford, P.C. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J. Am. Chem. Soc. 1996, 118, 5702. [Google Scholar]
- Fan, C.H.; Li, G.X.; Zhu, J.Q.; Zhu, D.X. A reagentless nitric oxide biosensro based on hemoglobin-DNA films. Anal. Chim. Acta 2000, 423, 95. [Google Scholar]
- Fan, C.H.; Chen, X.C.; Li, G.X.; Zhu, J.Q.; Zhu, D.X.; Scheer, H. Direct electrochemical charaterization of the interaction between haemoglobin and nitric oxide. Phys. Chem. Chem. Phys. 2000, 2, 4409. [Google Scholar]
- Bayachou, M.; Lin, R.; Cho, W.; Farmer, P.J. Electrochemical reduction of NO by myoglobin in surfactant film: characterization and reactivity of the nitroxyl (NO-) adduct. J. Am. Chem. Soc. 1998, 120, 9888. [Google Scholar]
- Barley, M.H.; Rhodes, M.R.; Meyer, T.J. Electrocatalytic Reduction of Nitrite to Nitrous Oxide and Ammonia Based on the N-Methylated, Cationic Iron Porphyrin Complexe [FeIII(H2O)(TMPyP)]5+. Inorg. Chem. 1987, 26, 1746. [Google Scholar]
- Herold, S.; Rehmann, F.K. Kinetic and mechanistic studies of the reactions of nitrogen monoxide and nitrite with ferryl myoglobin. J. Bio. Inorg. Chem. 2001, 6, 543. [Google Scholar]
- Lexa, D.; Saveant, J.M.; Schafer, H.J.; Su, K.B.; Vering, B.; Wang, D.L. Outer-sphere and inner-sphere processes in reductive elimination. Direct and indirect electrochemical reduction of vicinal dibromoalkanes. J. Am. Chem. Soc. 1990, 112, 6162. [Google Scholar]
| Porphyrin complexes | UV-Vis spectroscopy | |
|---|---|---|
| Soret band λ(nm) | Q bands λ(nm) | |
| FePyDP film on ITO in PBS (pH 7.4) | 428 | 515 |
| Fe(4-TMPyP) solution in PBS (pH 7.4) a | 422 | 490, 598 |
| FePyDP film on ITO in the presence of saturated NO | 425 | 557 |
| Substance | Concentration (1 × 10−5 M) | Relative peak current |
|---|---|---|
| Nitrite | 10 | 101.2 |
| L(+)-Ascorbate | 20 | 102.4 |
| Dopamine | 1 | 98.5 |
| Uric acid | 1 | 104.3 |
| D-cysteine | 1 | 105.6 |
| D(+)-Glucose | 20 | 97.1 |
© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lei, J.; Ju, H.; Ikeda, O. A Novel Supramolecular Assembly Film of Porphyrin Bound DNA: Characterization and Catalytic Behaviors Towards Nitric Oxide. Sensors 2005, 5, 171-184. https://doi.org/10.3390/s5040171
Lei J, Ju H, Ikeda O. A Novel Supramolecular Assembly Film of Porphyrin Bound DNA: Characterization and Catalytic Behaviors Towards Nitric Oxide. Sensors. 2005; 5(4):171-184. https://doi.org/10.3390/s5040171
Chicago/Turabian StyleLei, Jianping, Huangxian Ju, and Osamu Ikeda. 2005. "A Novel Supramolecular Assembly Film of Porphyrin Bound DNA: Characterization and Catalytic Behaviors Towards Nitric Oxide" Sensors 5, no. 4: 171-184. https://doi.org/10.3390/s5040171




