A COTS-Based Portable System to Conduct Accurate Substance Concentration Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Validation Instruments
2.2. Sample Preparation
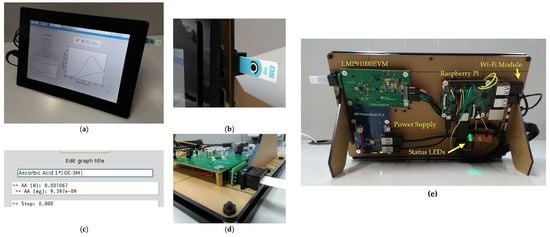
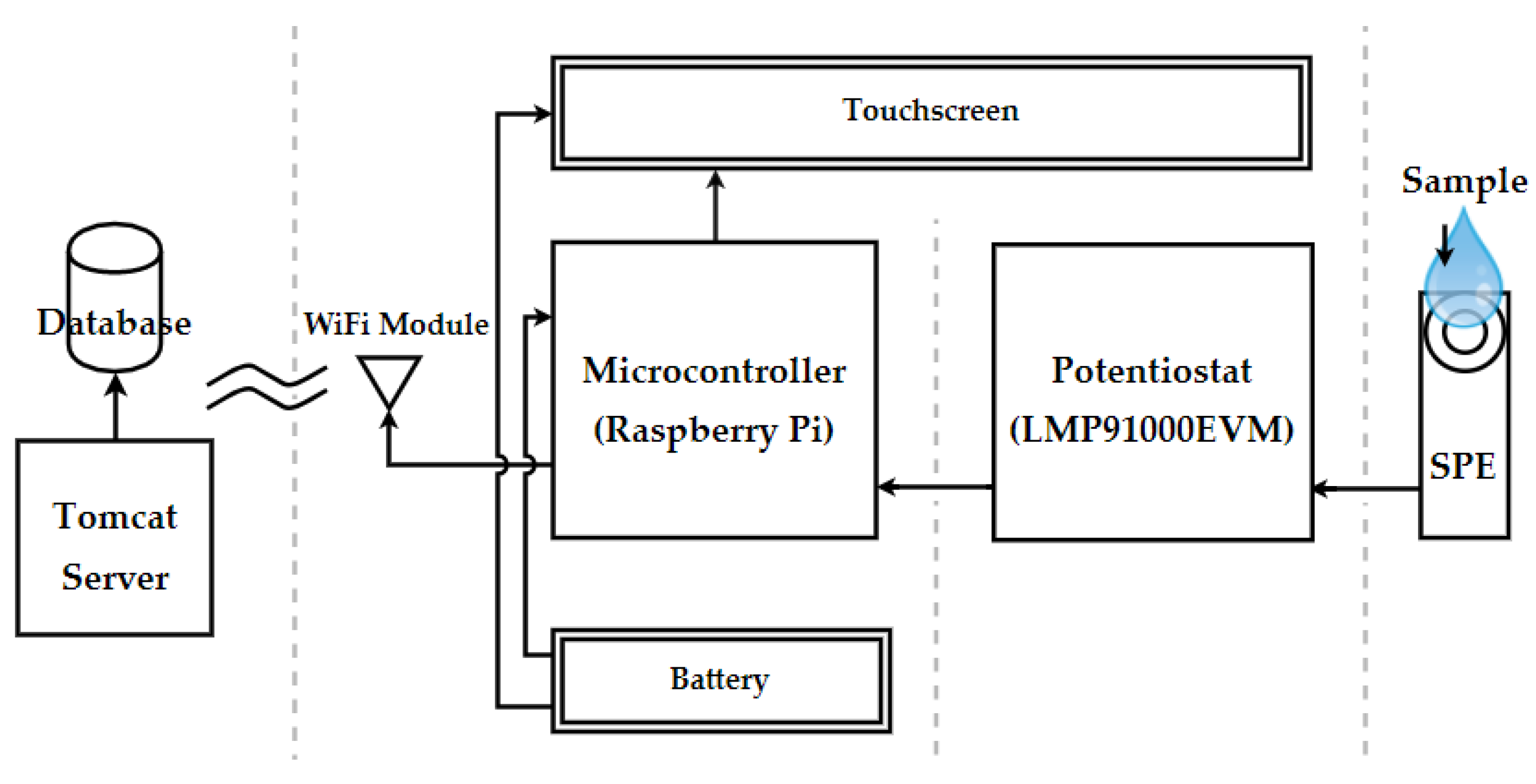
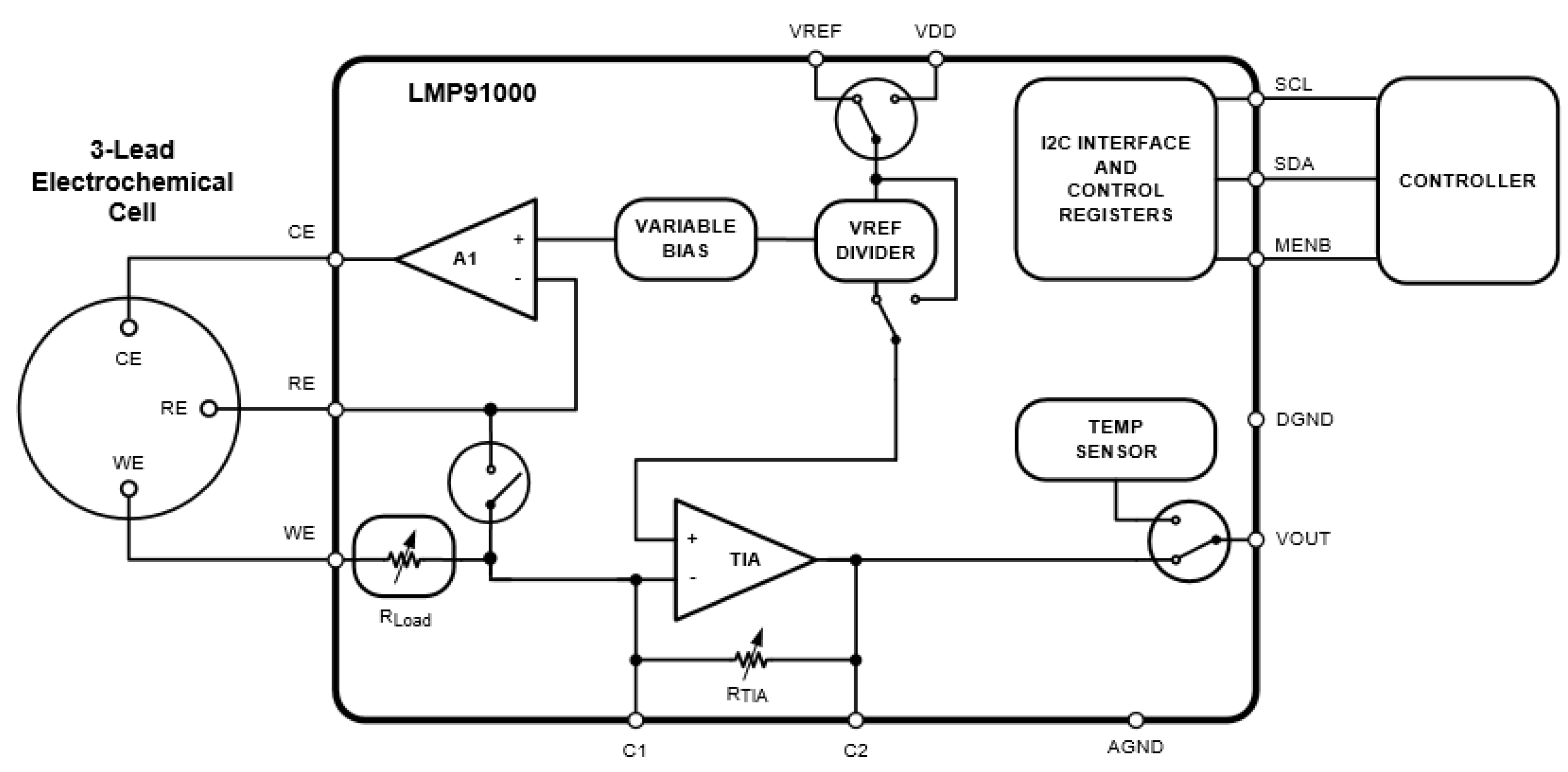
