Drawing Sensors with Ball-Milled Blends of Metal-Organic Frameworks and Graphite
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Synthetic Procedure of Pure MOFs
2.2. Preparation of M3HHTP2/Graphite Blended Pellets
2.3. Methods of Sensing Device Preparation by Mechanical Abrasion
2.4. General Methods and Materials for Sensing Studies and Measurements of Chemiresistance
2.5. Data Processing of Sensing Response
3. Results and Discussion
3.1. Characterization of Pure MOFs
3.2. Characterization of M3HHTP2 MOF/Graphite Blends
3.3. Fabrication of Sensing Devices
3.4. Sensing Results
3.4.1. Comparison of Chemiresistive Sensing Performance of Cu3HHTP2, Ball-Milled Cu3HHTP2, Cu3HHTP2/Graphite Blends
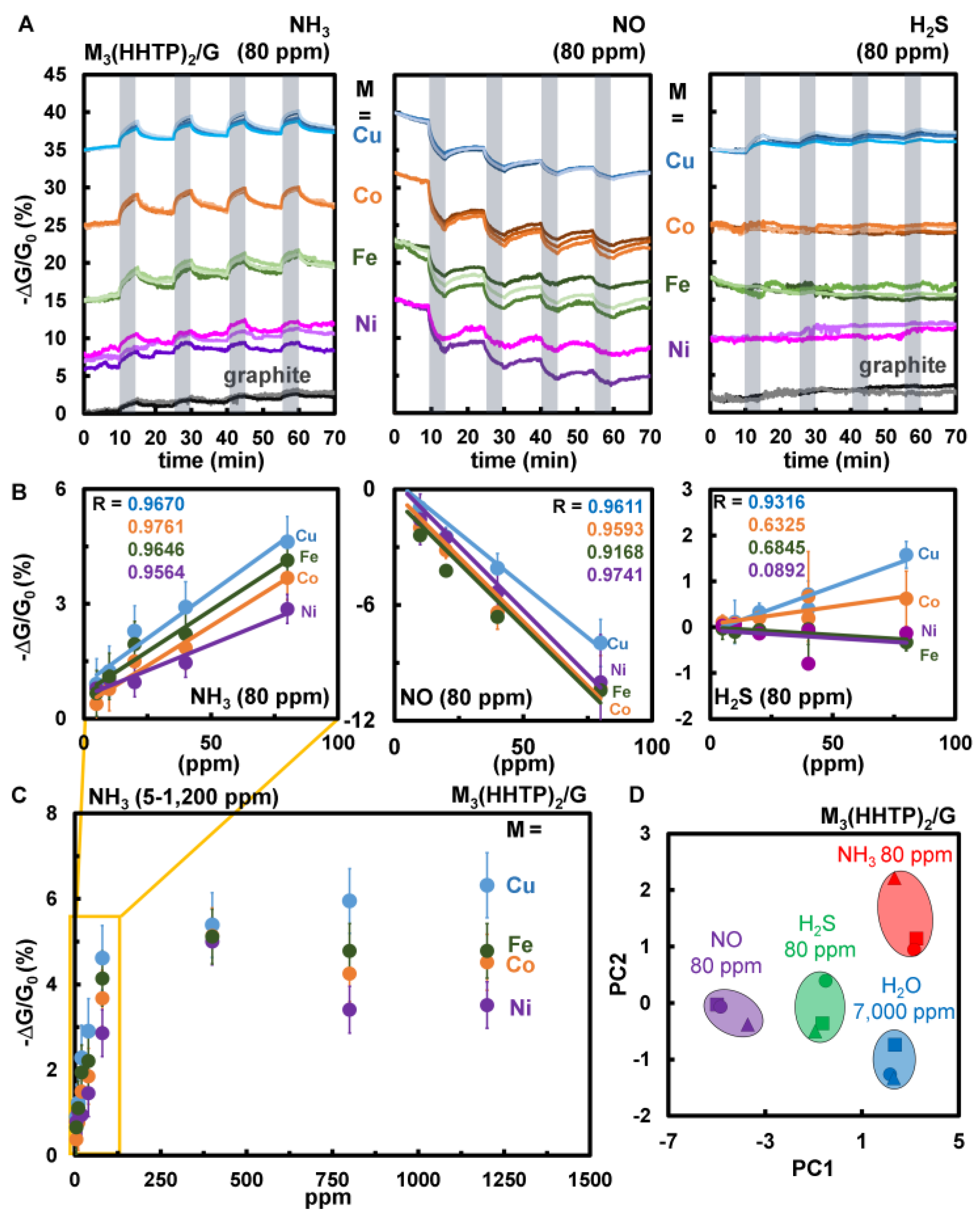
3.4.2. Chemiresistive Sensing Performance and Differentiation of NH3, NO, and H2S Using the M3HHTP2/Graphite Blends
3.4.3. Limits of Detection and Dynamic Range
3.4.4. Reproducibility Study and Scale Dependent Analysis of Cu3HHTP2/Graphite Blend
3.4.5. Proposed Chemiresistive Sensing Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Miura, N. Environmental Gas Sensing. Sens. Actuators B Chem. 1994, 20, 95–102. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward Innovations of Gas Sensor Technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Manzoli, A.; Steffens, C.; Paschoalin, R.T.; Correa, A.A.; Alves, W.F.; Leite, F.L.; Herrmann, P.S.P. Low-Cost Gas Sensors Produced by the Graphite Line-Patterning Technique Applied to Monitoring Banana Ripeness. Sensors 2011, 11, 6425–6434. [Google Scholar] [CrossRef] [PubMed]
- Duncan, V.T. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A Review of Breath Analysis for Diagnosis of Human Health. Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2016, 11, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Mirsky, V.M. Chemiresistors Based on Conducting Polymers: A Review on Measurement Techniques. Anal. Chim. Acta 2011, 687, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Lu, G.; Chen, J. Nanocarbon-Based Gas Sensors: Progress and Challenges. J. Mater. Chem. 2014, 2, 5573–5579. [Google Scholar] [CrossRef]
- Joo, S.; Brown, R.B. Chemical Sensors with Integrated Electronics. Chem. Rev. 2008, 108, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Mas-Torrent, M.; Rovira, C. Role of Molecular Order and Solid-State Structure in Organic Field-Effect Transistors. Chem. Rev. 2011, 111, 4833–4856. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.A.; Singh, A.; Daniels, K.; Vogt, T.; Chandrashekhar, M.V.S.; Koley, G. Impedance Spectroscopic Analysis of Nanoparticle Functionalized Graphene/P-Si Schottky Diode Sensors. Jpn. J. Appl. Phys. 2016, 55, 110312. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting Metal Oxides as Sensors for Environmentally Hazardous Gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide Materials for Development of Integrated Gas Sensors—A Comprehensive Review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in Sensor Applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An Overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Modi, A.; Koratkar, N.; Lass, E.; Wei, B.; Ajayan, P.M. Miniaturized Gas Ionization Sensors using Carbon Nanotubes. Nature 2003, 424, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, D.R.; Star, A. Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Sengupta, K.S.; Baruch, F.M.; Granz, D.C.; Ammu, S.; Manohar, K.S.; Whitten, E.J. A Hybrid Chemresistive Sensor System for the Detection of Organic Vapors. Sens. Actuators B Chem. 2011, 156, 715–722. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Chaekyu, K.; Li, X.; Rotello, M.V. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincă, M. Cu3(Hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; Jensen, K.E.; Pivak, P.A.; Mirica, K.A. Direct Self-Assembly of Conductive Nanorods of Metal-Organic Frameworks into Chemiresistive Devices on Shrinkable Polymer Films. Chem. Mater. 2016, 28, 5264–5268. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal-Organic Frameworks for Separations. Chem. Rev. 2011, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Sun, L.; Blood-Forsythe, M.A.; Er, S.; Wade, C.R.; Brozek, C.K.; Aspuru-Guzik, A.; Dincă, M. High Electrical Conductivity in Ni3(2,3,6,7,10,11-Hexaiminotriphenylene)2, a Semiconducting Metal-Organic Graphene Analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Tiana, D.; Walsh, A. Conductive metal-organic frameworks and networks: Fact or fantasy? Phys. Chem. 2012, 14, 13120–13132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Shao-Horn, Y.; Dincă, M. Conductive Mof Electrodes for Stable Supercapacitors with High Areal Capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, Z.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X. Metal Organic Frameworks for Energy Storage and Conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An Updated Roadmap for The Integration of Metal-Organic Frameworks with Electronic Devices and Chemical Sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Huang, J.; Zang, Y.; He, J.; Xu, G. Porous Field-Effect Transistors Based on a Semiconductive Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal-Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Clough, A.J.; Yoo, J.W.; Mecklenburg, M.H.; Marinescu, S.C. Two-Dimensional Metal-Organic Surfaces for Efficient Hydrogen Evolution from Water. J. Am. Chem. Soc. 2015, 137, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Dincă, M.; Surendranath, Y.; Nocera, D.G. Nickel-Borate Oxygen-Evolving Catalyst that Functions Under Benign Conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 10337–10341. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and The Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New Porous Crystals of Extended Metal-Catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical Sensors Based on Metal-Organic Frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-Led Design of New Porous Materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.G.; Xu, T.; Li, Z.Q.; Wang, W.; Wu, Y.; Jiang, X.; Tian, X.Y.; Zhang, L.D. Hierarchically Micro- and Mesoporous Metal-Organic Frameworks with Tunable Porosity. Angew. Chem. Int. Ed. 2008, 47, 9487–9491. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective Catalysis with Homochiral Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective Gas Adsorption and Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically Conductive Polymers and Composites for Biomedical Applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Mirica, K.A.; Azzarelli, J.M.; Weis, J.G.; Schnorr, J.M.; Swager, T.M. Rapid Prototyping of Carbon-Based Chemiresistive Gas Sensors on Paper. Proc. Natl. Acad. Sci. USA 2013, 110, E3265–E3270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Shin, T.S.; Choi, H.D.; Kwon, J.H.; Chung, Y.-C.; Yoon, H.G. Electrical Conductivity of Chemically Modified Multiwalled Carbon Nanotube/Epoxy Composites. Carbon 2005, 43, 23–30. [Google Scholar] [CrossRef]
