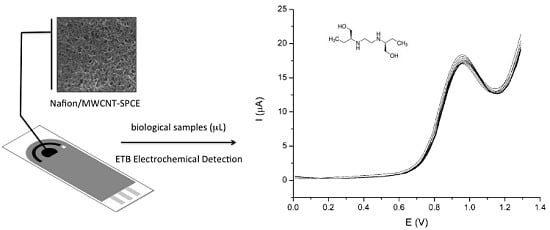
Development of a Nafion/MWCNT-SPCE-Based Portable Sensor for the Voltammetric Analysis of the Anti-Tuberculosis Drug Ethambutol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. SPCEs Modification
2.4. Analytical Procedure
3. Results and Discussion
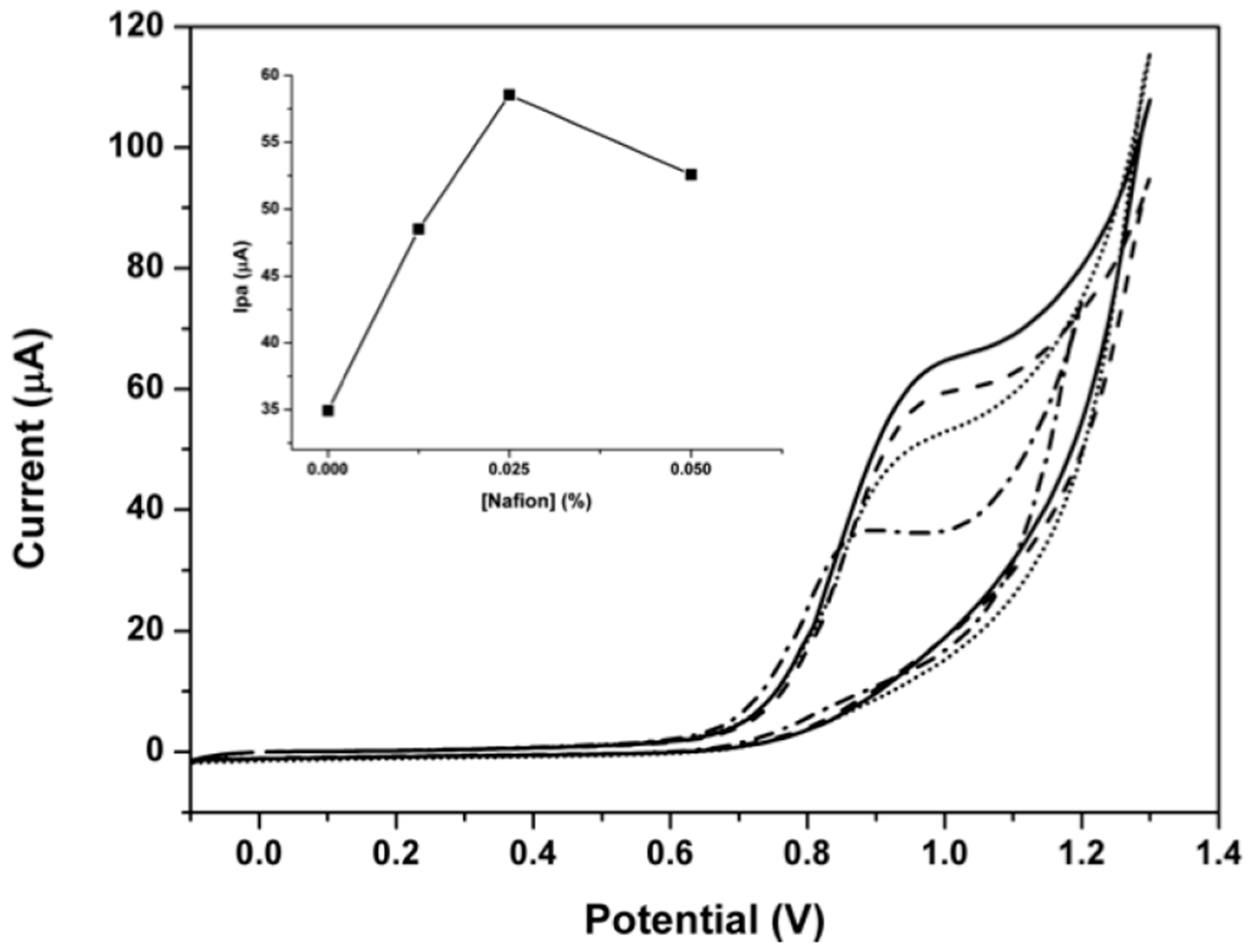
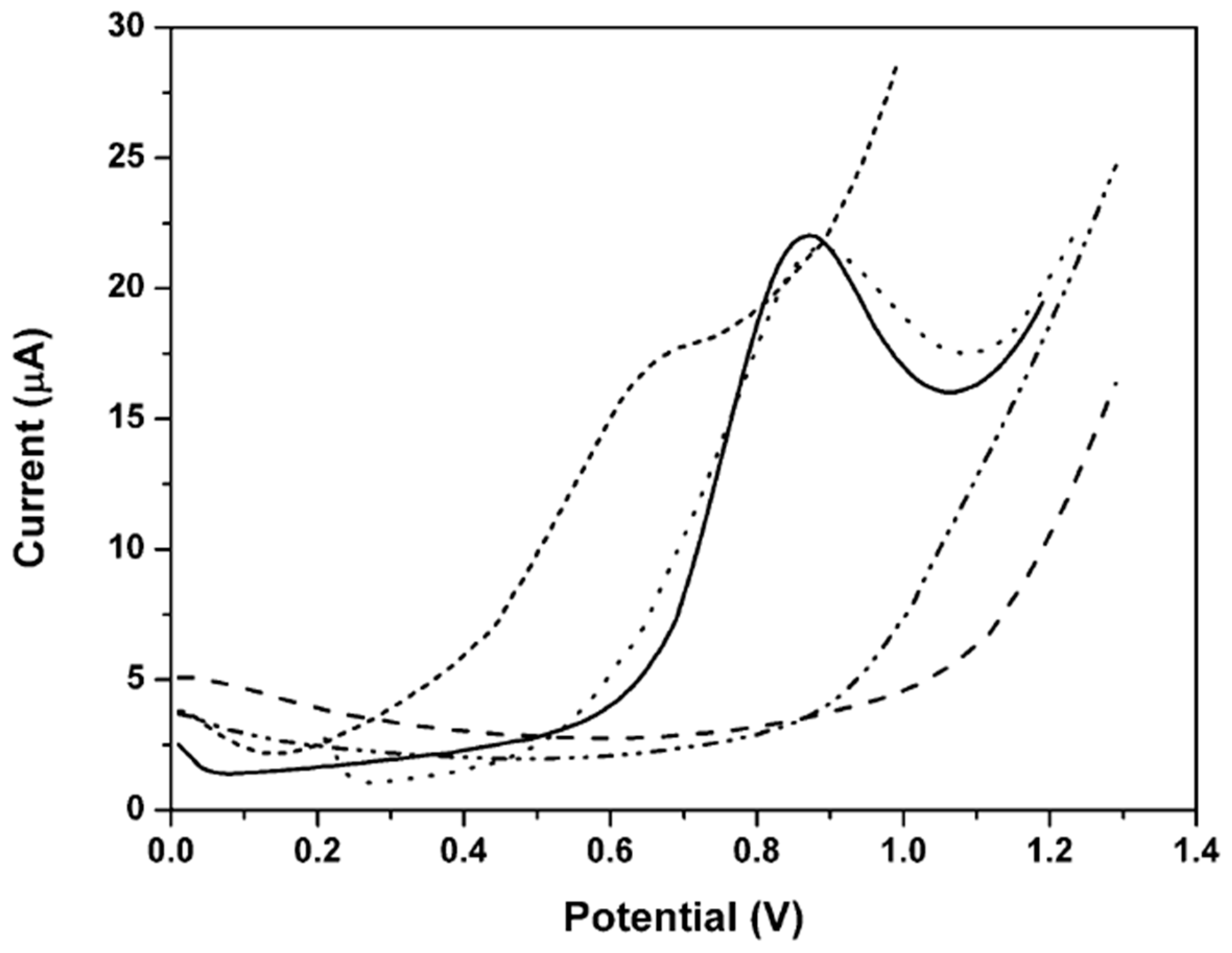
3.1. Optimization of the Modifier
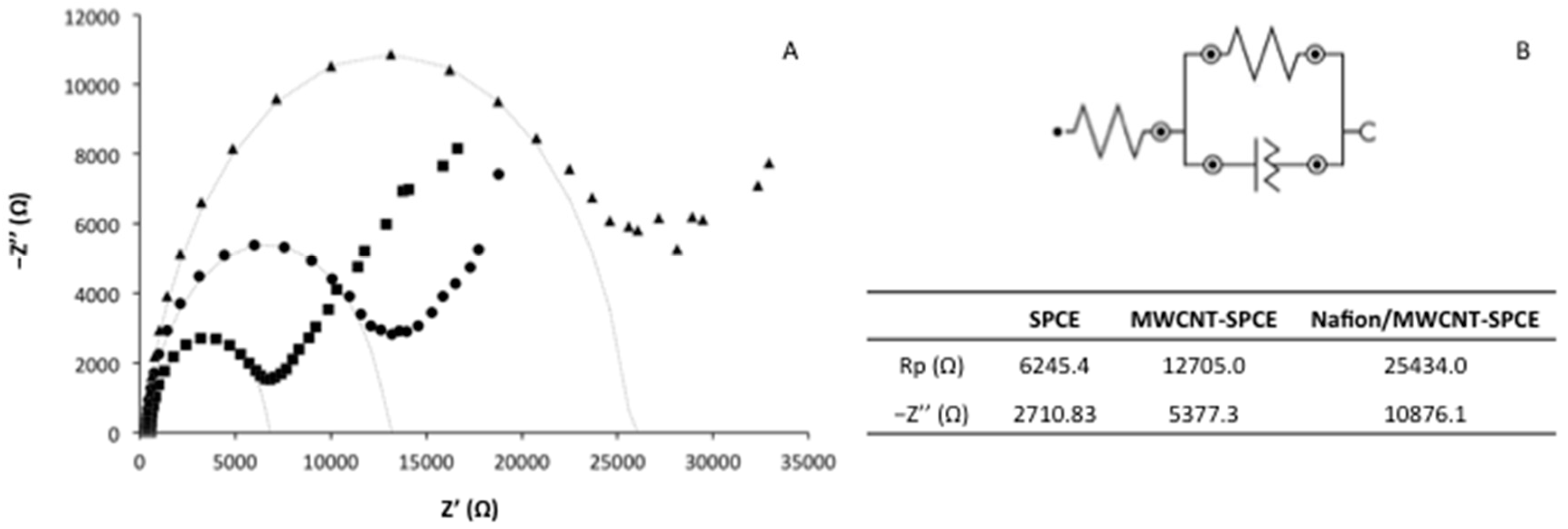
3.2. Characterization of the Nafion/MWCNT-SPCE Surface
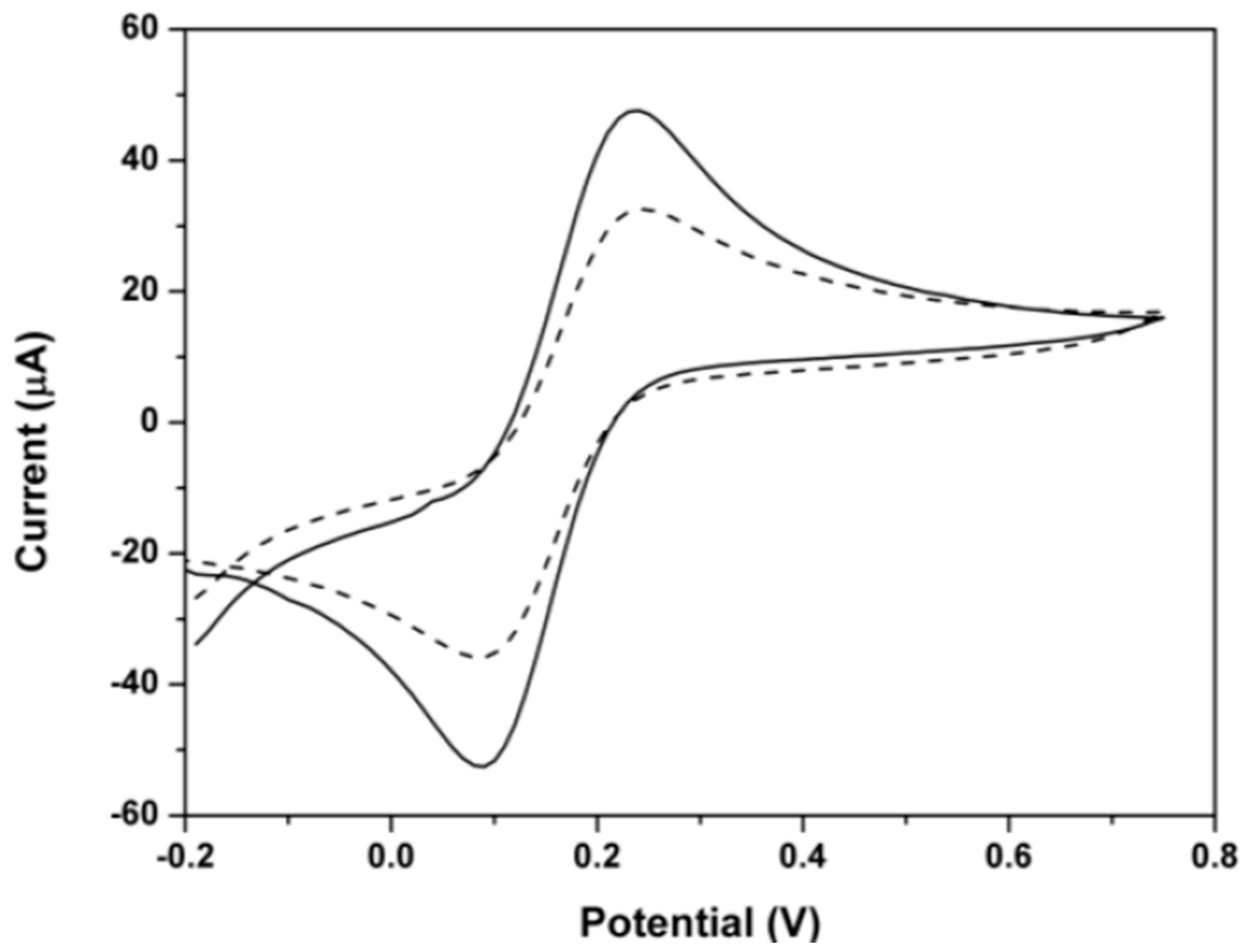
3.3. Electrochemical Behaviour of ETB
3.3.1. Influence of pH
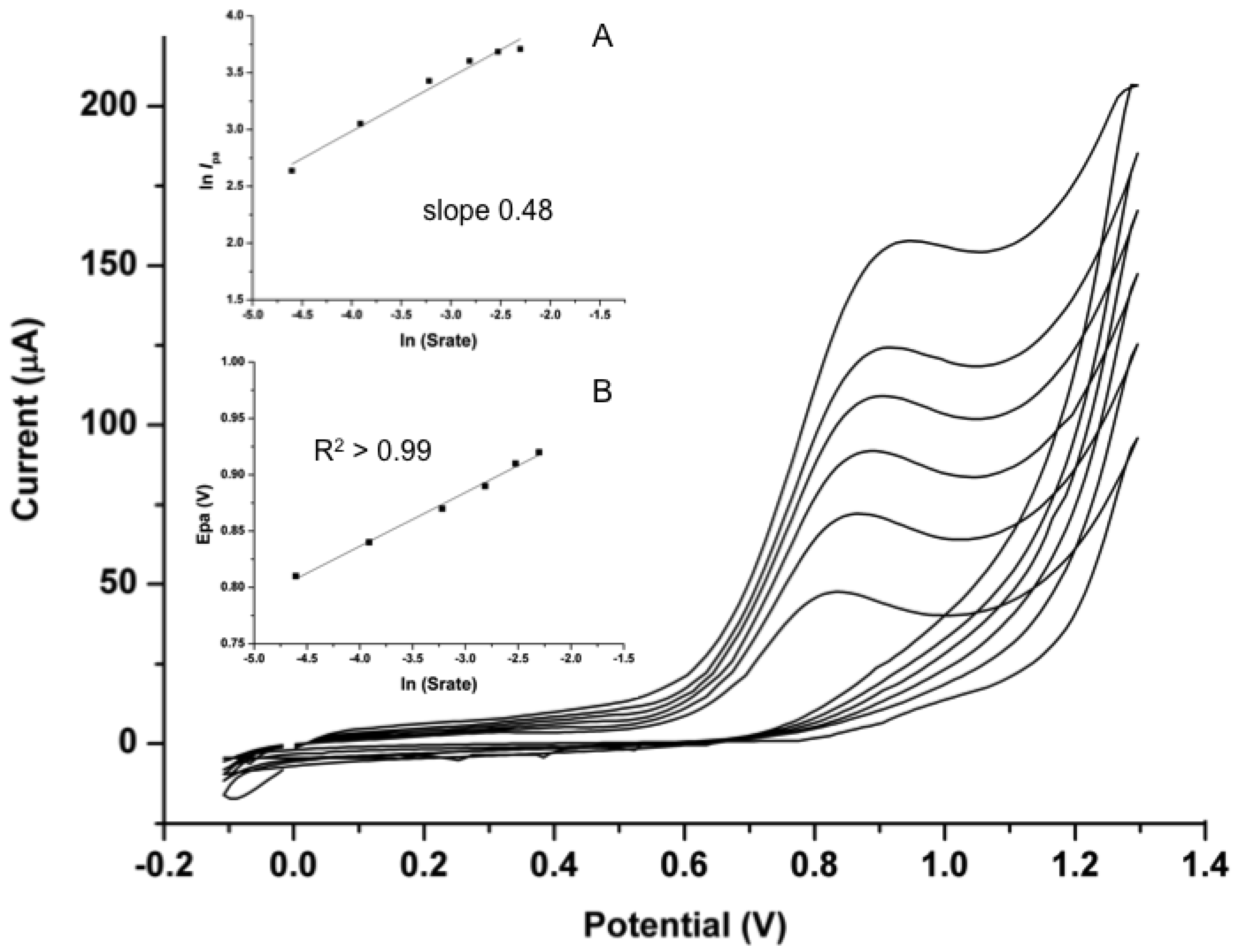
3.3.2. Oxidation Behaviour Analysis
3.3.3. Square Wave Voltammetry
3.4. Analytical Application to Biological Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abel, L.; El-Baghdadi, J.; Bousfiha, A.A.; Casanova, J.L.; Schurr, E. Human genetics of tuberculosis: A long and winding road. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 792–801. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation, Tuberculosis (TB), Global Tuberculosis Report 2015 (2015). Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 13 May 2016).
- Zumla, A.; Chakaya, J.; Centis, R.; D’Ambrosio, L.; Mwaba, P.; Bates, M.; Kapata, N.; Nyirenda, T.; Chanda, D.; Mfinanga, S.; et al. Tuberculosis treatment and management—An update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir. Med. 2015, 3, 220–234. [Google Scholar] [CrossRef]
