Efficient Donor Impurities in ZnO Nanorods by Polyethylene Glycol for Enhanced Optical and Glutamate Sensing Properties
Abstract
:1. Introduction
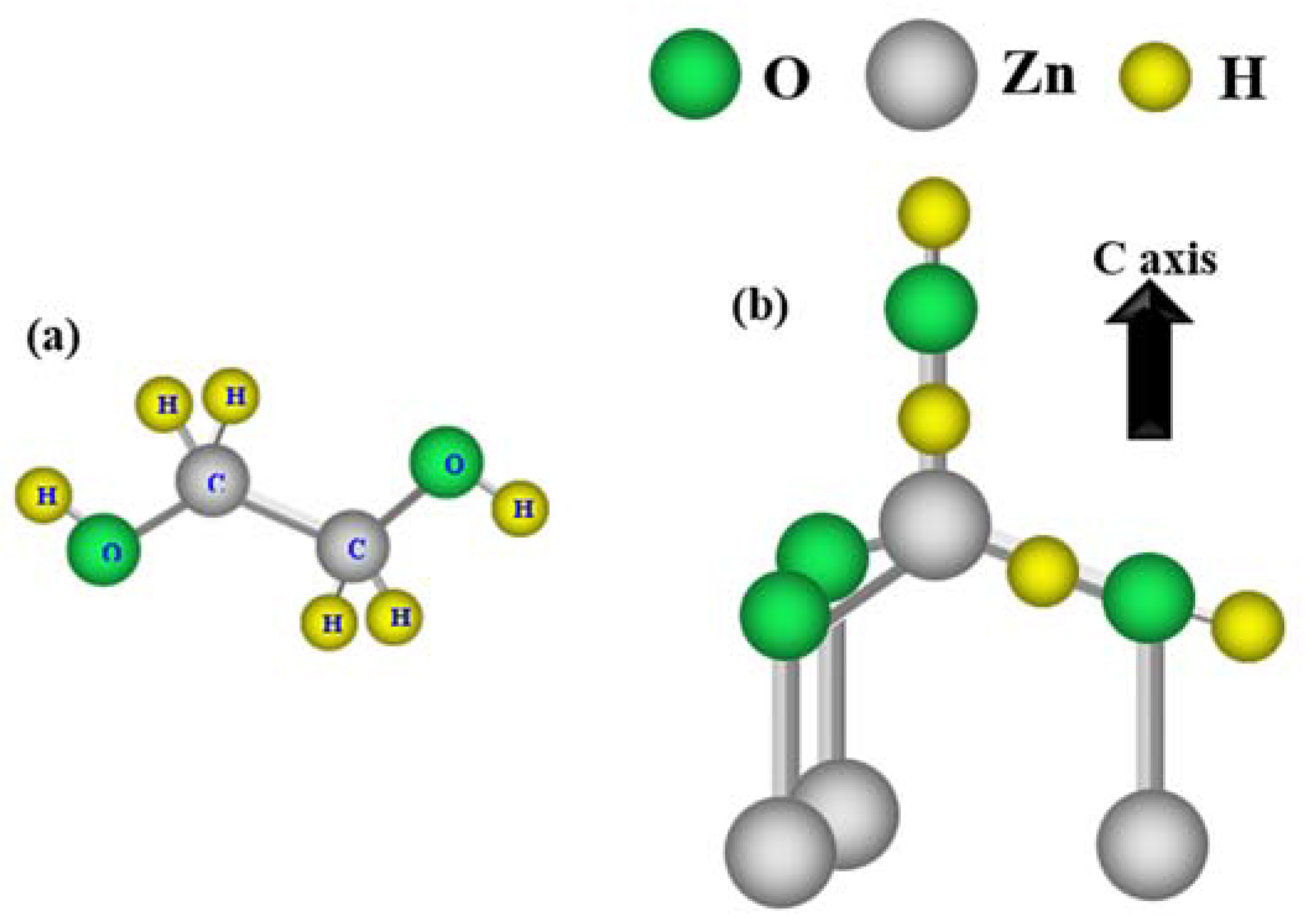
2. Experimental Section
2.1. Chemicals
2.2. Growth of the Modified ZNRs on Au Coated Glass
2.3. Characterization
2.4. Immobilization of ZNRs with GluOx and Electrochemical Measurement
3. Results and Discussion
3.1. Morphology and Structural Analysis
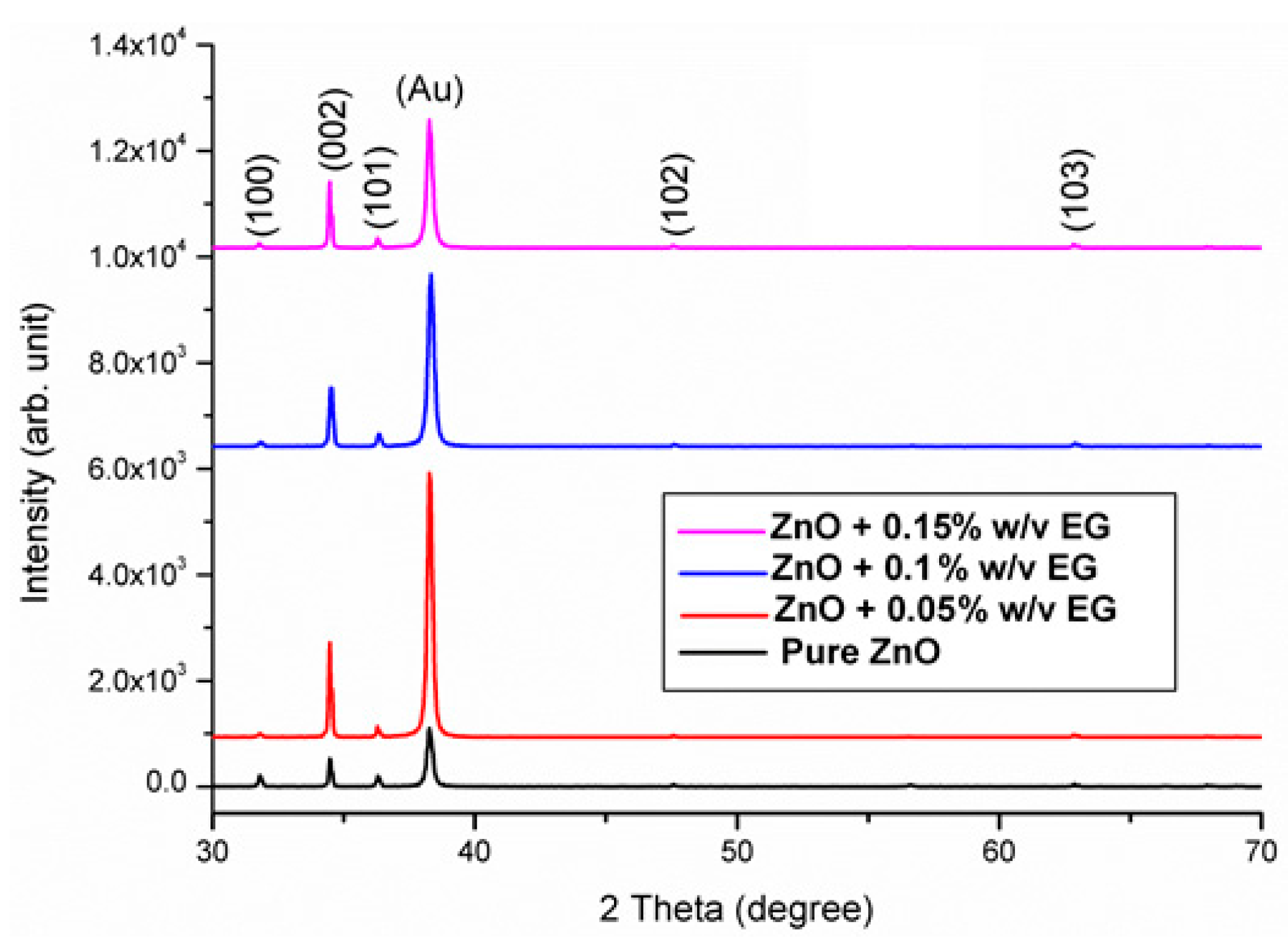
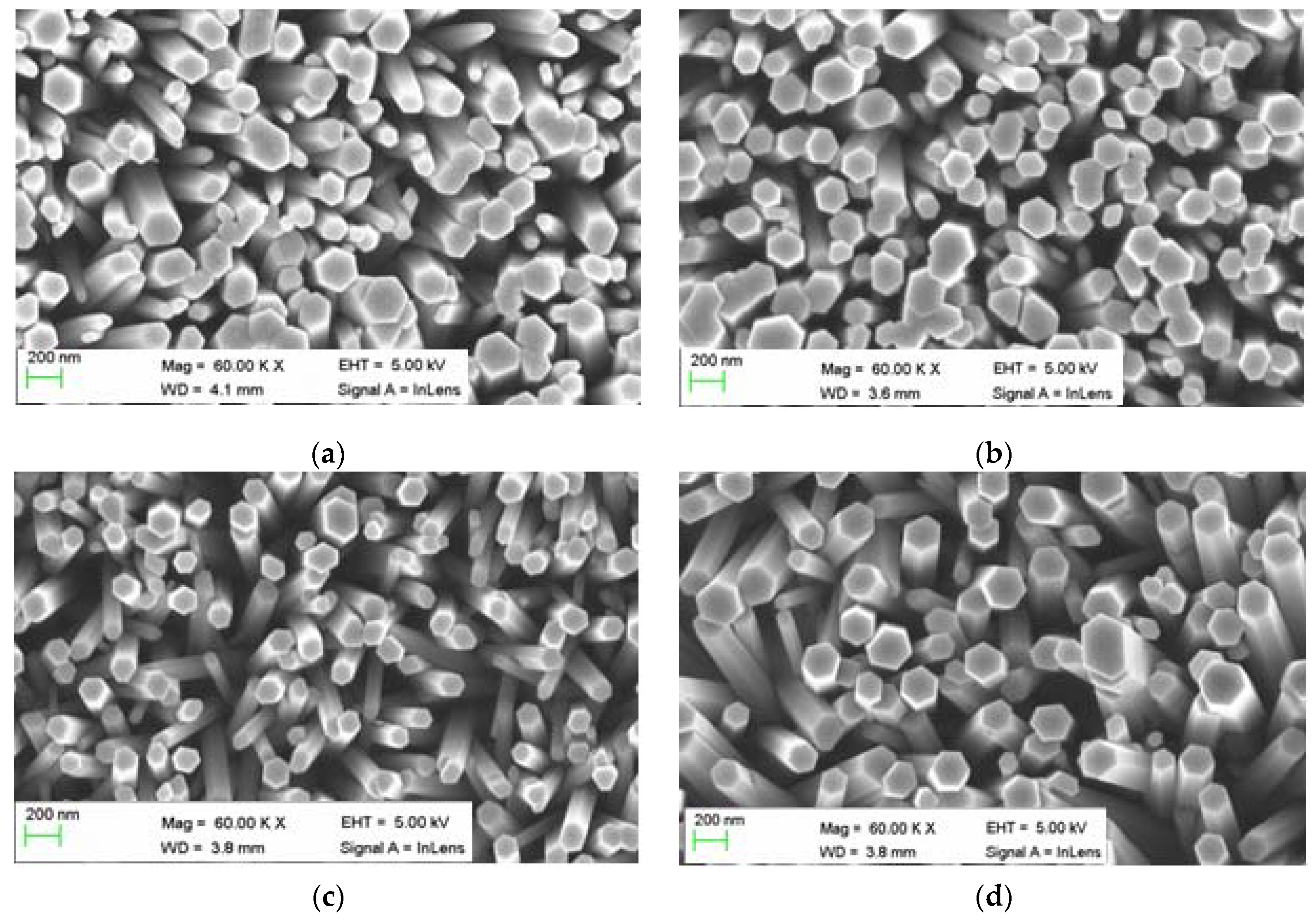
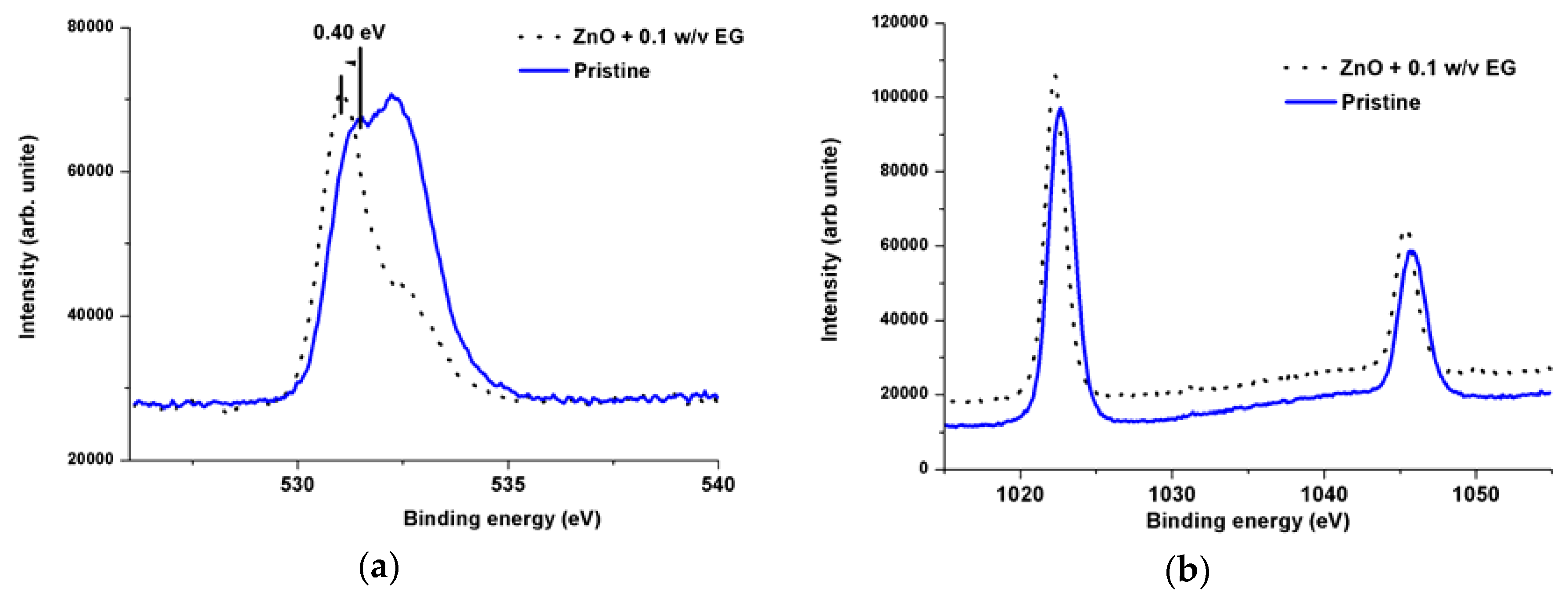

3.2. Optical Characteristics

3.3. Mott-Schottky Analysis

| ZNRs | Vfb (V) | Nd (cm−3) |
|---|---|---|
| Pristine | −1.09 | 2.81 × 1019 |
| +0.05% (w/v) EG | −1.1 | 5.37 × 1019 |
| +0.1% (w/v) EG | −0.87 | 1.39 × 1020 |
| +0.15% (w/v) EG | −0.82 | 7.58 × 1019 |
3.4. The Detection of Glu by Modified ZNRs

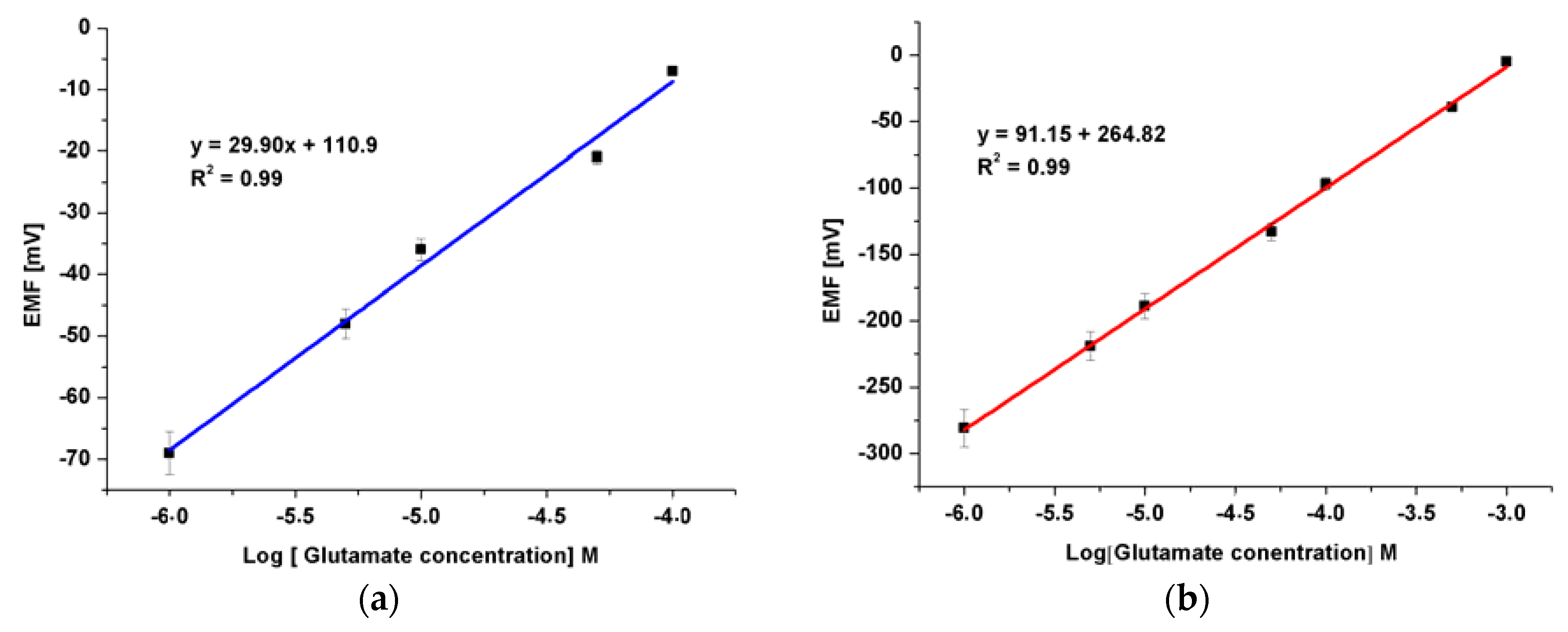
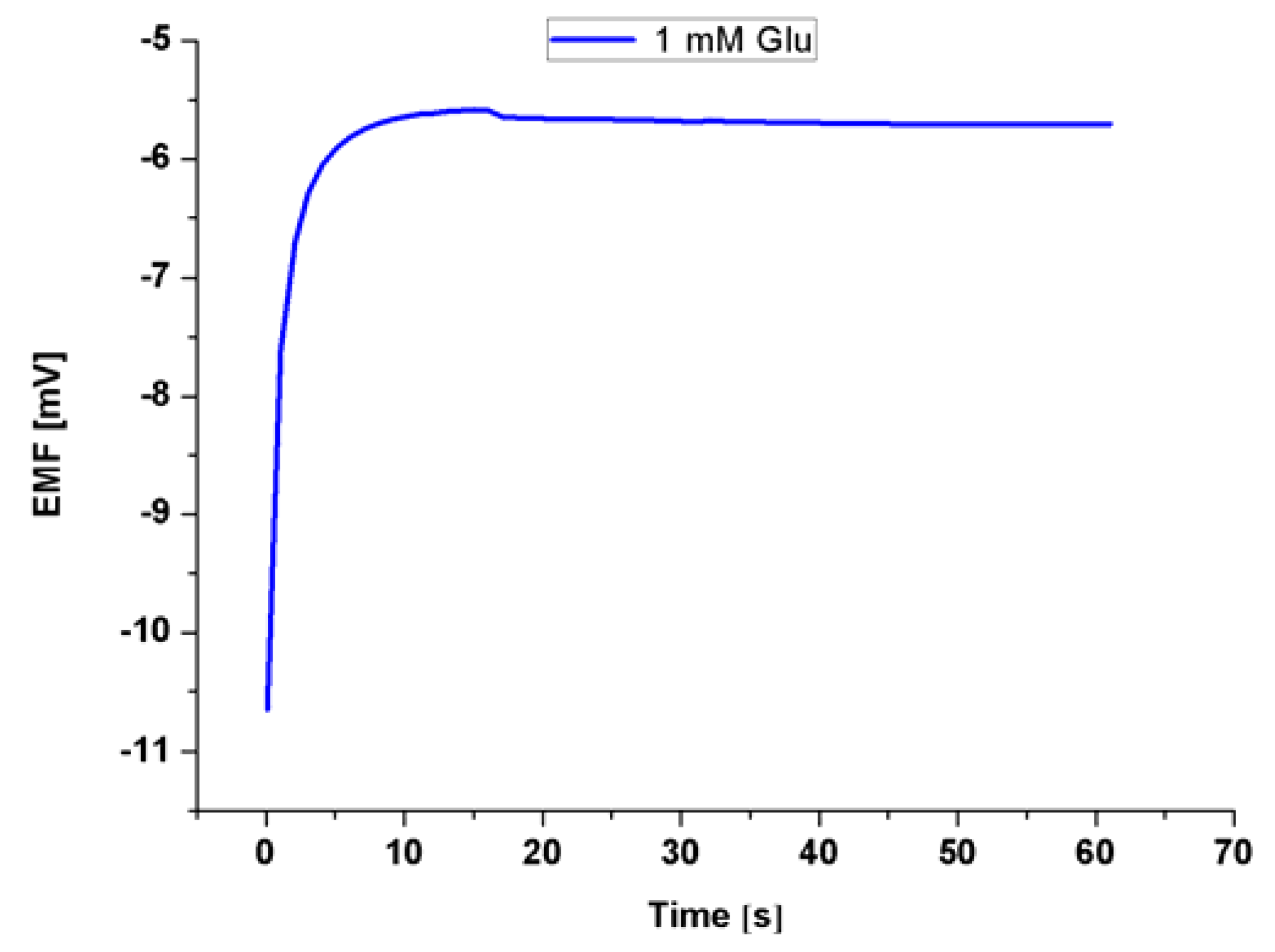
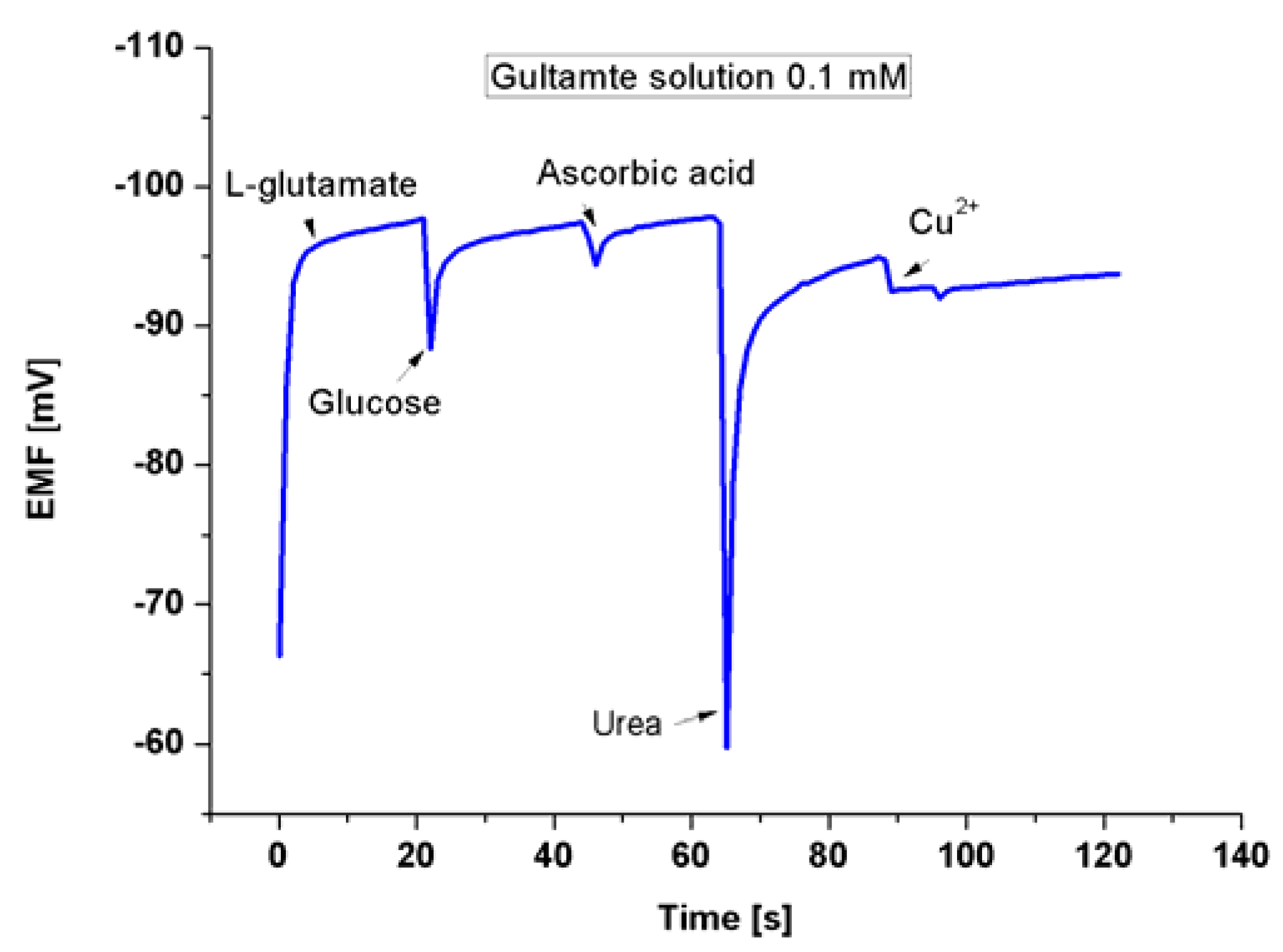

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [PubMed]
- Jinap, S.; Hajeb, P. Glutamate. Its applications in food and contribution to health. Appetite 2010, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, A.; Mulller, A.; Passarge, M.; Liebs, P.; Honeck, H.; Muller, H.G. A novel L-glutamate oxidase from Streptomyces endus purification and properties. Eur. J. Biochem. 