Single-Shot Detection of Neurotransmitters in Whole-Blood Samples by Means of the Heat-Transfer Method in Combination with Synthetic Receptors
Abstract
:1. Introduction
2. Materials and Methods
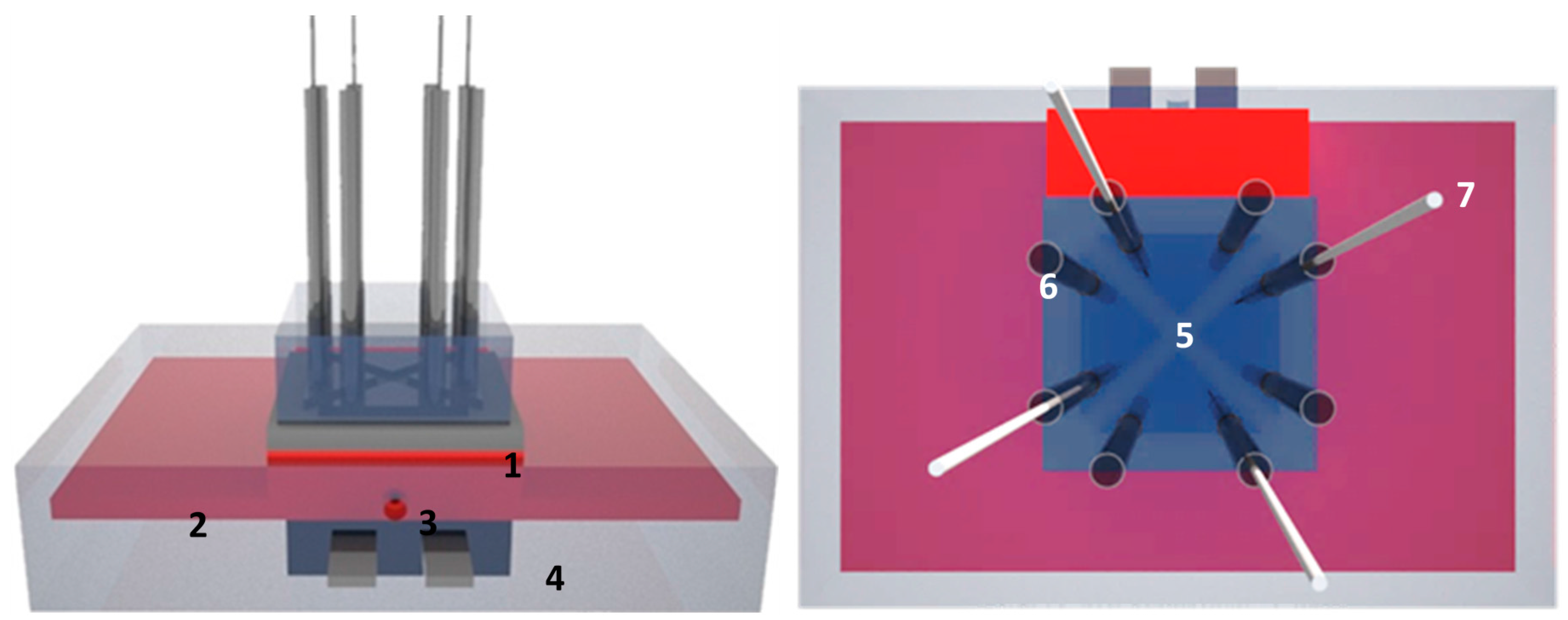
2.1. Design of the Four Chamber-HTM Platform
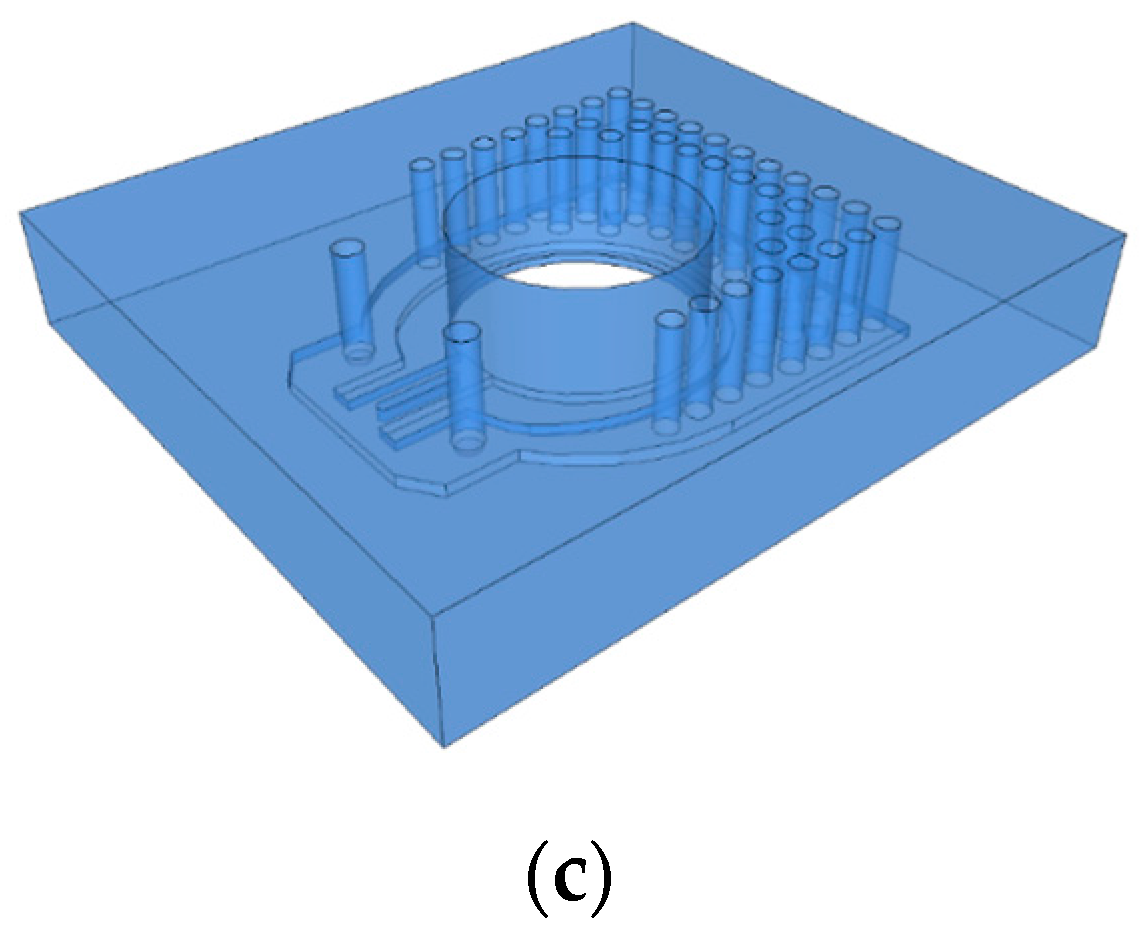
2.2. Design of a Single-Shot Device
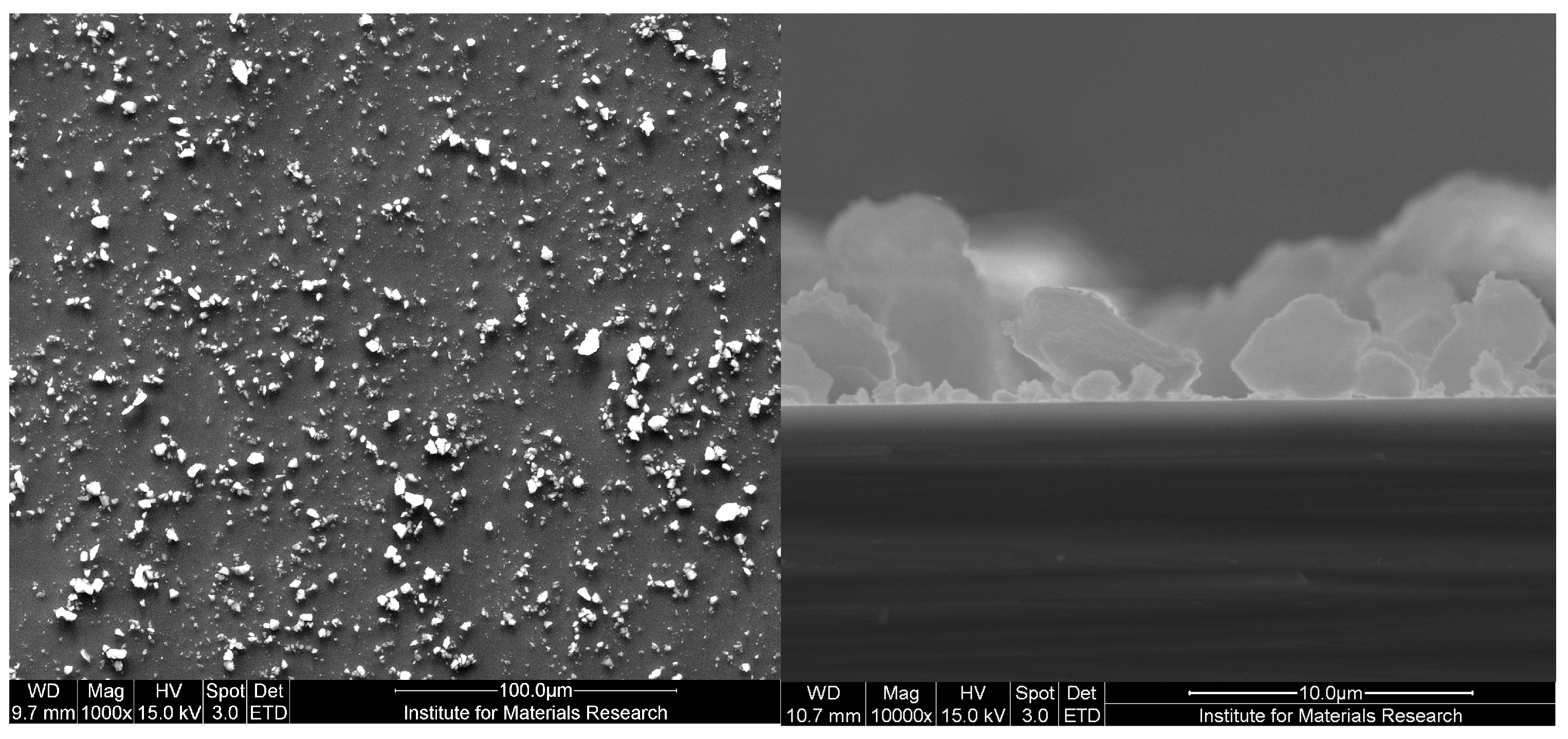
2.3. Receptor Layer
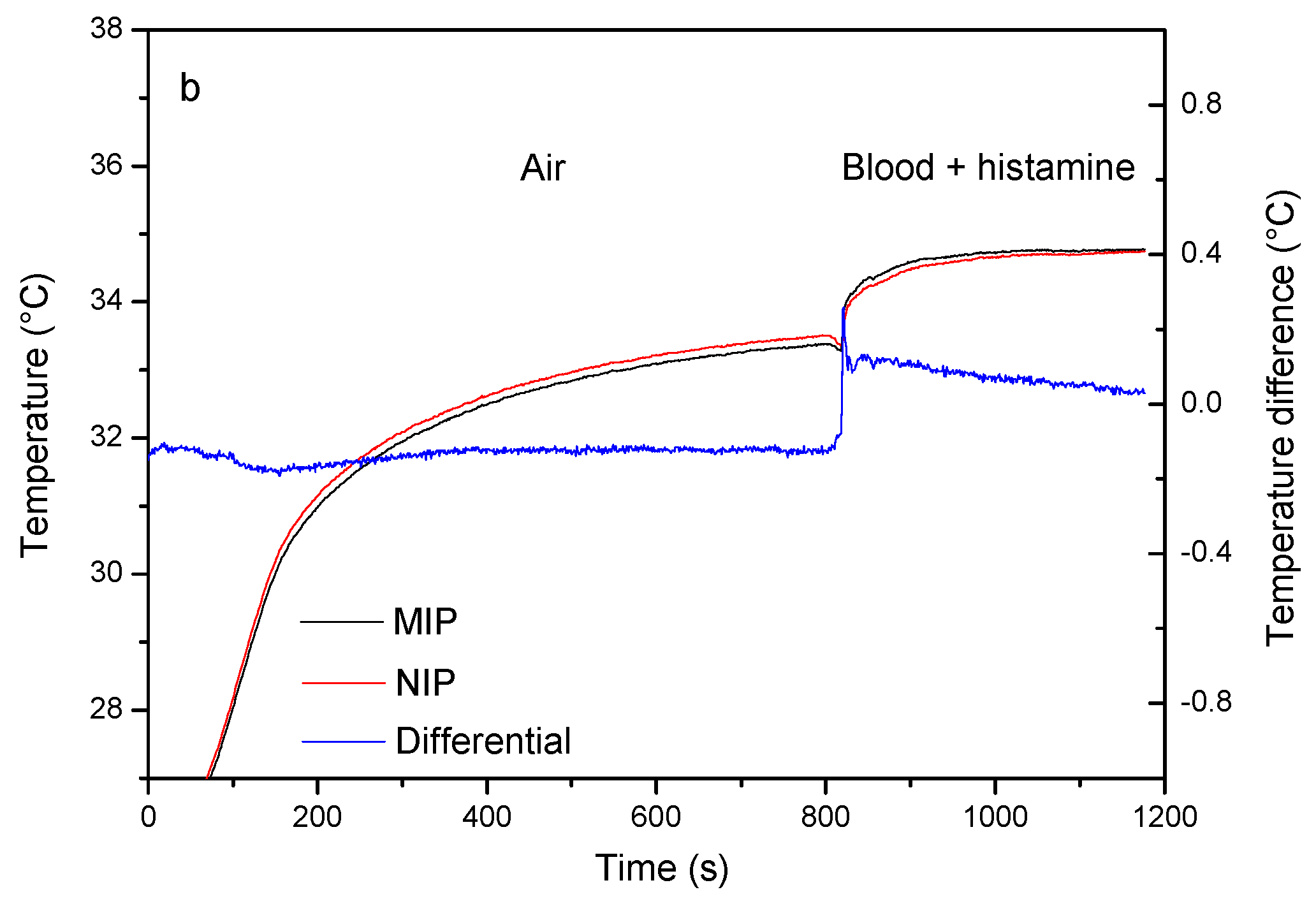
2.4. Proof-of-Principle Experiments in Whole Blood Samples
2.5. Single-Shot Serotonin Measurement in Whole Blood Samples
2.6. Optical Characterization
3. Results and Discussion
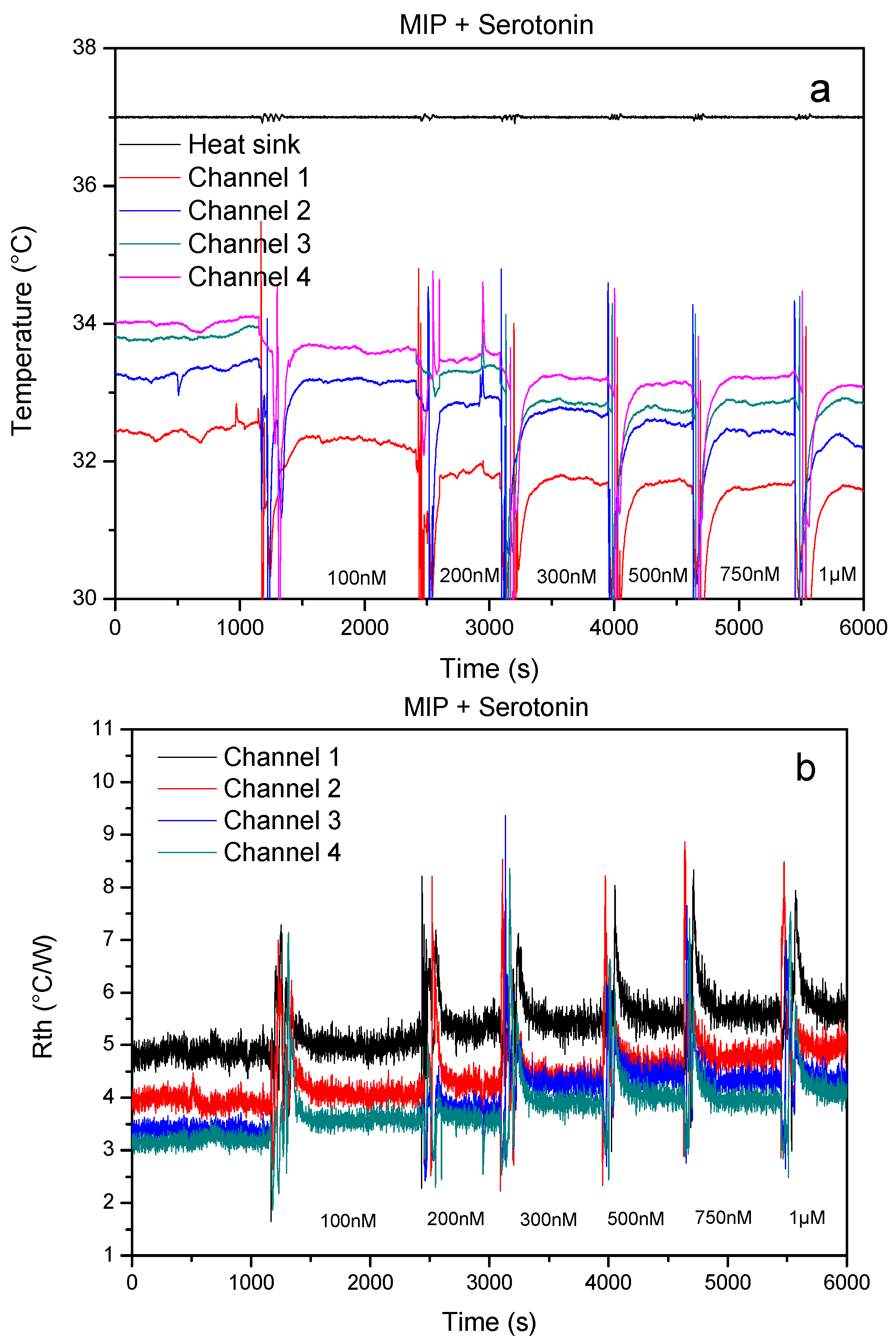
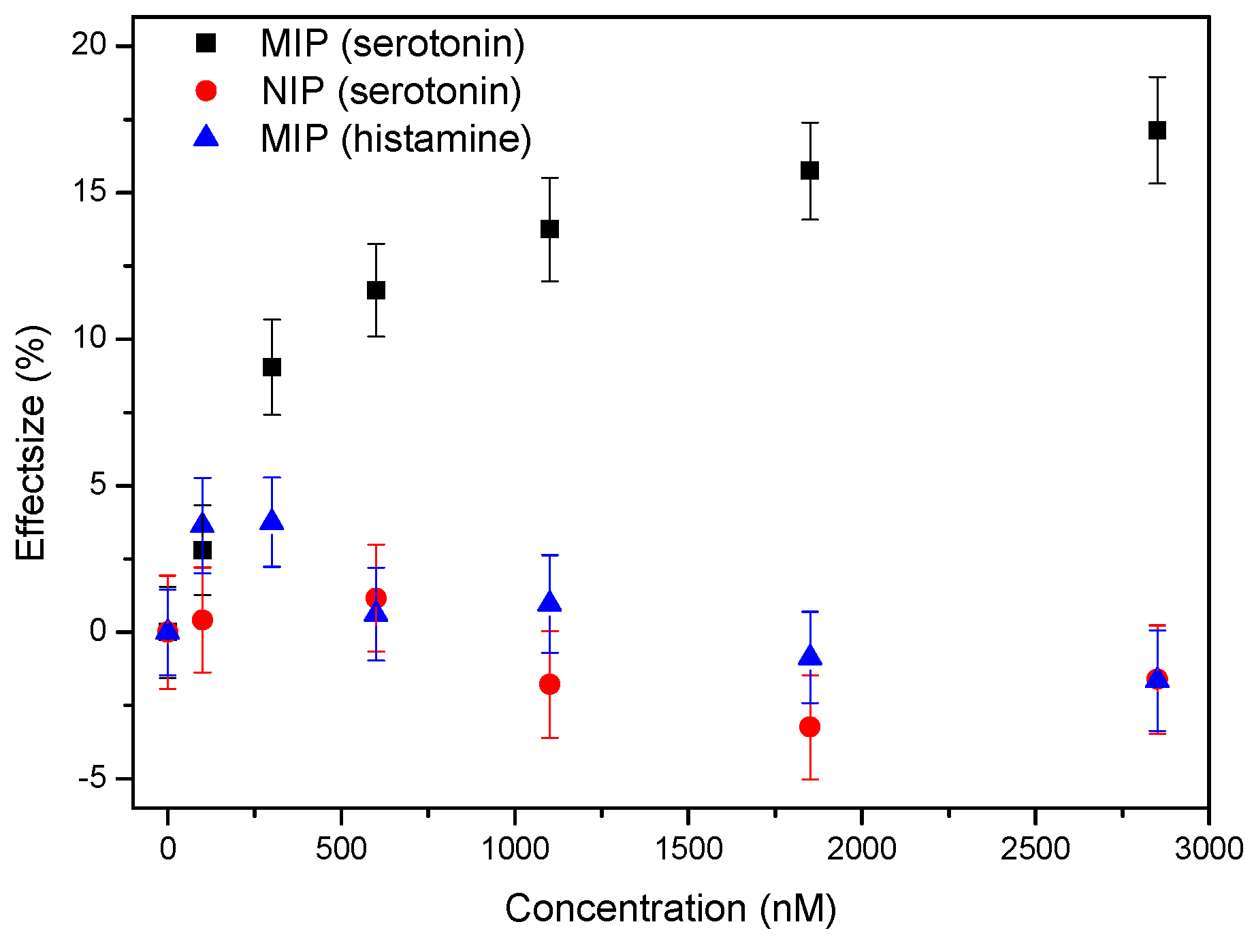
3.1. Proof-of-Principle: Thermal MIP-Based Detection of Serotonin in Whole Blood
3.2. Proof-of-Principle: Thermal MIP-Based Detection of Serotonin in Whole Blood
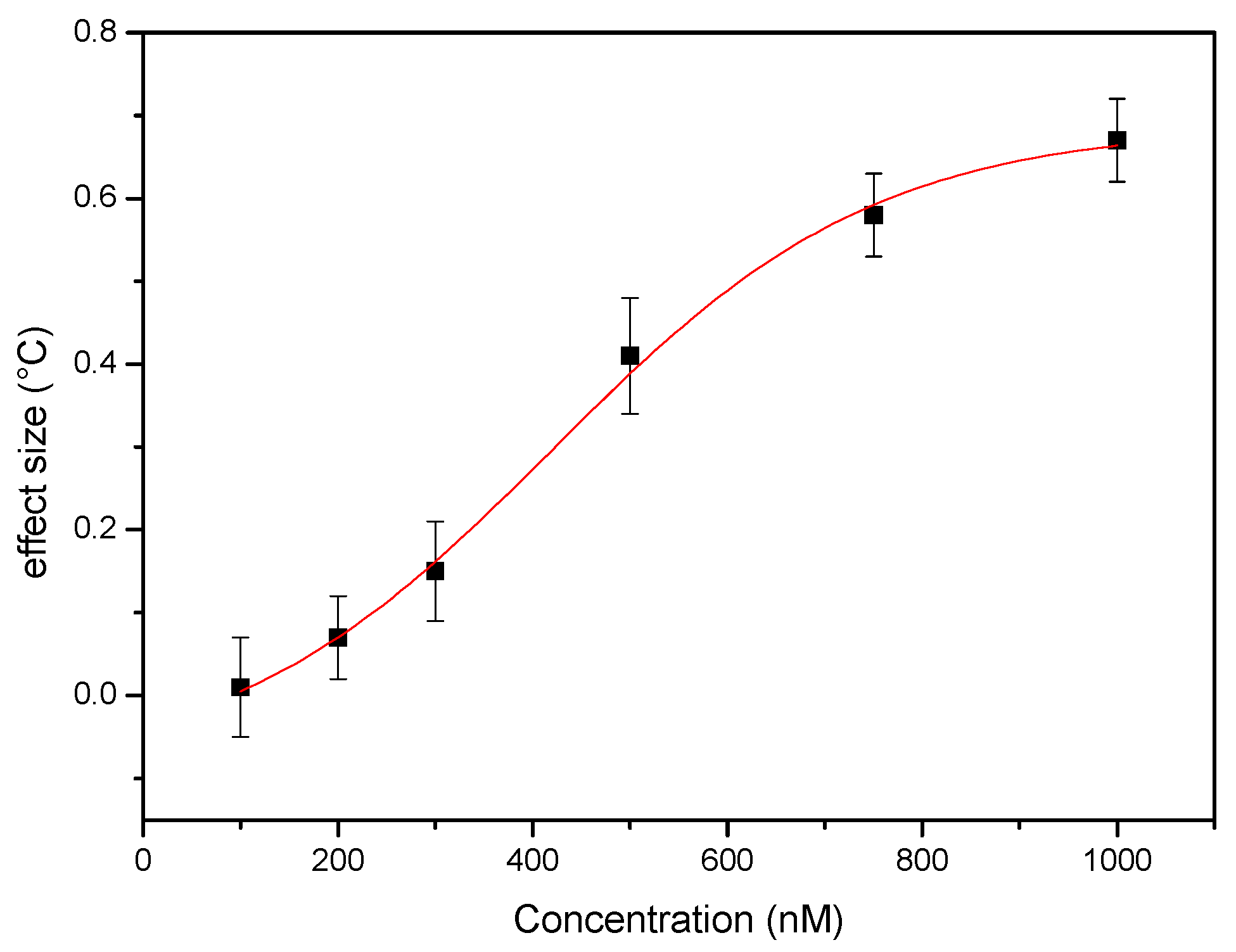
3.3. Single-Shot Detection of Serotonin in Whole Blood Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaswani, M.; Linda, F.K.; Ramesh, S. Role of Selective Serotonin Reuptake Inhibitors in Psychiatric Disorders: A Comprehensive Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 85–102. [Google Scholar] [CrossRef]