- Zumla, A.I.; Gillespie, S.H.; Hoelscher, M.; Philips, P.P.; Cole, S.T.; Abubakar, I.; McHugh, T.D.; Schito, M.; Maeurer, M.; Nunn, A.J. New antituberculosis drugs, regimens, and adjunct therapies: Needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 327–340. [Google Scholar] [CrossRef]
- Zumla, A.; Nahid, P.; Cole, S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013, 12, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, N.; Karpoormath, R.V.; Goyal, R.N. Electroanalysis of antitubercular drugs in pharmaceutical dosage forms and biological fluids: A review. Anal. Chim. Acta 2015, 853, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, M.C.; Calvino, R.; Abrigo, C. Ion interaction reagent reversed-phase high-performance liquid chromatography determination of anti-tuberculosis drugs and metabolites in biological fluids. J. Chromatogr. B Biomed. Sci. Appl. 2001, 754, 477–486. [Google Scholar] [CrossRef]
- Hee, K.H.; Seo, J.J.; Lee, L.S. Development and validation of liquid chromatography tandem mass spectrometry method for simultaneous quantification of first line tuberculosis drugs and metabolites in human plasma and its application in clinical study. J. Pharm. Biomed. Anal. 2015, 102, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, X.; Wei, Q.; Liu, Y.; Liu, P.; Ma, A.; Zou, F. Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the simultaneous determination of five first-line antituberculosis drugs in plasma. Anal. Bioanal. Chem. 2013, 405, 6323–6335. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.; Zhou, H.; Zhang, Y.L.; Hybertson, B.; Randolph, T.; Christians, U. Quantification of isoniazid and acetylisoniazid in rat plasma and alveolar macrophages by liquid chromatography-tandem mass spectrometry with on-line extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 847, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Acedo-Valenzuela, M.I.; Espinosa-Mansilla, A.; Muñoz De La Peña, A.; Cañada-Cañada, F. Determination of antitubercular drugs by micellar electrokinetic capillary chromatography (MEKC). Anal. Bioanal. Chem. 2002, 374, 432–436. [Google Scholar] [PubMed]
- Liu, Y.; Fu, Z.; Wang, L. Capillary electrophoresis analysis of isoniazid using luminol-periodate potassium chemiluminescence system. Luminescence 2011, 26, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Shi, B.; Ai, X.; He, Z. Chemiluminescence detection of isoniazid using Ru(phen)32+-isoniazid-Ce(IV) system. J. Pharm. Biomed. Anal. 2004, 36, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Abolhasani, J.; Hassanzadeh, J. Potassium permanganate-acridine yellow chemiluminescence system for the determination of fluvoxamine, isoniazid and ceftriaxone. Luminescence 2014, 29, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Yang, J.Y.; Du, L.M.; Wu, H.; Li, C.F. Determination of ethambutol by a sensitive fluorescent probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Dong, C. Simultaneous determination of trace of loxacin, ciprofloxacin, and sparfloxacin by micelle TLC-fluorimetry. J. Chromatogr. Sci. 2004, 42, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Csiba, A. Colorimetric determination of isoniazid in aqueous solutions and biological fluids. Acta Pharm. Hung. 1989, 59, 205–212. [Google Scholar] [PubMed]