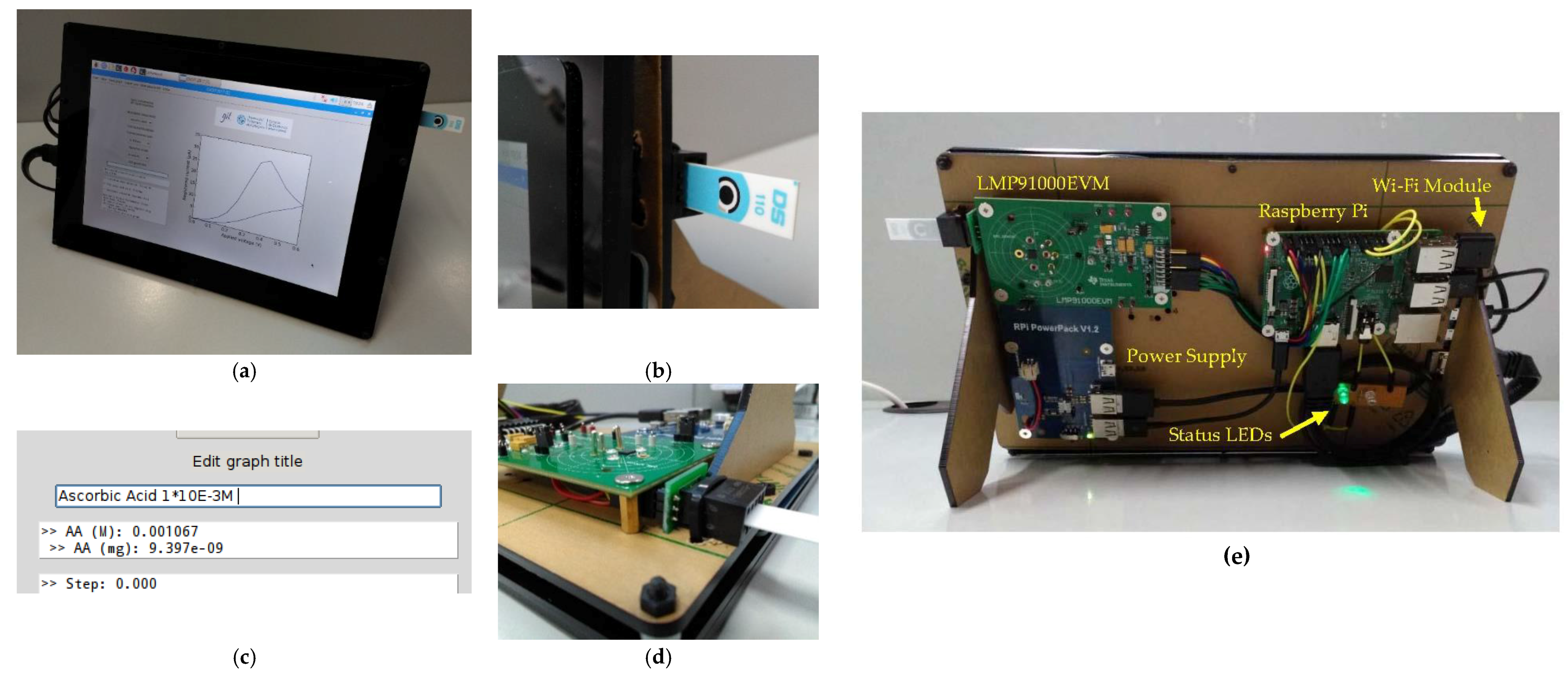
2.3. System Architecture
2.4. Software Description
3. Results
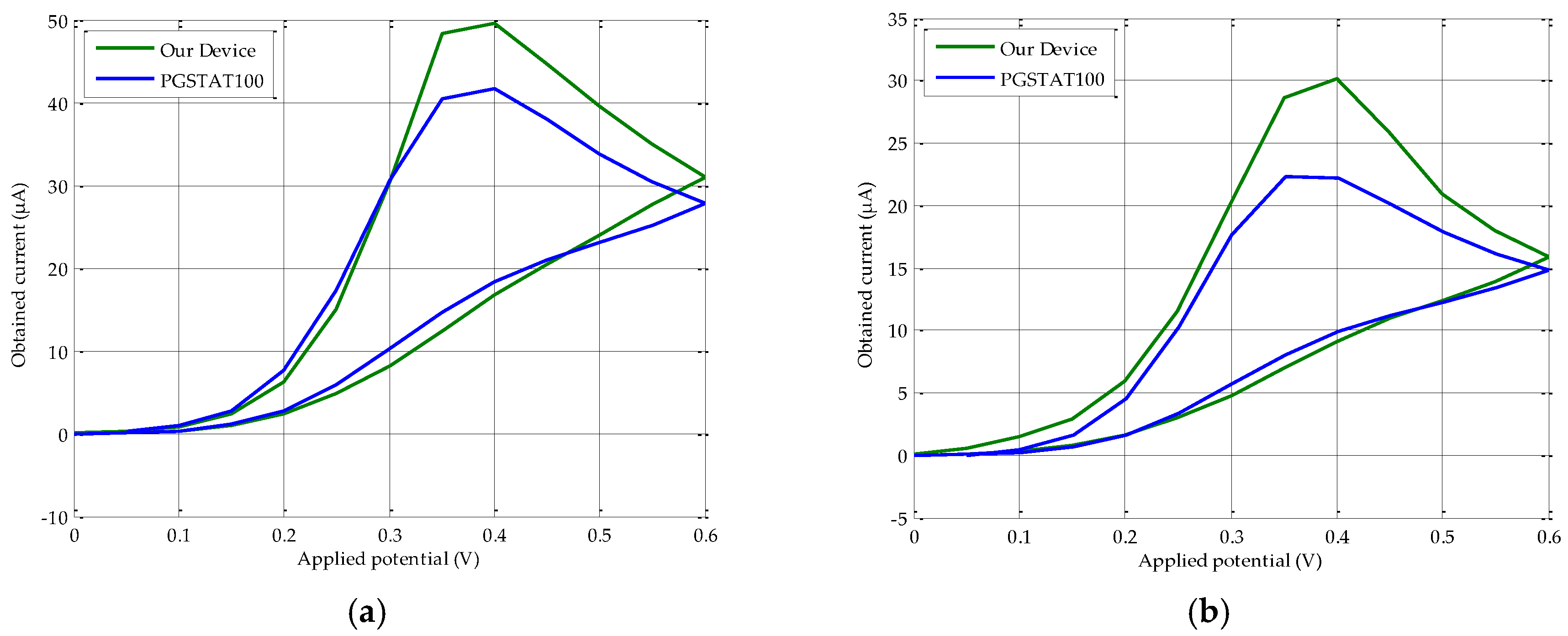
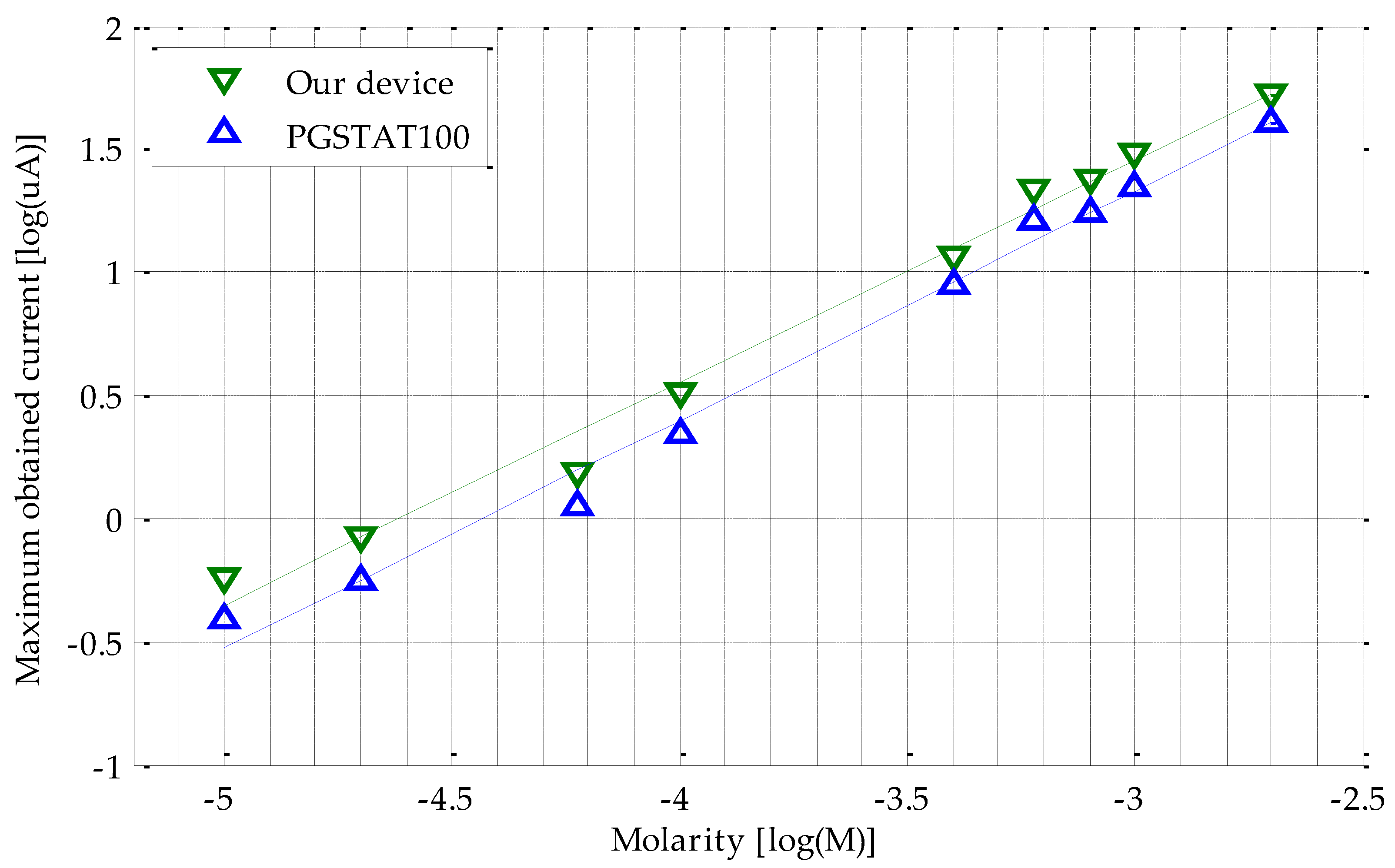
3.1. Calibration Line, Range and Reliability
3.2. Repeatability
3.3. Sensor Reusability
4. Discussion
- 1)
- Our calibration curve is thoroughly valid for interpolating values different from the proposed ones in this work. That is, if the maximum current measured is 0.5 μA, a molarity of 1.13 × 10−4 M is reached, as shown in Figure 6.
- 2)
- The current peaks obtained for the PGSTAT100 and our device can be introduced in Equations (3) and (4) to derive the same molarity.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lykkesfeldt, J. Determination of Ascorbic Acid and Dehydroascorbic Acid in Biological Samples by High-Performance Liquid Chromatography Using Subtraction Methods: Reliable Reduction with Tris[2-carboxyethyl]phosphine Hydrochloride. Anal. Biochem. 2000, 282, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Measuring Protein Concentration through Absorption Spectrophotometry. Available online: http://w3.marietta.edu/~spilatrs/biol309/labexercises/Spectrophotometry.pdf (accessed on 25 October 2017).