- Ayuso-Mateos, J.L.; Vázquez-Barquero, J.L.; Dowrick, C.; Lehtinen, V.; Dalgard, O.S.; Casey, P.; Wilkinson, C.; Lasa, L.; Page, H.; Dunn, G.; et al. Depressive Disorders in Europe: Prevalence Figures from the ODIN Study. Br. J. Psychiatry 2001, 179, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The Size and Burden of Mental Disorders and Other Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed]
- Kema, I.P.; De Vries, E.G.E.; Muskiet, F.A.J. Clinical Chemistry of Serotonin and Metabolites. J. Chromatogr. B Biomed. Sci. Appl. 2000, 747, 33–48. [Google Scholar] [CrossRef]
- Amara, S.G.; Sonders, M.S. Neurotransmitter Transporters as Molecular Targets for Addictive Drugs. Drug Alcohol Depend. 1998, 51, 87–96. [Google Scholar] [CrossRef]
- Rothman, R.B.; Blough, B.E.; Baumann, M.H. Dual Dopamine/serotonin Releasers as Potential Medications for Stimulant and Alcohol Addictions. AAPS J. 2007, 9, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, M.S.; Zhou, M.; Lira, A.; Hen, R.; Gingrich, J.A. Early-Life Blockade of the 5-HT Transporter Alters Emotional Behavior in Adult Mice. Science 2004, 306, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Cleare, A.J. Reduced whole blood serotonin in major depression. Depress. Anxiety 1997, 5, 108–111. [Google Scholar] [CrossRef]