- Franke, E.M.; Koplin, J.T.; Ulrich, S. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Highly Sensitive and Selective Chemiresistor Gas/Vapor Sensors Based on Polyaniline Nanocomposite: A Comprehensive Review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Gou, P.; Kraut, N.D.; Feigel, I.M.; Bai, H.; Morgan, G.J.; Chen, Y.; Tang, Y.; Bocan, K.; Stachel, J.; Berger, L.; et al. Carbon Nanotube Chemiresistor for Wireless pH Sensing. Sci. Rep. 2014, 4, 4468. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Dincă, M. Metal-Organic Frameworks as Active Materials in Electronic Sensor Devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Liu, S.F.; Swager, T.M.; Dincă, M. Chemiresistive Sensor Arrays from Conductive 2D Metal-Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, J.S.; Hwang, S.; Yun, W.S.; Song, D.; Lee, J.; Jeong, N.C. Multiple Coordination Exchanges for Room-Temperature Activation of Open-Metal Sites in Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 24743–24752. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, W.S.; Kim, M.B.; Kim, J.Y.; Bae, Y.S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal-Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Pavanello, M.A.; Claeys, C.; Martino, J.A. Mircoelectronics Technology and Devices—SBMicro 2010: Issue 1; Electrochemical Society: Pennington, NJ, USA, 2010; Volume 1. [Google Scholar]
- Petroski, H. The Pencil A History of Design and Circumstance; Alfred A. Knopf INC.: New York, NY, USA, 2014; p. 434. [Google Scholar]
- Mirica, K.A.; Weis, J.G.; Schnorr, J.M.; Esser, B.; Swager, T.M. Mechanical Drawing of Gas Sensors on Paper. Angew. Chem. Int. Ed. Engl. 2012, 51, 10740–10745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazier, K.M.; Mirica, K.A.; Walish, J.J.; Swager, T.M. Fully-Drawn Carbon-Based Chemical Sensors on Organic and Inorganic Surfaces. Lab Chip 2014, 14, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. The Role of Morphology and Crystallographic Structure of Metal Oxides in Response of Conductometric-Type Gas Sensors. Mater. Sci. Eng. R Rep. 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Shimizu, Y.; Egashira, M. Basic Aspects and Challenges of Semiconductor Gas Sensors. MRS Bull. 2013, 24, 18–24. [Google Scholar] [CrossRef]
- Lee, S.P. Electrodes for Semiconductor Gas Sensors. Sensors 2017, 17, 683. [Google Scholar] [CrossRef] [PubMed]
- Jurs, P.C.; Bakken, G.A.; McClelland, H.E. Computational Methods for the Analysis of Chemical Sensor Array Data from Volatile Analytes. Chem. Rev. 2000, 100, 2649–2678. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis; Springer Science & Business Media: Berlin, Germany, 2013; p. 271. [Google Scholar]
- Table AC 1—Permissible Exposure Limits for Chemical Contaminants. Available online: https://www.dir.ca.gov/title8/5155table_ac1.html (accessed on 19 September 2017).
