Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Exfoliation of Graphene
2.3. Preparation of ZnO-G Composites
2.4. Characterizations
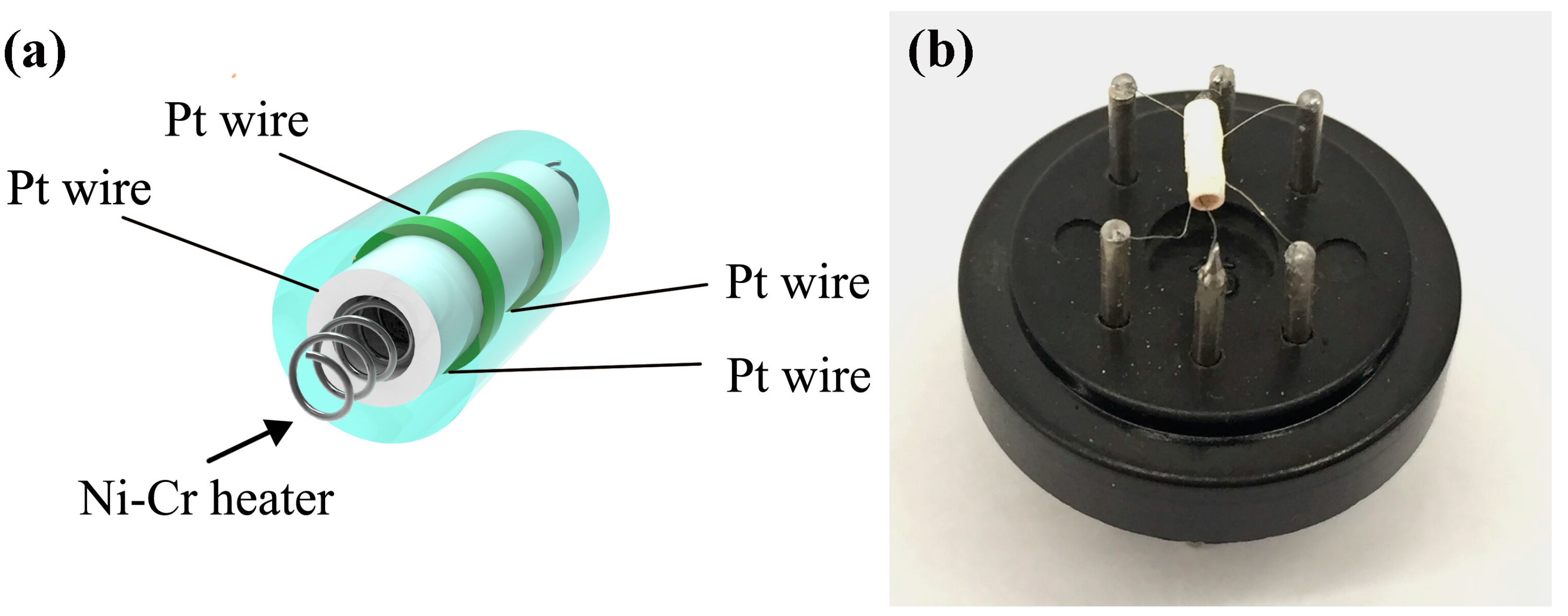
2.5. Fabrication and Gas Sensing Measurements
3. Results
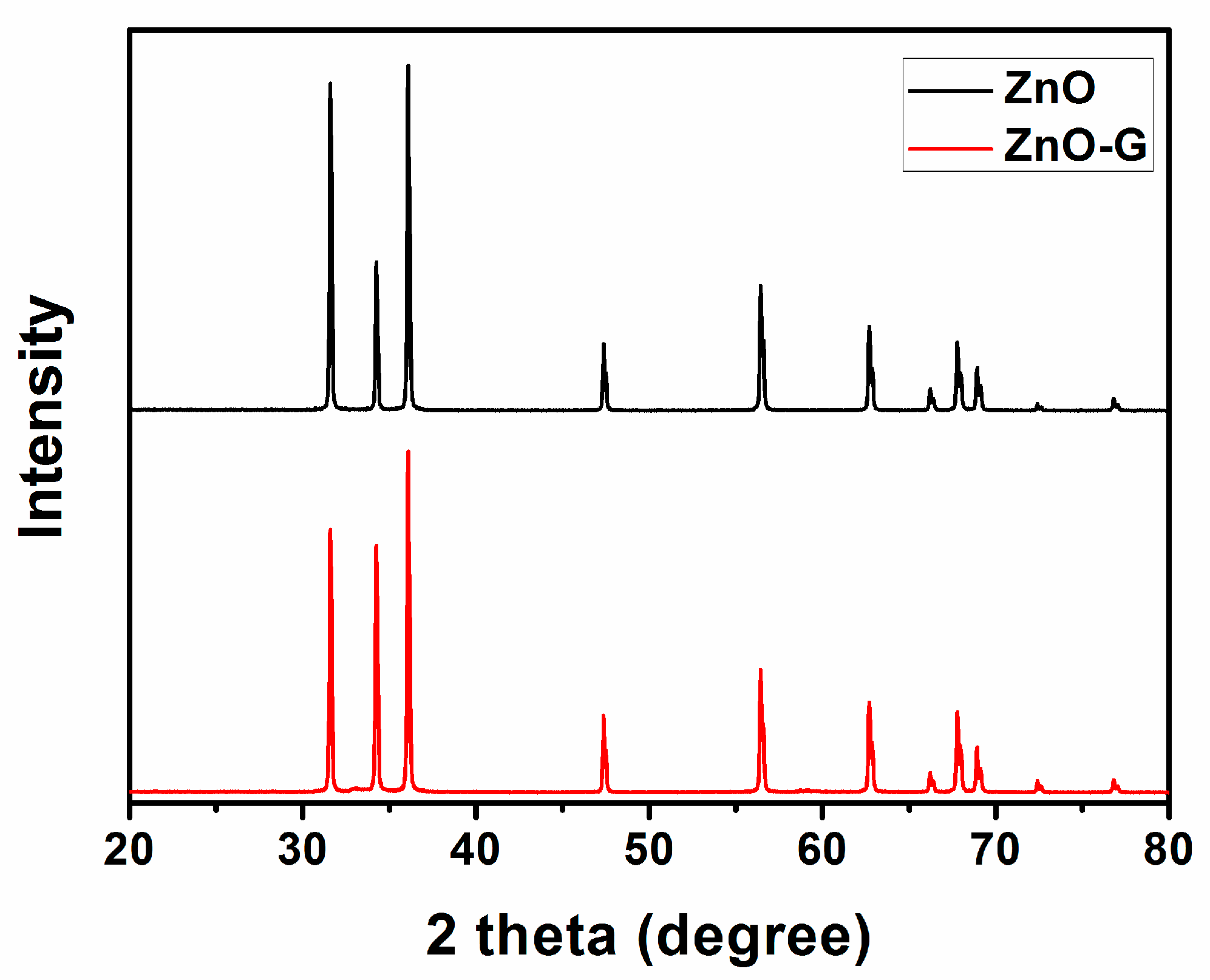
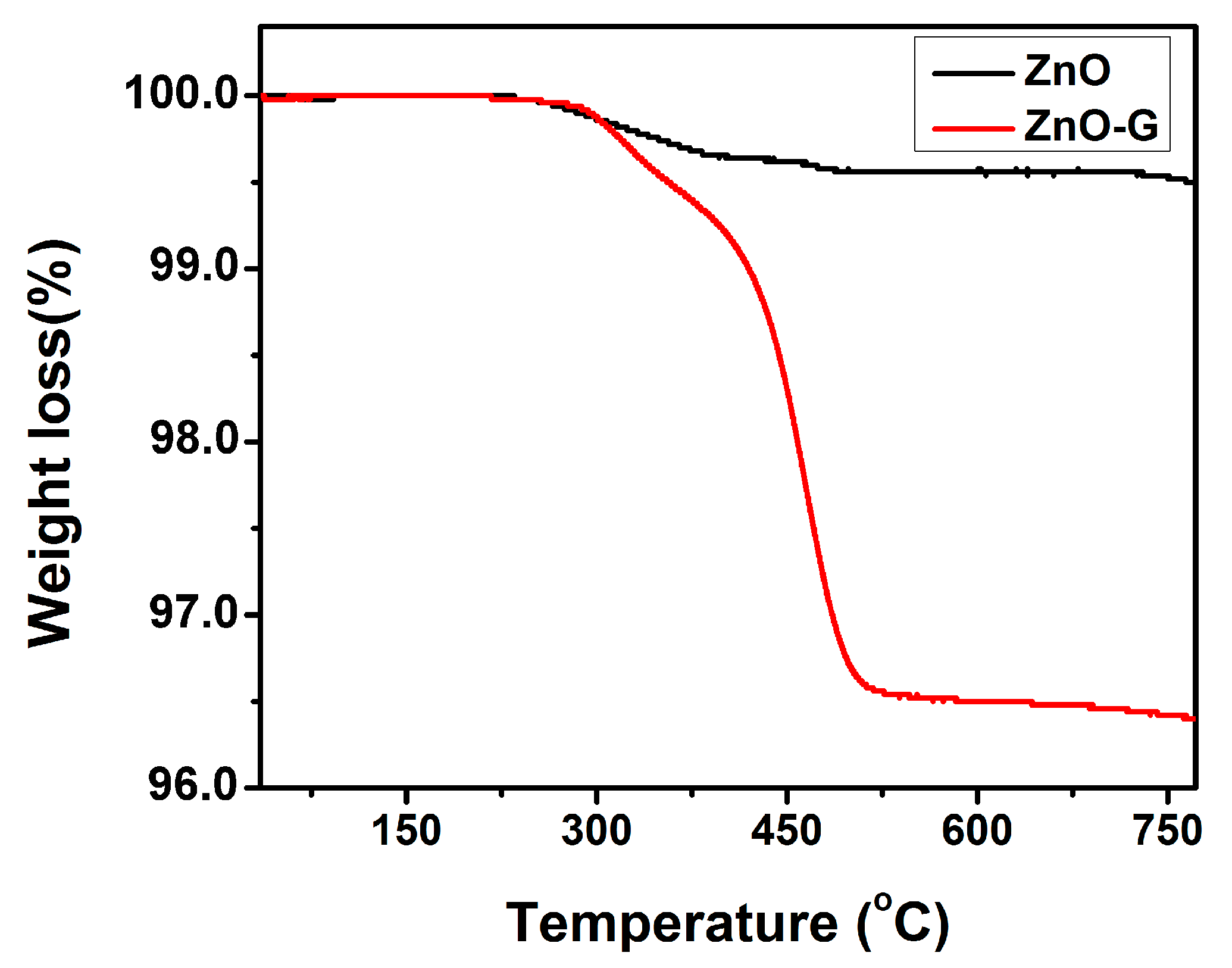
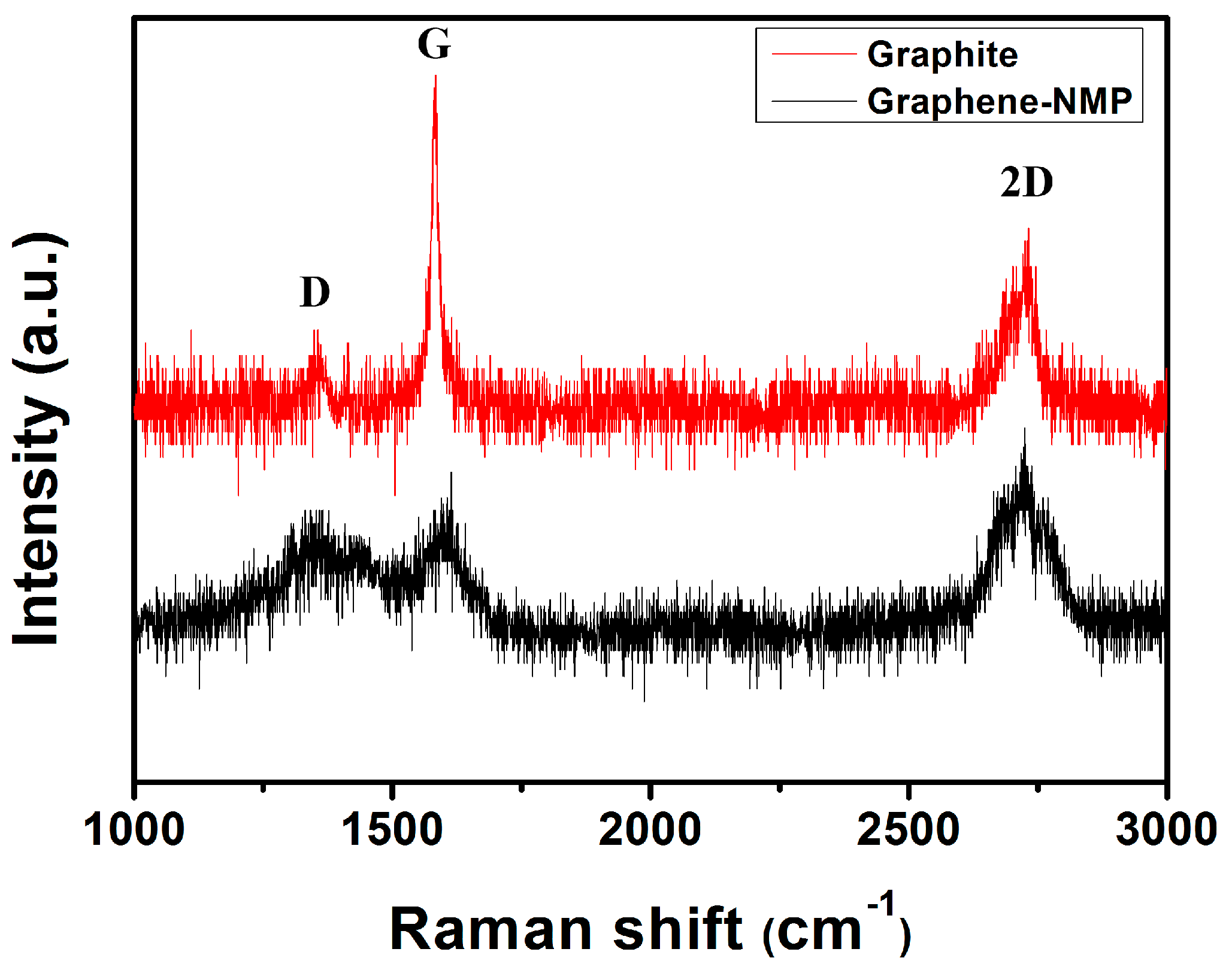
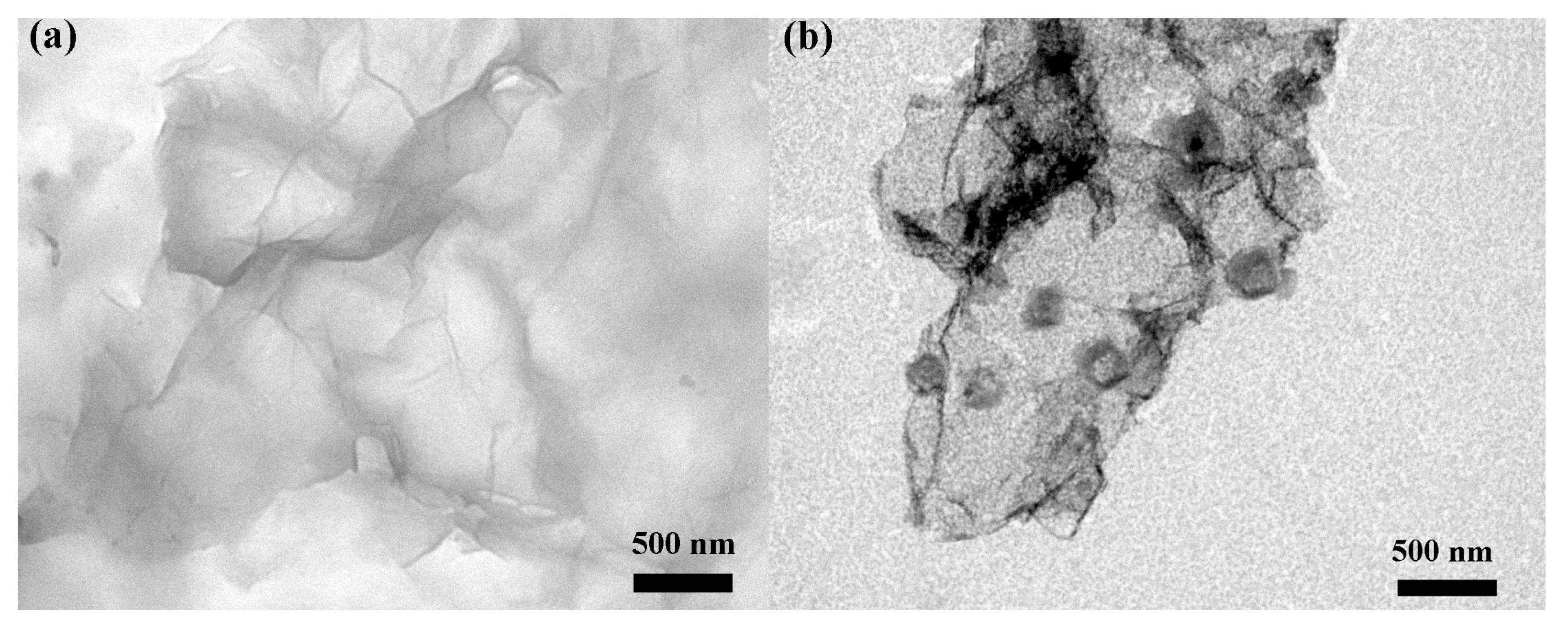
3.1. Structural and Morphological Characteristics
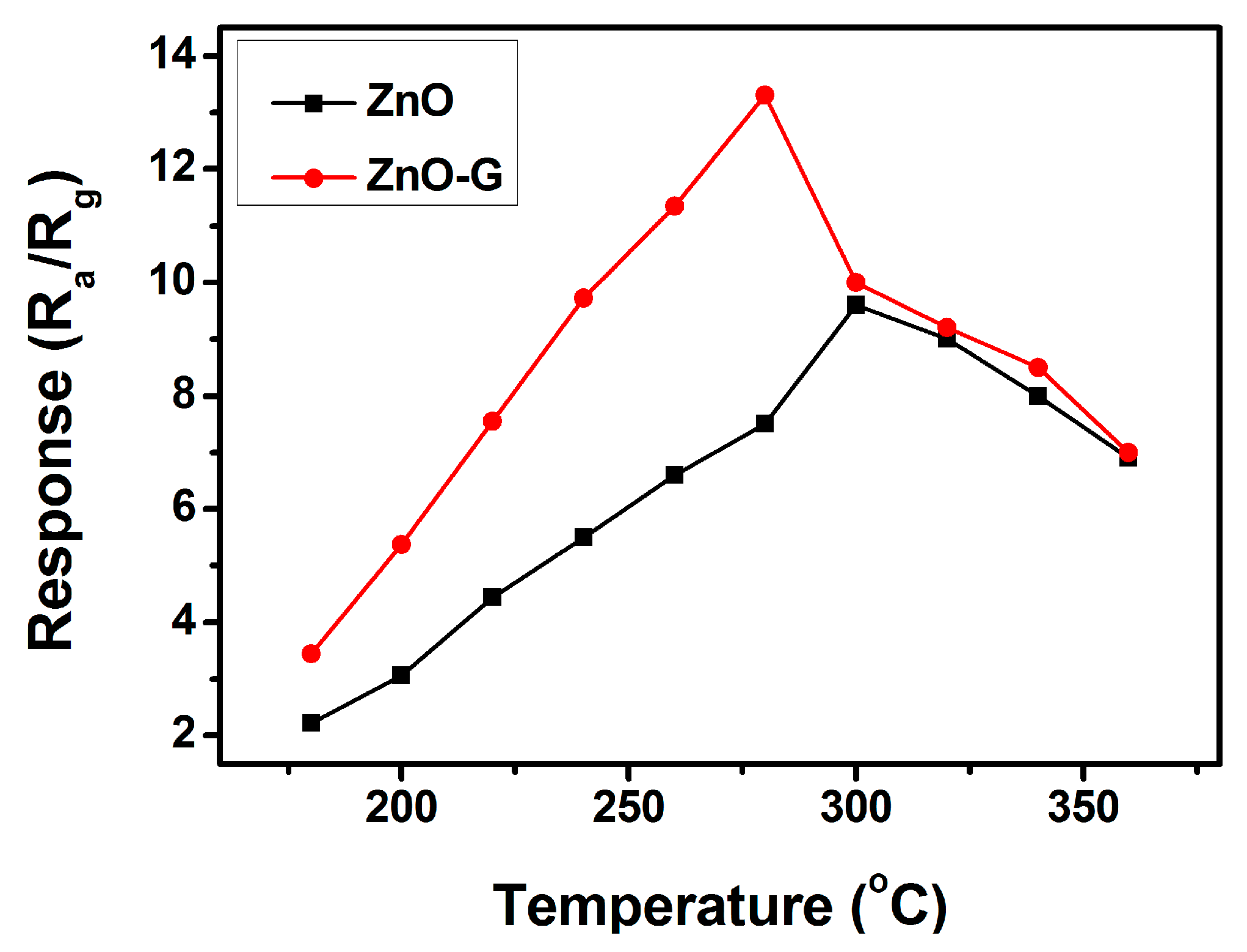
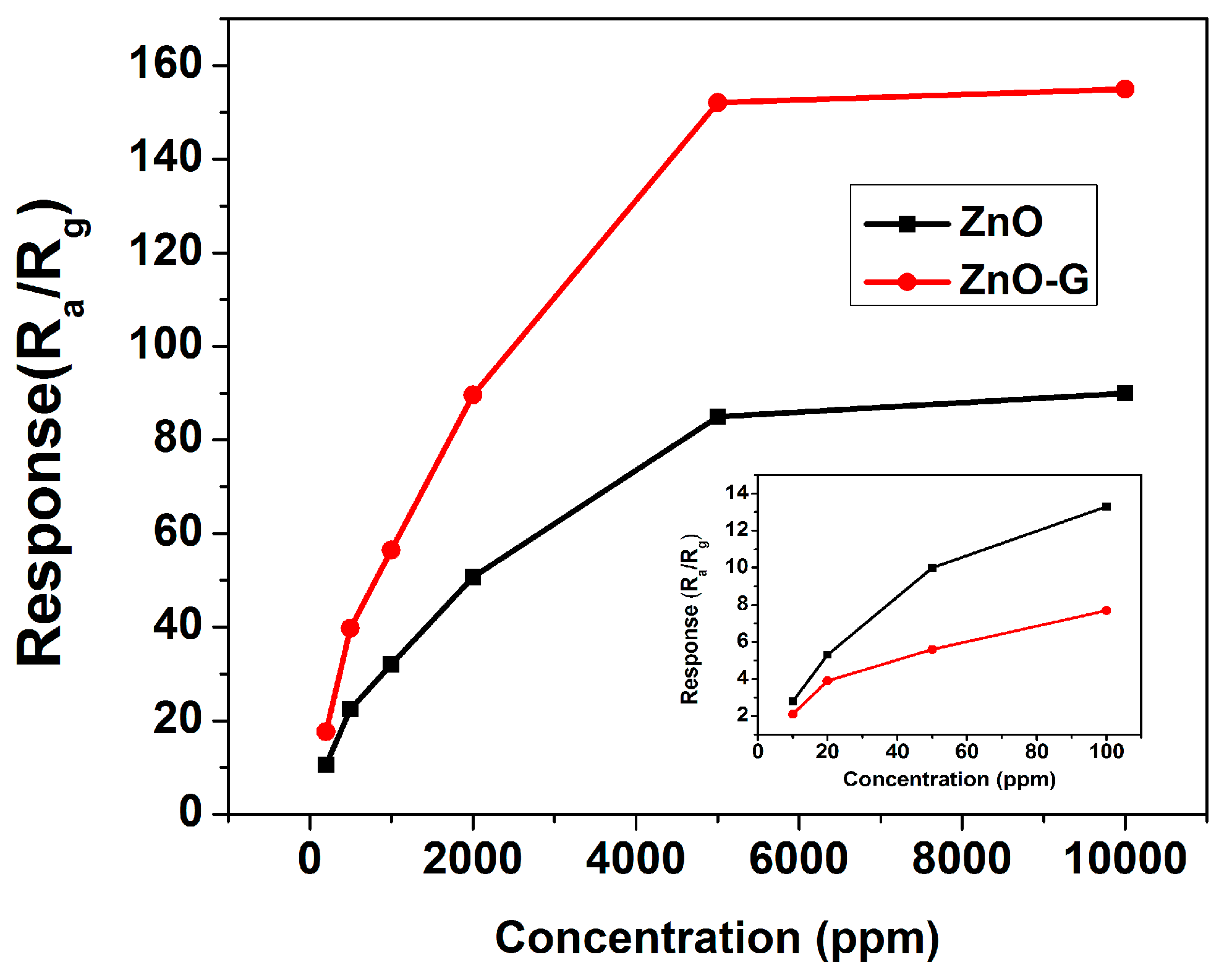
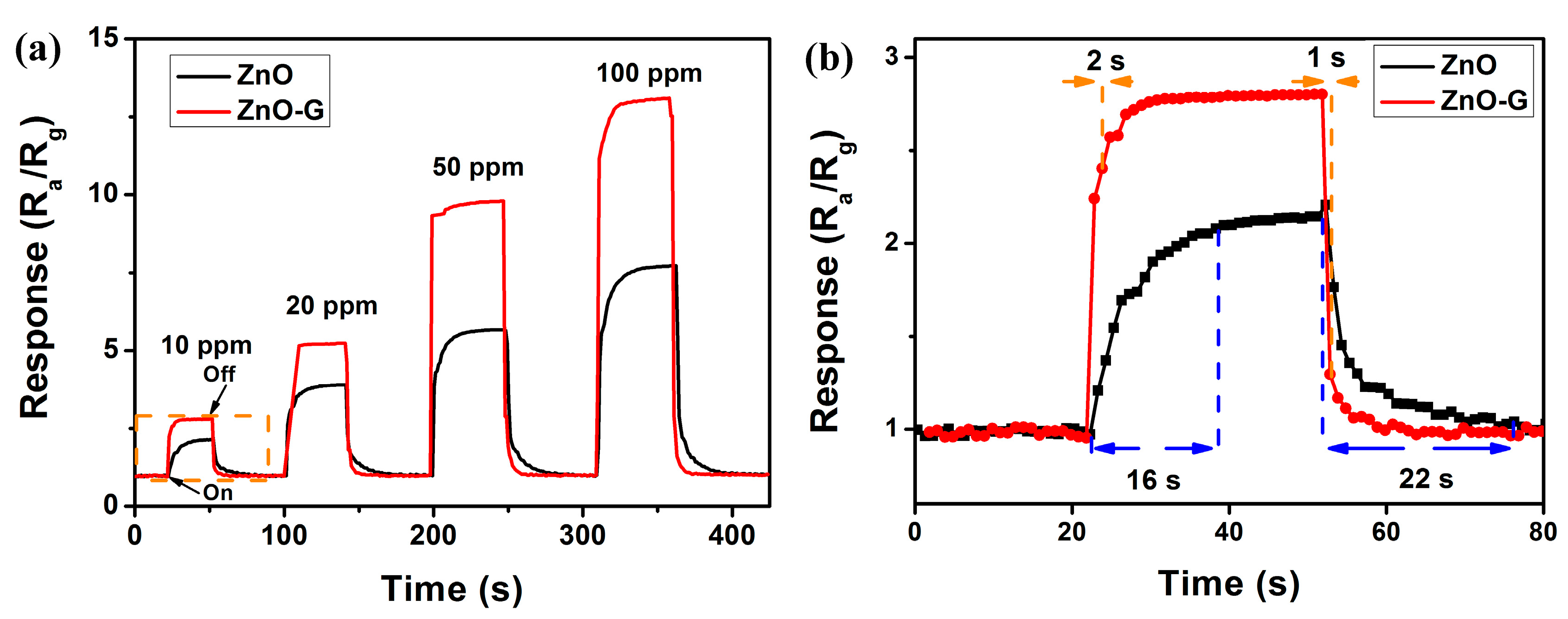
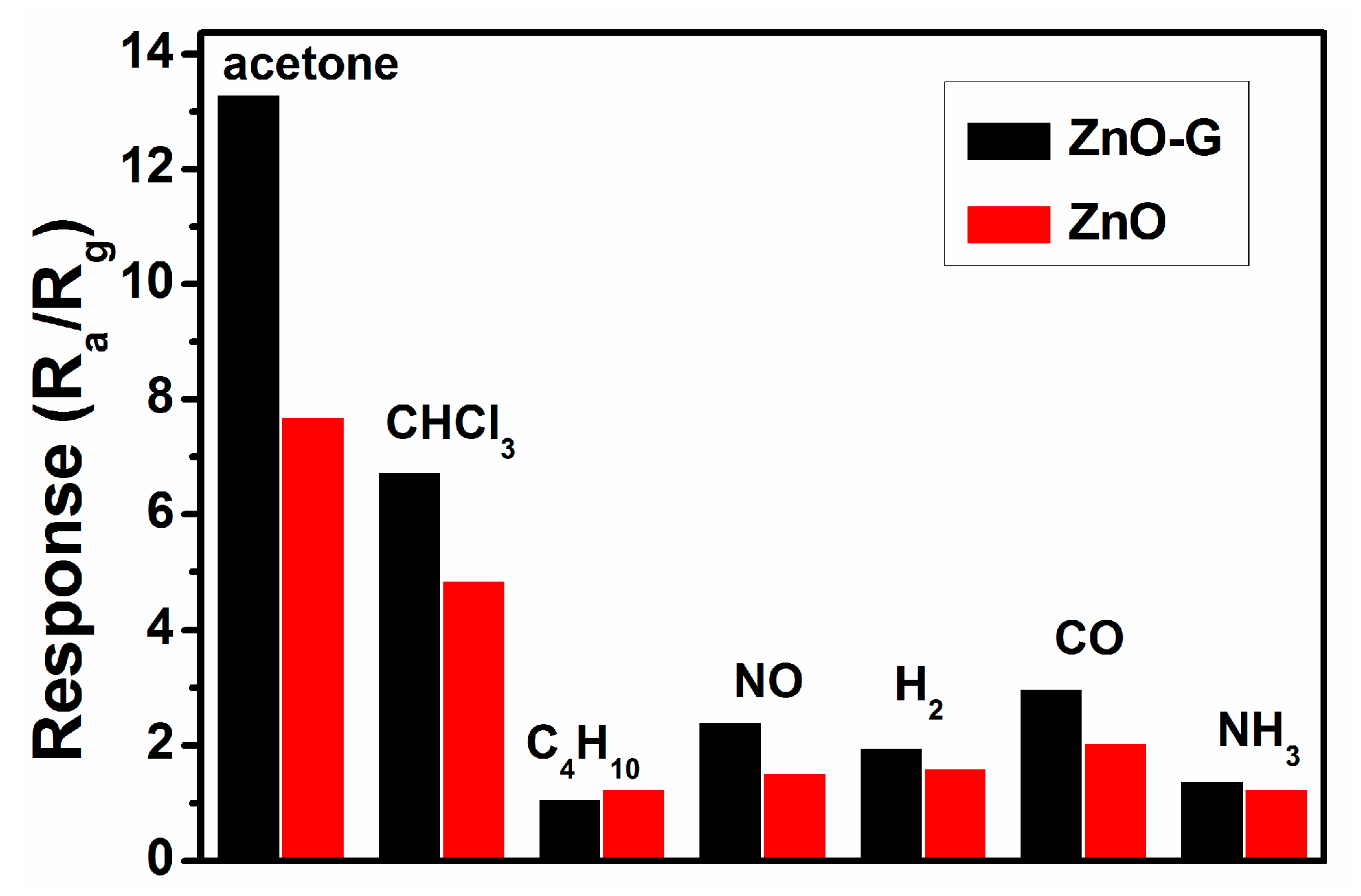
3.2. Acetone Sensing Properties
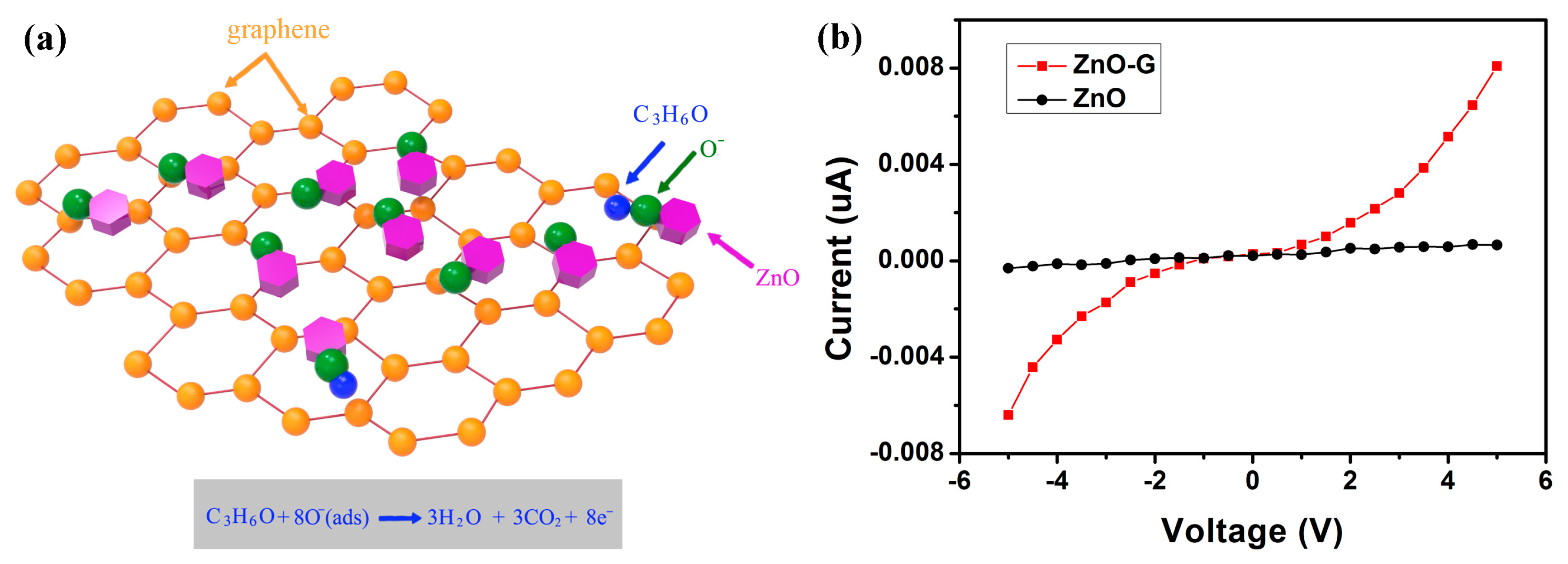
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polleux, J.; Gurlo, A.; Barsan, N.; Weimar, U.; Antonietti, M.; Niederberger, M.M. Template-Free Synthesis and Assembly of Single-Crystalline Tungsten Oxide Nanowires and their Gas-Sensing Properties. Angew. Chem. Int. Ed. 2006, 45, 267–271. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Pongpaiboonkul, S.; Phokharatkul, D.; Hodakc, J.H.; Wisitsoraat, A.; Hodak, S.K. Enhancement of H2S-sensing performances with Fe-doping in CaCu3Ti4O12 thin films prepared by a sol-gel method. Sens. Actuators B 2016, 224, 118–127. [Google Scholar] [CrossRef]
- Wang, L.L.; Lou, Z.; Fei, T.; Zhang, T. Templating synthesis of ZnO hollow nanospheres loaded with Au nanoparticles and their enhanced gas sensing properties. J. Mater. Chem. 2012, 22, 4767–4771. [Google Scholar] [CrossRef]
- Owen, O.E.; Trapp, V.E.; Skutches, C.L.; Mozzoli, M.A.; Hoeldtke, R.D.; Boden, G.; Reichard, G.A. Acetone metabolism during diabetic ketoacidosis. Diabetes 1982, 31, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Reichard, G.A.; Skutches, C.L.; Hoeldtke, R.D.; Owen, O.E. Acetone metabolism inhumans during diabetic ketoacidosis. Diabetes 1986, 35, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Zhou, J.; Song, J.H.; Liu, J.; Xu, N.S.; Wang, Z.L. Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.D.; Yan, H.Q.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choietal, H.-J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323–331. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P.D. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Liu, T.M.; Zeng, W.; Liu, D.J.; Chen, Y.; Wang, Z.C. Gas-sensing property improvement of ZnO by hierarchical flower-like architectures. Mater. Lett. 2011, 65, 3384–3387. [Google Scholar] [CrossRef]
- Rai, P.; Song, H.M.; Kim, Y.S.; Song, M.K.; Oh, P.R.; Yoon, J.M.; Yu, Y.T. Microwave assisted hydrothermal synthesis of single crystalline ZnO nanorods for gas sensor application. Mater. Lett. 2012, 68, 90–93. [Google Scholar] [CrossRef]