- Maurer-Spurej, E.; Pittendreigh, C.; Misri, S. Platelet serotonin levels support depression scores for women with postpartum depression. J. Psychiatry Neurosci. 2007, 32, 23–29. [Google Scholar] [PubMed]
- Wulsin, L.R.; Musselman, D.; Otte, C.; Bruce, E.; Ali, S.; Whooley, A. Depression and Whole Blood Serotonin in Patients with Coronary Heart Disease from the Heart and Soul Study. Psychosom. Med. 2009, 71, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Linnoila, M.; Umhau, J.C.; Rawlings, R.; George, D.T.; Salem, N. Essential Fatty Acids Predict Metabolites of Serotonin and Dopamine in Cerebrospinal Fluid among Healthy Control Subjects, and Early- and Late-Onset Alcoholics. Biol. Psychiatry 1998, 44, 235–242. [Google Scholar] [CrossRef]
- Wilhelm, K.; Mitchell, P.B.; Niven, H.; Finch, A.; Wedgwood, L.; Scimone, A.; Blair, I.P.; Parker, G.; Schofield, P.R. Life Events, First Depression Onset and the Serotonin Transporter Gene. Br. J. Psychiatry 2006, 188, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.M. Signal-to-Noise Optimization of HPLC-Fluorometric Systems and Their Application to the Analysis of Indoles. Adv. Exp. Med. Biol. 1991, 294, 51–61. [Google Scholar] [PubMed]