- Albert, D.B.; Martens, C.S. Determination of low-molecular-weight organic acid concentrations in seawater and pore-water samples via HPLC. Mar. Chem. 1997, 56, 27–37. [Google Scholar] [CrossRef]
- Conte, L.S.; Moret, S.; Purcaro, G. HPLC in food analysis. Chromatogr. Sci. Ser. 2011, 101, 561–660. [Google Scholar] [CrossRef]
- Yang, J.; Xu, G.; Zheng, Y.; Kong, H.; Pang, T.; Lv, S.; Yang, Q. Diagnosis of liver cancer using HPLC-based metabonomics avoiding false-positive result from hepatitis and hepatocirrhosis diseases. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 813, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Floriani, G.; Gasparetto, J.C.; Pontarolo, R.; Gonçalves, A.G. Development and validation of an HPLC-DAD method for simultaneous determination of cocaine, benzoic acid, benzoylecgonine and the main adulterants found in products based on cocaine. Forensic Sci. Int. 2014, 235, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Lazerges, M.; Bedioui, F. Analysis of the evolution of the detection limits of electrochemical DNA biosensors. Anal. Bioanal. Chem. 2013, 405, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Lautner, G.; Gyurcsányi, R.E. Electrochemical Detection of miRNAs. Electroanalysis 2014, 26, 1224–1235. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.F.; Ciou, W.L.; Chen, P.Y. Voltammetric study of polyviologen and the application of polyviologen-modified glassy carbon electrode in amperometric detection of vitamin C. J. Appl. Electrochem. 2008, 38, 1285–1292. [Google Scholar] [CrossRef]
- Hasslacher, C.; Kulozik, F.; Platten, I. Accuracy of self monitoring blood glucose systems in a clinical setting: Application of new planned ISO-Standards. Clin. Lab. 2013, 59, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernandez-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Fadda, A.; Schirra, M.; Bazzu, G.; Serra, P.A. Detection of postharvest changes of ascorbic acid in fresh-cut melon, kiwi, and pineapple, by using a low cost telemetric system. Food Chem. 2012, 135, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Metrohm-Autolab. Available online: www.metrohm-autolab.com/ (accessed on 25 October 2017).
- DropSens. Available online: http://www.dropsens.com/en/home.html (accessed on 8 February 2018).
- PalmSens. Available online: https://www.palmsens.com/ (accessed on 22 December 2017).
- Dryden, M.D.M.; Wheeler, A.R. DStat: A versatile, open-source potentiostat for electroanalysis and integration. PLoS ONE 2015, 10, e0140349. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.N. Building a microcontroller based potentiostat: A inexpensive and versatile platform for teaching electrochemistry and instrumentation. J. Chem. Educ. 2016, 93, 1320–1322. [Google Scholar] [CrossRef]
- Rowe, A.A.; Bonham, A.J.; White, R.J.; Zimmer, M.P.; Yadgar, R.J.; Hobza, T.M.; Honea, J.W.; Ben-Yaacov, I.; Plaxco, K.W. CheapStat: An Open-Source, “Do-It-Yourself” Potentiostat for Analytical and Educational Applications. PLoS ONE 2011, 6, e23783. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.H.; Umasankar, Y.; Gonzalez, P.J.; Alfonso, A.; Bhansali, S. Multimodal technique to eliminate humidity interference for specific detection of ethanol. Biosens. Bioelectron. 2017, 87, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Umasankar, Y.; Jalal, A.H.; Gonzalez, P.J.; Chowdhury, M.; Alfonso, A.; Bhansali, S. Wearable alcohol monitoring device with auto-calibration ability for high chemical specificity. In Proceedings of the BSN 2016—13th Annual Body Sensor Networks Conference, San Francisco, CA, USA, 14–17 June 2016; pp. 353–358. [Google Scholar]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Yndart, A.; Jayant, R.D.; Sagar, V.; Atluri, V.; Bhansali, S.; Nair, M. Electrochemical sensing method for point-of-care cortisol detection in human immunodeficiency virus-infected patients. Int. J. Nanomed. 2015, 10, 677–685. [Google Scholar] [CrossRef]
- Makiewicz, P.; Matias, D.; Jaskuła, M.; Biegun, M.; Penkala, K.; Mijowska, E.; El Fray, M.; Podolski, J. Accuracy analysis of measurements in electrochemical biosensing. In IFMBE Proceedings; Springer: Singapore, 2016; Volume 55, pp. 370–373. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Deutsch, M.J.; Weeks, C.E. Micro-fluorometric assay for vitamin C. J. Assoc. Off. Agric. Chem. 1965, 48, 1248–1256. [Google Scholar]
- Texas Instruments. Available online: http://www.ti.com/tool/LMP91000EVM (accessed on 5 December 2017).