1989, 182, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.B.; Khudaish, E.A.; Hart, A.L. Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part 1. An adsorption-controlled mechanism. Electrochim. Acta 1998, 43, 579–588. [Google Scholar] [CrossRef]
- Monge-Acuña, A.A.; Fornaguera-Trías, J. A high performance liquid chromatography method with electrochemical detection of gamma-aminobutyric acid, glutamate and glutamine in rat brain homogenates. J. Neurosci. Methods 2009, 183, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Marvin, J.S.; Borghuis, B.G.; Tian, L.; Cichon, J.; Harnett, M.T.; Akerboom, J.; Gordus, A.; Renninger, S.L.; Chen, T.-W.; Bargmann, C.I.; et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 2013, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.-C.; Noel, V.; Berggren, M. Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Wu, M.; Meng, S.; Wang, Q.; Si, W.; Huang, W.; Dong, X. Nickel−cobalt oxide decorated three-dimensional graphene as an enzyme mimic for glucose and calcium detection. ACS Appl. Mater. Interfaces 2015, 7, 21089–21094. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Liu, C.; Chen, H.; Shi, H.; Wang, L.; Chen, P.; Huang, W.; Dong, X. Free-standing electrochemical electrode based on Ni(OH)2/3D graphene foam for non-enzymatic glucose detection. Nanoscale 2014, 6, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Desnica, U.V. Doping limits in II-Vl compounds-challenges, problems and solutions. Prog. Cryst. Growth Charact. 1998, 36, 291–357. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupoto, Z.H.; Khranovskyy, V.; Willander, M.; Nur, O. Habit-modifying additives and their morphological consequences on photoluminescence and glucose sensing properties of ZnO nanostructures, grown via aqueous chemical synthesis. Vacuum 2015, 116, 21–26. [Google Scholar] [CrossRef]
- Tian, C.; Li, W.; Pan, K.; Zhang, Q.; Tian, G.; Zhou, W.; Fu, H. One pot synthesis of Ag nanoparticle modified ZnO microspheres in ethylene glycol medium and their enhanced photocatalytic performance. J. Solid State Chem. 2010, 183, 2720–2725. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Mujawar, S.H.; Ganesan, V.; Patil, P.S. Surfactant-mediated growth of nanostructured zinc oxide thin films via electrodeposition and their photoelectrochemical performance. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chen, L.; Li, D.; Yang, W.; Yang, P.; Liu, Z.; Chen, A.; Liu, C.C. Different morphologies of ZnO nanorods and their sensing property. Sens. Actuators B Chem. 2010, 146, 129–137. [Google Scholar]
- Ibupoto, Z.H.; Khun, K.; Lu, J.; Willander, M. The synthesis of CuO nanoleaves, structural characterization, and their glucose sensing application. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Khun, K.; Ibupoto, Z.H.; Liu, X.; Beni, V.; Willander, M. The ethylene glycol template assisted hydrothermal synthesis of Co3O4 nanowires; structural characterization and their application as glucose non-enzymatic sensor. Mat. Sci. Eng. B 2015, 194, 94–100. [Google Scholar] [CrossRef]
- Limpijumnong, S.; Zhang, S.B. Resolving hydrogen binding sites by pressure—A first-principles prediction for ZnO. App. Phys. Lett. 2005, 86, 151910. [Google Scholar] [CrossRef]
- Mora-Seró, I.; Fabregat-Santiago, F.; Denier, B.; Bisquert, J.; Tena-Zaera, R.; Elias, J.; Lévy-Clément, C. Determination of carrier density of ZnO nanowires by electrochemical techniques. App. Phys. Lett. 2006, 89, 203117. [Google Scholar] [CrossRef]
- Qin, S.; Zeyden, M.; Oldenziel, W.H.; Cremers, T.I.F.H.; Westerink, B.H.C. Microsensors for in vivo measurement of glutamate in brain tissue. Sensors 2008, 8, 6860–6884. [Google Scholar] [CrossRef]