- Camilleri, M.; Katzka, D.A. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. Genetic Epidemiology and Pharmacogenetics in Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1075–G1084. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, D.; Gattavecchia, E.; Gandolfi, M. Thin-Layer Chromatographic Determination of Indolic Tryptophan Metabolites in Human Urine Using Sep-Pak C18 Extraction. J. Chromatogr. B Biomed. Sci. Appl. 1982, 231, 283–289. [Google Scholar] [CrossRef]
- Patel, B.A.; Arundell, M.; Parker, K.H.; Yeoman, M.S.; O’Hare, D. Simple and Rapid Determination of Serotonin and Catecholamines in Biological Tissue Using High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005, 818, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.B.; Minzenberg, M.J.; Wilkinson, L.O. Extracellular Serotonin and 5-Hydroxyindoleacetic Acid in Hypothalamus of the Unanesthetized Rat Measured by In Vivo Dialysis Coupled to High-Performance Liquid Chromatography with Electrochemical Detection: Dialysate Serotonin Reflects Neuronal Release. Brain Res. 1989, 499, 281–290. [Google Scholar] [CrossRef]
- Wagner, J.; Vitali, P.; Palfreyman, M.G.; Zraika, M.; Huot, S. Simultaneous Determination of 3,4-Dihydroxyphenylalanine, 5-Hydroxytryptophan, Dopamine, 4-Hydroxy-3-Methoxyphenylalanine, Norepinephrine, 3,4-Dihydroxyphenylacetic Acid, Homovanillic Acid, Serotonin, and 5-Hydroxyindoleacetic Acid in Rat Cerebrospinal Fl. J. Neurochem. 