- Anno, Y.; Maekawa, Y.T.; Tamaki, J.; Asano, Y.; Hayashi, K.; Miura, N. Zinc-oxide-based semiconductor sensors for detecting acetone and caproaldehyde in the vapour of consommé soup. Sens. Actuators B 1995, 25, 623–627. [Google Scholar] [CrossRef]
- Sahay, P.P. Zinc oxide thin film gas sensor for detection of acetone. J. Mater. Sci. 2005, 40, 4383–4385. [Google Scholar] [CrossRef]
- Chang, S.J.; Hsueh, T.J.; Chen, I.C.; Huang, B.R. Highly sensitive ZnO nanowire CO sensors with the adsorption of Au nanoparticles. Nanotechnology 2008, 19, 175502. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, T.; Yuan, M.X.; Kang, M.H.; Lu, G.Y.; Wang, R.; Fan, H.T.; He, Y.; Yang, H.B. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene–metal particle composites. J. Phys. Chem. C. 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Wang, L.L.; Jackman, J.A.; Ng, W.B.; Cho, N.-J. Flexible, graphene-coated biocomposite for highly sensitive, real-time molecular detection. Adv. Funct. Mater. 2016, in press. [Google Scholar]
- Xiao, Y.; Yang, Q.Y.; Wang, Z.Y.; Zhang, R.; Gao, Y.; Sun, P.; Sun, Y.F.; Lu, G.Y. Improvement of NO2 gas sensing performance based on discoid tinoxide modified by reduced graphene oxide. Sens. Actuators B 2016, 227, 419–426. [Google Scholar] [CrossRef]
- Ye, Z.; Tai, H.L.; Xie, T.; Yuan, Z.; Liu, C.H.; Jiang, Y.D. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B 2016, 223, 149–156. [Google Scholar] [CrossRef]
- Wang, L.L.; Ng, W.B.; Jackman, J.A.; Cho, N.-J. Graphene gunctionalized natural microcapsules: modular building blocks for ultrahigh sensitivity bioelectronic platforms. Adv. Funct. Mater. 2016, 26, 2097–2103. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Mart_nez-Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chia, P.J.; Chua, L.L.; Zhao, L.H.; Png, R.Q.; Sivaramakrishnan, S.; Zhou, M.; Goh, R.G.S.; Friend, R.H.; Wee, A.T.S.; et al. Band-like transport in surface-functionalized highly solution-processable graphene nanosheets. Adv. Mater. 2008, 20, 3440–3446. [Google Scholar] [CrossRef]