- Yao, S.; Liu, D.; Ge, K.; Chent, K.; Nie, L. A novel glutamine biosensor system based on a conductance-surface acoustic wave frequency response. Enzyme Microb. Technol. 1995, 17, 413–417. [Google Scholar] [CrossRef]
- Wang, B.G.; Shi, E.W.; Zhong, W.Z. Understanding and controlling the morphology of ZnO crystallites under hydrothermal conditions. Cryst. Res. Technol. 1997, 32, 659–667. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Ono, L.K.; Cuenya, B.R.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Synthesis and characterization of Ag- or Sb-doped ZnO nanorods by a facile hydrothermal route. J. Phys. Chem. C 2010, 114, 12401–12408. [Google Scholar] [CrossRef]
- Rai, P.; Kim, Y.-S.; Song, H.-M.; Song, M.-K.; Yu, Y.-T. The role of gold catalyst on the sensing behavior of ZnO nanorods for CO and NO2 gases. Sens. Actuators B Chem. 2012, 165, 133–142. [Google Scholar] [CrossRef]
- Rebsdat, S.; Mayer, D. Ethylene Glycol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Z.; Zhang, Y.; Kai, M.; Fang, H.; Chen, G.H.; Wei, X.H. Hydrogen bonding interactions between ethylene glycol and water: density, excess molar volume, and spectral study. Sci. Chin. Ser B Chem. 2008, 51, 420–426. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Weber, J. Effect of uniaxial stress on vibrational modes of hydrogen in ZnO. Phys. Rev. B 2006, 73, 035208. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Börrnert, F.; Weber, J. Photoconductivity and infrared absorption study of hydrogen-related shallow donors in ZnO. Phys. Rev. B 2005, 73, 085212. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed]
- Kunat, M.; Girol, S.G.; Burghaus, U.; Wöll, C. The Interaction of water with the oxygen-terminated, polar surface of ZnO. J. Phys. Chem. B 2003, 107, 14350–14356. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Hydrogen as a cause of doping in zinc oxide. Phys. Rev. Lett. 2000, 85, 1012. [Google Scholar] [CrossRef] [PubMed]
- Djurisic, A.B.; Leung, Y.H. Optical properties of ZnO nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Tsiarapas, C.; Girginoudi, D.; Georgoulas, N. Effect of hydrogen on ZnO films and Au/ZnO schottky contacts. Semicond. Sci. Technol. 2014, 29, 045012. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Han, P.G.; Xu, J.; Chen, D.Y.; Wei, D.Y.; Ma, Z.Y.; Chen, K.J.; Xu, L.; Huang, X.F. Hydrogen passivation effect on enhanced luminescence from nanocrystalline Si/SiO2 multilayers. Chin. Phys. Lett. 2007, 24, 2657. [Google Scholar]