- El-Brashy, A.M.; El-Ashry, S.M. Colorimetric and titrimetric assay of isoniazid. J. Pharm. Biomed. Anal. 1992, 10, 421–426. [Google Scholar] [CrossRef]
- Cheemalapati, S.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M. Enhanced electrocatalytic oxidation of isoniazid at electrochemically modified rhodium electrode for biological and pharmaceutical analysis. Colloids Surf. B Biointerfaces 2014, 121, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kawde, A.N.; Temerk, Y.; Farhan, N. Adsorptive stripping voltammetry of antibiotics rifamycin SV and rifampicin at renewable pencil electrodes. Acta Chim. Slov. 2014, 61, 398–405. [Google Scholar] [PubMed]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S. Voltammetric techniques for the assay of pharmaceuticals—A review. Anal. Biochem. 2011, 408, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.A.; Kwon, J.; Kim, D.; Yang, S. A rapid, sensitive and selective electrochemical biosensor with concanavalin A for the preemptive detection of norovirus. Biosens. Bioelectron. 2015, 64, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Sharma, A.; Bhand, S. Ultrasensitive detection of streptomycin using flow injection analysis-electrochemical quartz crystal nanobalance (FIA-EQCN) biosensor. Biosens. Bioelectron. 2015, 67, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Metters, J.P.; Randviir, E.P.; Banks, C.E. Screen-printed back-to-back electroanalytical sensors. Analyst 2014, 139, 5339–5349. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.M.; Gorodetsky, A.A. Electrochemical sensors: Taking charge of detection. Nat. Chem. 2012, 4, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry: A review. Anal. Chim. Acta 2008, 622, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Saleh Ahammad, A.J.; Lee, J.J.; Rahman, M.A. Electrochemical sensors based on carbon nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef] [PubMed]
- García-González, R.; Fernández-Abedul, M.T.; Costa-García, A. Nafion® modified-screen printed gold electrodes and their carbon nanostructuration for electrochemical sensors applications. Talanta 2013, 107, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Palanisamy, S.; Chen, S.M. Highly selective dopamine electrochemical sensor based on electrochemically pretreated graphite and nafion composite modified screen printed carbon electrode. J. Colloid Interface Sci. 2013, 411, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, J.; Liu, Y.; Wu, S.; Song, G.; Ye, B. Electrochemical oxidation behavior of colchicine on a graphene oxide-Nafion composite film modified glassy carbon electrode. Analyst 2011, 136, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Bo, X.; Guo, L. Electrochemical behaviors and determination of isoniazid at ordered mesoporous carbon modified electrode. Sens. Actuator B Chem. 2011, 155, 837–842. [Google Scholar] [CrossRef]
- Pan, X.; Wang, L.; Gründemann, D.; Sweet, D.H. Interaction of Ethambutol with human organic cation transporters of the SLC22 family indicates potential for drug-drug interactions during antituberculosis therapy. Antimicrob. Agents Chemother. 2013, 57, 5053–5059. [Google Scholar] [CrossRef] [PubMed]
- Ngece, R.F.; West, N.; Ndangili, P.M.; Olowu, R.A.; Williams, A.; Hendricks, N.; Mailu, S.; Baker, P.; Iwuoha, E. A silver Nanoparticle/Poly (8-Anilino-1-Naphthalene Sulphonic Acid) Bioelectrochemical Biosensor System for the Analytical Determination of Ethambutol. Int. J. Electrochem. Sci. 2011, 6, 1820–1834. [Google Scholar]
- Perantoni, C.B.; Carbogim, L.G.S.; Semaan, F.S.; Matos, R.C.; Lowinsohn, D. Flow Injection Analysis of Ethambutol in Antituberculosis Drugs Using a Graphite-Paraffin Electrode as Amperometric Detector. Electroanal 2011, 23, 2582–2585. [Google Scholar] [CrossRef]
- Rooney, M.B.; Coomber, D.C.; Bond, A.M. Achievement of Near-Reversible Behavior for the [Fe(CN)6]3−/4− Redox Couple Using Cyclic Voltammetry at Glassy Carbon, Gold, and Platinum Macrodisk Electrodes in the Absence of Added Supporting Electrolyte. Anal. Chem. 2000, 72, 3486–3491. [Google Scholar] [CrossRef] [PubMed]
- Lufrano, F.; Staiti, P.; Minutoli, M. Evaluation of nafion based double layer capacitors by electrochemical impedance spectroscopy. J. Power Sources 2003, 124, 314–320. [Google Scholar] [CrossRef]
- Lai, C.M.; Lin, J.C.; Ting, F.P.; Chyou, S.D.; Hsueh, K.L. Contribution of Nafion loading to the activity of catalysts and the performance of PEMFC. Int. J. Hydrog. Energy 2008, 33, 4132–4137. [Google Scholar] [CrossRef]