1982, 38, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Morgadinho, M.T.; Fontes Ribeiro, C.A.; Macedo, T.R.A. Influence of the Sample Preparation Method on the Serotonin Determination in Plasma and Platelets. Biomed. Chromatogr. 2004, 18, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Csipai, P.; Geerets, B.; Weustenraed, A.; van Grinsven, B.; Thoelen, R.; Gruber, J.; De Ceuninck, W.; Cleij, T.J.; Troost, F.J.; et al. Heat-Transfer-Based Detection of L-Nicotine, Histamine, and Serotonin Using Molecularly Imprinted Polymers as Biomimetic Receptors. Anal. Bioanal. Chem. 2013, 405, 6453–6460. [Google Scholar] [CrossRef] [PubMed]
- Wackers, G.; Vandenryt, T.; Cornelis, P.; Kellens, E.; Thoelen, R.; De Ceuninck, W.; Losada-Pérez, P.; van Grinsven, B.; Peeters, M.; Wagner, P. Array Formatting of the Heat-Transfer Method (HTM) for the Detection of Small Organic Molecules by Molecularly Imprinted Polymers. Sensors 2014, 14, 11016–11030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haupt, K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003, 75, 376 A–383 A. [Google Scholar] [CrossRef]
- Wulff, G. The Role of Binding-Site Interactions in the Molecular Imprinting of Polymers. Trends Biotechnol. 1993, 11, 85–87. [Google Scholar] [CrossRef]