- Park, S.; Mun, Y.; An, S.; Lee, W.I.; Lee, C. Enhanced photoluminescence of Au-functionalized ZnO nanorods annealed in a hydrogen atmosphere. J. Lumin. 2014, 147, 5–8. [Google Scholar] [CrossRef]
- Lorite, I.; Wasik, J.; Michalsky, T.; Schmidt-Grund, R.; Esquinazi, P. Hydrogen influence on the electrical and optical properties of ZnO thin films grown under different atmospheres. Thin Solid Films 2014, 556, 18–22. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of germanium. Phys. Stat. Sol. 1966, 15, 627. [Google Scholar] [CrossRef]
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chen, K.-H.; Shen, C.-H.; Ganguly, A.; Chen, L.-C.; Wu, J.-J.; Wen, H.-I.; Pong, W.-F. Anomalous blue shift in emission spectra of ZnO nanorods with sizes beyond quantum confinement regime. Appl. Phys. Lett. 2006, 88, 241905. [Google Scholar] [CrossRef]
- Rajeshwar, K. Encyclopedia of Electrochemistry: Semiconductor Electrodes and Photoelectrochemistry; Licht, S., Ed.; Wiley-VCH: Weinheim, Germany, 2002; Chapter 1; pp. 1–53. [Google Scholar]
- Tornow, J.; Schwarzburg, K. Transient electrical response of dye-sensitized ZnO nanorod solar Cells. J. Phys. Chem. C 2007, 111, 8692–8698. [Google Scholar] [CrossRef]