- Lima, A.E.B.; Luz, G.E.; Batista, N.C.; Longo, E.; Cavalcante, L.S.; Santos, R.S. Determination of Ethambutol in Aqueous Medium Using an Inexpensive Gold Microelectrode Array as Amperometric Sensor. Electroanal 2015, 28, 985–989. [Google Scholar] [CrossRef]
- Shao, C.; Wei, Y.; Li, S.; Li, C. Determination of ethambutol at glassy carbon electrode modified with multiwall carbon nanotubes. Chem. Anal. 2008, 53, 511–521. [Google Scholar]
- Perantoni, C.B.; Azevedo, A.B.R.; Vaz, F.A.S.; Oliveira, M.A.L.; Matos, R.C.; Lowinsohn, D. Flow injection analysis of ethambutol in synthetic urine using a graphite-polyurethane composite electrode as an amperometric detector. Cent. Eur. J. Chem. 2013, 11, 1668–1673. [Google Scholar] [CrossRef]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, R.A.S.; Quinaz, M.B. Development of a Nafion/MWCNT-SPCE-Based Portable Sensor for the Voltammetric Analysis of the Anti-Tuberculosis Drug Ethambutol. Sensors 2016, 16, 1015. https://doi.org/10.3390/s16071015
Couto RAS, Quinaz MB. Development of a Nafion/MWCNT-SPCE-Based Portable Sensor for the Voltammetric Analysis of the Anti-Tuberculosis Drug Ethambutol. Sensors. 2016; 16(7):1015. https://doi.org/10.3390/s16071015
Chicago/Turabian StyleCouto, Rosa A. S., and Maria Beatriz Quinaz. 2016. "Development of a Nafion/MWCNT-SPCE-Based Portable Sensor for the Voltammetric Analysis of the Anti-Tuberculosis Drug Ethambutol" Sensors 16, no. 7: 1015. https://doi.org/10.3390/s16071015