- Xu, Y.X.; Bai, H.; Lu, G.W.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5867. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Chen, S.; Wang, L.L.; Jiang, K.; Shen, G.Z. An ultra-sensitive and rapid response speed graphene pressure sensors for electronic skin and health monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; Mcgovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar]
- Tang, Z.H.; Zhuang, J.; Wang, X. Exfoliation of graphene from graphite and their self-assembly at the oil-water Interface. Langmuir 2010, 26, 9045–9049. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, N.; Wei, Y.; Zhang, G. An in situ gelatin-assisted hydrothermalsynthesis of ZnO-reduced graphene oxide composites with enhanced photo-catalytic performance under ultraviolet and visible light. RSC Adv. 2014, 4, 7933–7943. [Google Scholar] [CrossRef]
- Gul, K.; Sohni, S.; Waqar, M.; Ahmad, F.; Nik Norulaini, N.A.; Mohd. Omar, A.K. Functionalization of magnetic chitosan with graphene oxide for removal of cationic and anionic dyes from aqueous solution. Carbohydr. Polym. 2016, 152, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Raman imaging of graphene. Solid State Commun. 2007, 143, 44–46. [Google Scholar] [CrossRef]
- Oh, E.; Choi, H.Y.; Jung, S.H.; Cho, S.; Kim, J.C.; Lee, K.H.; Kang, S.W.; Kim, J.; Yun, J.Y.; Jeong, S.H. High-performance NO2 gas sensor based on ZnO nanorod grown by ultrasonic irradiation. Sens. Actuators B 2009, 141, 239–243. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.t.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; Kohld, P.A. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482. [Google Scholar] [CrossRef]
- Yi, J.; Lee, J.M.; Park, W.I. Vertically aligned ZnO nanorods and graphene hybridarchitectures for high-sensitive flexible gas sensors. Sens. Actuators B 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. Acetone sensing characteristics of ZnO hollow spheres prepared by one-pot hydrothermal reaction. Mater. Lett. 2012, 86, 168–170. [Google Scholar] [CrossRef]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon 2012, 50, 385–394. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Cen, Y.; Du, Y.; Ruan, S. Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites. Sensors 2016, 16, 1876. https://doi.org/10.3390/s16111876
Zhang H, Cen Y, Du Y, Ruan S. Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites. Sensors. 2016; 16(11):1876. https://doi.org/10.3390/s16111876
Chicago/Turabian StyleZhang, Hao, Yuan Cen, Yu Du, and Shuangchen Ruan. 2016. "Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites" Sensors 16, no. 11: 1876. https://doi.org/10.3390/s16111876