- Mosbach, K.; Ramström, O. The Emerging Technique of Molecular Imprinting and Its Future Impact on Biotechnology. Nat. Biotechnol. 1996, 14, 163–170. [Google Scholar] [CrossRef]
- Van Grinsven, B.; Eersels, K.; Peeters, M.; Losada-Pérez, P.; Vandenryt, T.; Cleij, T.J.; Wagner, P. The Heat-Transfer Method: A Versatile Low-Cost, Label-Free, Fast, and User-Friendly Readout Platform for Biosensor Applications. ACS Appl. Mater. Interfaces 2014, 6, 13309–13318. [Google Scholar] [CrossRef] [PubMed]
- Eersels, K.; van Grinsven, B.; Khorshid, M.; Somers, V.; Püttmann, C.; Stein, C.; Barth, S.; Diliën, H.; Bos, G.M.J.; Germeraad, W.T.V.; et al. Heat-Transfer-Method-Based Cell Culture Quality Assay through Cell Detection by Surface Imprinted Polymers. Langmuir 2015, 31, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Diliën, H.; Peeters, M.; Royakkers, J.; Harings, J.; Cornelis, P.; Wagner, P.; Steen Redeker, E.; Banks, C.E.; Eersels, K.; van Grinsven, B.; et al. Label-Free Detection of Small Organic Molecules by Molecularly Imprinted Polymer Functionalized Thermocouples: Toward In Vivo Applications. ACS Sens. 2017, 2, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.L.