- Talke, H.; Schubert, G.E. Enzymatic urea determination in the blood and serum in the Warburg optical test. Klinische Wochenschrift 1965, 43, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Wachiratianchai, S.; Bhumiratana, A.; Udomsopagit, S. Isolation, purification, and characterization of L-glutamate oxidase from streptomyces sp. 18G. Elec. J. Biotec. 2004, 7, 277–284. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhag, S.; Khun, K.; Khranovskyy, V.; Liu, X.; Willander, M.; Nur, O. Efficient Donor Impurities in ZnO Nanorods by Polyethylene Glycol for Enhanced Optical and Glutamate Sensing Properties. Sensors 2016, 16, 222. https://doi.org/10.3390/s16020222
Elhag S, Khun K, Khranovskyy V, Liu X, Willander M, Nur O. Efficient Donor Impurities in ZnO Nanorods by Polyethylene Glycol for Enhanced Optical and Glutamate Sensing Properties. Sensors. 2016; 16(2):222. https://doi.org/10.3390/s16020222
Chicago/Turabian StyleElhag, Sami, Kimleang Khun, Volodymyr Khranovskyy, Xianjie Liu, Magnus Willander, and Omer Nur. 2016. "Efficient Donor Impurities in ZnO Nanorods by Polyethylene Glycol for Enhanced Optical and Glutamate Sensing Properties" Sensors 16, no. 2: 222. https://doi.org/10.3390/s16020222