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the Sensitivity of Chemiresistor Gas Sensors Based on Pristine Carbon Nanotubes to Detect Low-ppb Ammonia Concentrations in the Environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.-Q.; Liu, L.-W.; Pan, G.-B. Ammonia Chemiresistor Sensor Based on Poly(3-Hexylthiophene) Film Oxidized by Nitrosonium Hexafluorophosphate. Chem. Lett. 2012, 41, 1569–1570. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia Sensors and Their Applications—A Review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Grange, C.S.; Meijer, A.J.; Ward, M.D. Trinuclear Ruthenium Dioxolene Complexes Based on the Bridging Ligand Hexahydroxytriphenylene: Electrochemistry, Spectroscopy, and Near-Infrared Electrochromic Behaviour Associated with a Reversible Seven-Membered Redox Chain. Dalton Trans. 2010, 39, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Stassen, I.; Bueken, B.; Reinsch, H.; Oudenhoven, J.F.M.; Wouters, D.; Hajek, J.; Van Speybroeck, V.; Stock, N.; Vereecken, P.M.; Van Schaijk, R.; De Vos, D.; Ameloot, R. Towards Metal-Organic Framework Based Field Effect Chemical Sensors: UiO-66-NH2 for Nerve Agent Detection. Chem. Sci. 2016, 7, 5827–5832. [Google Scholar] [CrossRef]
- Easun, T.L.; Moreau, F.; Yan, Y.; Yang, S.; Schroder, M. Structural and Dynamic Studies of Substrate Binding in Porous Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46, 239–274. [Google Scholar] [CrossRef] [PubMed]
- Fruhberger, B.; Stirling, N.; Grillo, F.G.; Ma, S.; Ruthven, D.; Lad, R.J.; Frederick, B.G. Detection and Quantification of Nitric Oxide in Human Breath Using a Semiconducting Oxide Based Chemiresistive Microsensor. Sens. Actuators B Chem. 2001, 76, 226–234. [Google Scholar] [CrossRef]
- Pandey, K.S.; Kim, H.K.; Tang, K. T. A Review of Sensor-Based Methods for Monitoring Hydrogen Sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Chandran, G.T.; Li, X.; Ogata, A.; Penner, R.M. Electrically Transduced Sensors Based on Nanomaterials (2012–2016). Anal. Chem. 2017, 89, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Davydovskaya, P.; Ranft, A.; Lotsch, B.V.; Pohle, R. Analyte Detection with Cu-BTC Metal-Organic Framework Thin Films by Means of Mass-Sensitive and Work-Function-Based Readout. Anal. Chem. 2014, 86, 6948–6958. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, M.; Aykanat, A.; Smith, M.K.; Mirica, K.A. Drawing Sensors with Ball-Milled Blends of Metal-Organic Frameworks and Graphite. Sensors 2017, 17, 2192. https://doi.org/10.3390/s17102192
Ko M, Aykanat A, Smith MK, Mirica KA. Drawing Sensors with Ball-Milled Blends of Metal-Organic Frameworks and Graphite. Sensors. 2017; 17(10):2192. https://doi.org/10.3390/s17102192
Chicago/Turabian StyleKo, Michael, Aylin Aykanat, Merry K. Smith, and Katherine A. Mirica. 2017. "Drawing Sensors with Ball-Milled Blends of Metal-Organic Frameworks and Graphite" Sensors 17, no. 10: 2192. https://doi.org/10.3390/s17102192