- Raspberry Pi 2 Model B. Available online: https://www.raspberrypi.org/products/raspberry-pi-2-model-b/ (accessed on 9 November 2017).
- Dropsens DS110 SPE. Available online: http://www.dropsens.com/en/pdfs_productos/new_brochures/110-c110.pdf (accessed on 8 February 2018).
- LMP91000 Sensor AFE System: Configurable AFE Potentiostat for Low-Power Chemical Sensing Applications. Available online: http://www.ti.com/lit/ds/symlink/lmp91000.pdf (accessed on 8 February 2018).
- J Aznar Poveda Software Repository—OPEN SOURCE CVGIT. Available online: https://github.com/GITUPCT/CVGIT.git (accessed on 16 December 2017).
- IO Rodeo SPE Adapter. Available online: https://iorodeo.com/collections/cheapstat-open-source-potentiostat/products/cheapstat-screen-printed-electrode-adapters?variant=12478343814 (accessed on 16 December 2017).
- Harris, D.C. Quantitative Chemical Analysis; WH Freeman: New York, NY, USA, 2007; Volume 42, ISBN 0716770415. [Google Scholar]




| Raspberry Pi | Connection Name | Protocol | LMP91000EVM |
|---|---|---|---|
| 19 | MOSI | SPI | 7 |
| 21 | MISO | SPI | 5 |
| 23 | SCLK | SPI | 3 |
| 24 | CE0 | SPI | 1 |
| 3 | SDA | I2C | 11 |
| 5 | SCL | I2C | 12 |
| 9, 14, 6, 39 * | GND * | - | 2, 4, 8, 10 * |
| 2 | 5V | - | 14 |
| 1 | 3.3V | - | 13 |
| % of VREF | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 |
| Potential Step (V) | 0.00 | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | 0.35 | 0.40 | 0.45 | 0.50 | 0.55 | 0.60 |
| REFCN Register (Binary) | 10110000 | 10110010 | 10110011 | 10110100 | 10110101 | 10110110 | 10110111 | 10111000 | 10111001 | 10111010 | 10111011 | 10111100 | 10111101 |
| Molarity (M) | PGSTAT100 x̄ (µA) ± RSD (%) | Our Device x̄ (µA) ± RSD (%) |
|---|---|---|
| 2 × 10−3 | 41.99 µA ± 1.81% | 47.51 µA ± 4.60% |
| 6 × 10−5 | 1.12 µA ± 1.20% | 1.55 µA ± 4.26% |
| Uses | Max. Current (µA) | Deviation in Respect to the 1st Use (µA) |
|---|---|---|
| 1st | 40.02 | 0 |
| 2nd | 39.29 | 0.73 |
| 3rd | 38.93 | 1.09 |
| 4th | 38.14 | 1.88 |
| 5th | 40.62 | 0.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.-J.; Garcia-Haro, J.; Otero, T.F. A COTS-Based Portable System to Conduct Accurate Substance Concentration Measurements. Sensors 2018, 18, 539. https://doi.org/10.3390/s18020539
Aznar-Poveda J, Lopez-Pastor JA, Garcia-Sanchez A-J, Garcia-Haro J, Otero TF. A COTS-Based Portable System to Conduct Accurate Substance Concentration Measurements. Sensors. 2018; 18(2):539. https://doi.org/10.3390/s18020539
Chicago/Turabian StyleAznar-Poveda, Juan, Jose Antonio Lopez-Pastor, Antonio-Javier Garcia-Sanchez, Joan Garcia-Haro, and Toribio Fernández Otero. 2018. "A COTS-Based Portable System to Conduct Accurate Substance Concentration Measurements" Sensors 18, no. 2: 539. https://doi.org/10.3390/s18020539