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T.; et al. Thermal Detection of Histamine with a Graphene Oxide Based Molecularly Imprinted Polymer Platform Prepared by Reversible Addition–fragmentation Chain Transfer Polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Van Grinsven, B.; Vanden Bon, N.; Strauven, H.; Grieten, L.; Murib, M.; Monroy, K.L.J.; Janssens, S.D.; Haenen, K.; Schöning, M.J.; Vermeeren, V.; et al. Heat-Transfer Resistance at Solid-Liquid Interfaces: A Tool for the Detection of Single-Nucleotide Polymorphisms in DNA. ACS Nano 2012, 6, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Hayashi, K.; Seyama, M.; Inoue, S.; Tamechika, E. Cooperative Suction by Vertical Capillary Array Pump for Controlling Flow Profiles of Microfluidic Sensor Chips. Sensors 2012, 12, 14053–14067. [Google Scholar] [CrossRef] [PubMed]
- Bodas, D.; Khan-Malek, C. Hydrophilization and Hydrophobic Recovery of PDMS by Oxygen Plasma and Chemical treatment—An SEM Investigation. Sens. Actuators B Chem. 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Yao, M.; Fang, J. Hydrophilic PEO-PDMS for Microfluidic Applications. J. Micromech. Microeng. 2012, 22, 025012. [Google Scholar] [CrossRef]
- Peeters, M.; Troost, F.J.; van Grinsven, B.; Horemans, F.; Alenus, J.; Murib, M.S.; Keszthelyi, D.; Ethirajan, A.; Thoelen, R.; Cleij, T.J.; et al. MIP-Based Biomimetic Sensor for the Electronic Detection of Serotonin in Human Blood Plasma. Sens. Actuators B Chem. 2012, 171, 602–610. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandenryt, T.; Van Grinsven, B.; Eersels, K.; Cornelis, P.; Kholwadia, S.; Cleij, T.J.; Thoelen, R.; De Ceuninck, W.; Peeters, M.; Wagner, P. Single-Shot Detection of Neurotransmitters in Whole-Blood Samples by Means of the Heat-Transfer Method in Combination with Synthetic Receptors. Sensors 2017, 17, 2701. https://doi.org/10.3390/s17122701
Vandenryt T, Van Grinsven B, Eersels K, Cornelis P, Kholwadia S, Cleij TJ, Thoelen R, De Ceuninck W, Peeters M, Wagner P. Single-Shot Detection of Neurotransmitters in Whole-Blood Samples by Means of the Heat-Transfer Method in Combination with Synthetic Receptors. Sensors. 2017; 17(12):2701. https://doi.org/10.3390/s17122701
Chicago/Turabian StyleVandenryt, Thijs, Bart Van Grinsven, Kasper Eersels, Peter Cornelis, Safira Kholwadia, Thomas J. Cleij, Ronald Thoelen, Ward De Ceuninck, Marloes Peeters, and Patrick Wagner. 2017. "Single-Shot Detection of Neurotransmitters in Whole-Blood Samples by Means of the Heat-Transfer Method in Combination with Synthetic Receptors" Sensors 17, no. 12: 2701. https://doi.org/10.3390/s17122701






