Patterns of Sponge Biodiversity in the Pilbara, Northwestern Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collation of Sponge Occurrence Data
2.3. Collection Effort
2.4. Community Composition
2.5. Taxonomic Distinctness Analysis
3. Results
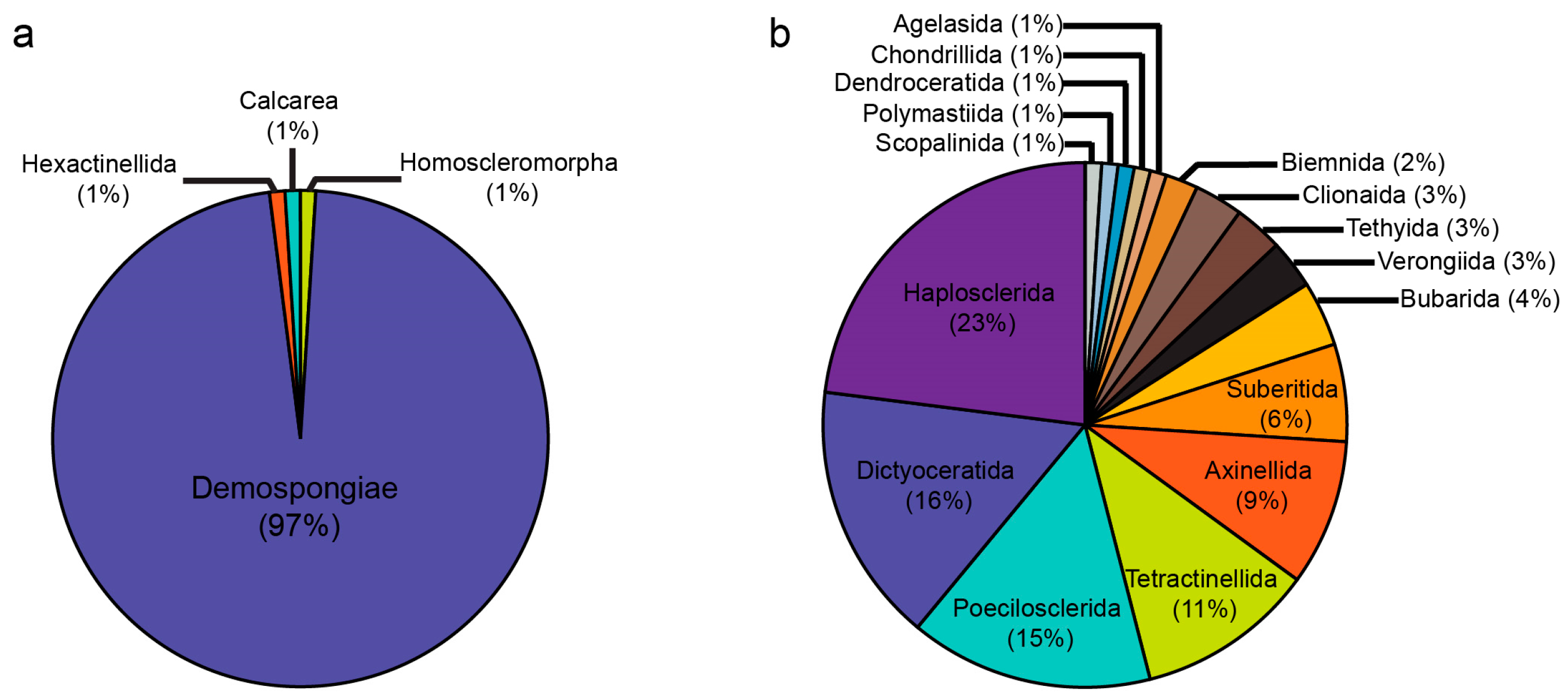
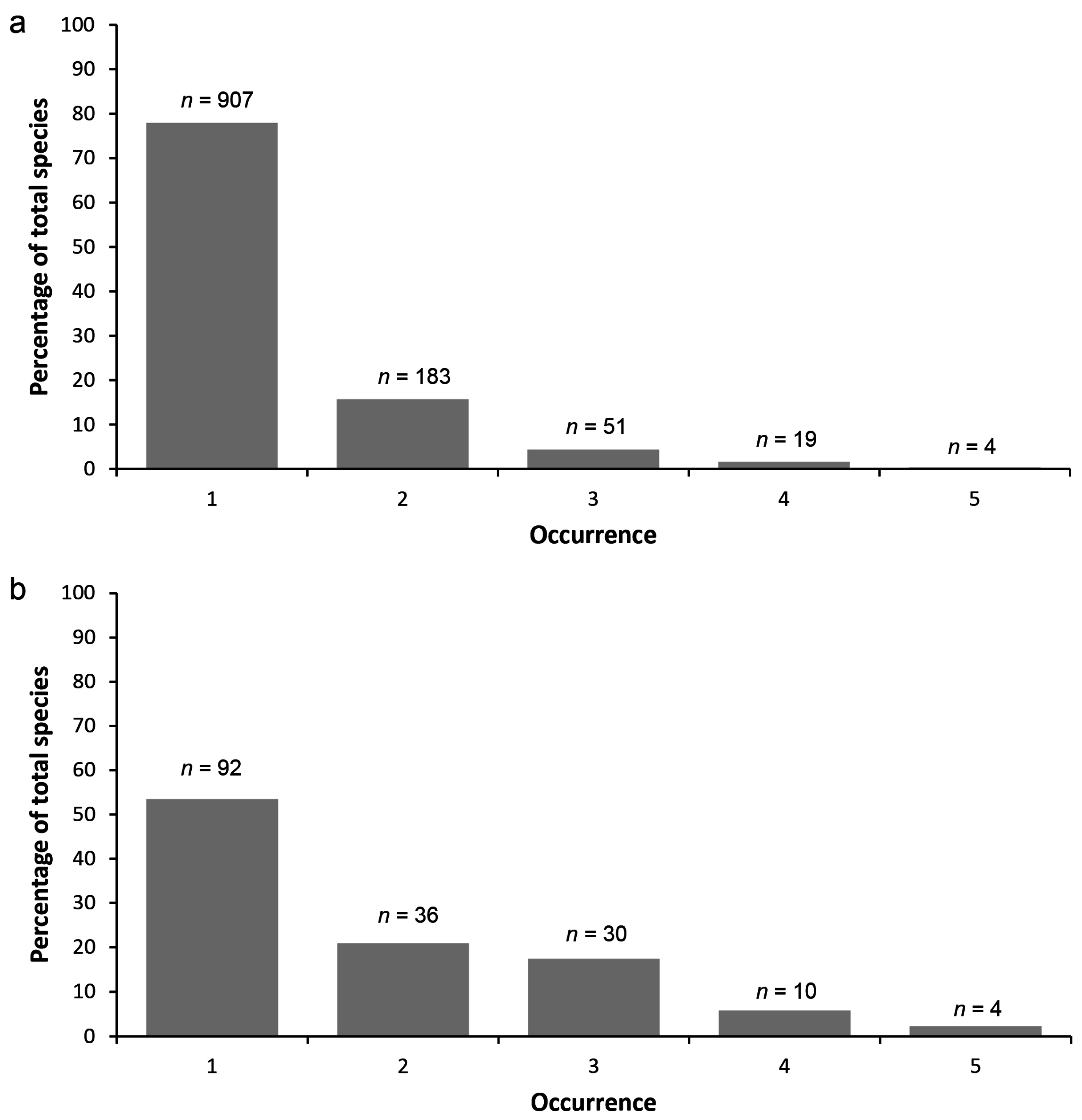
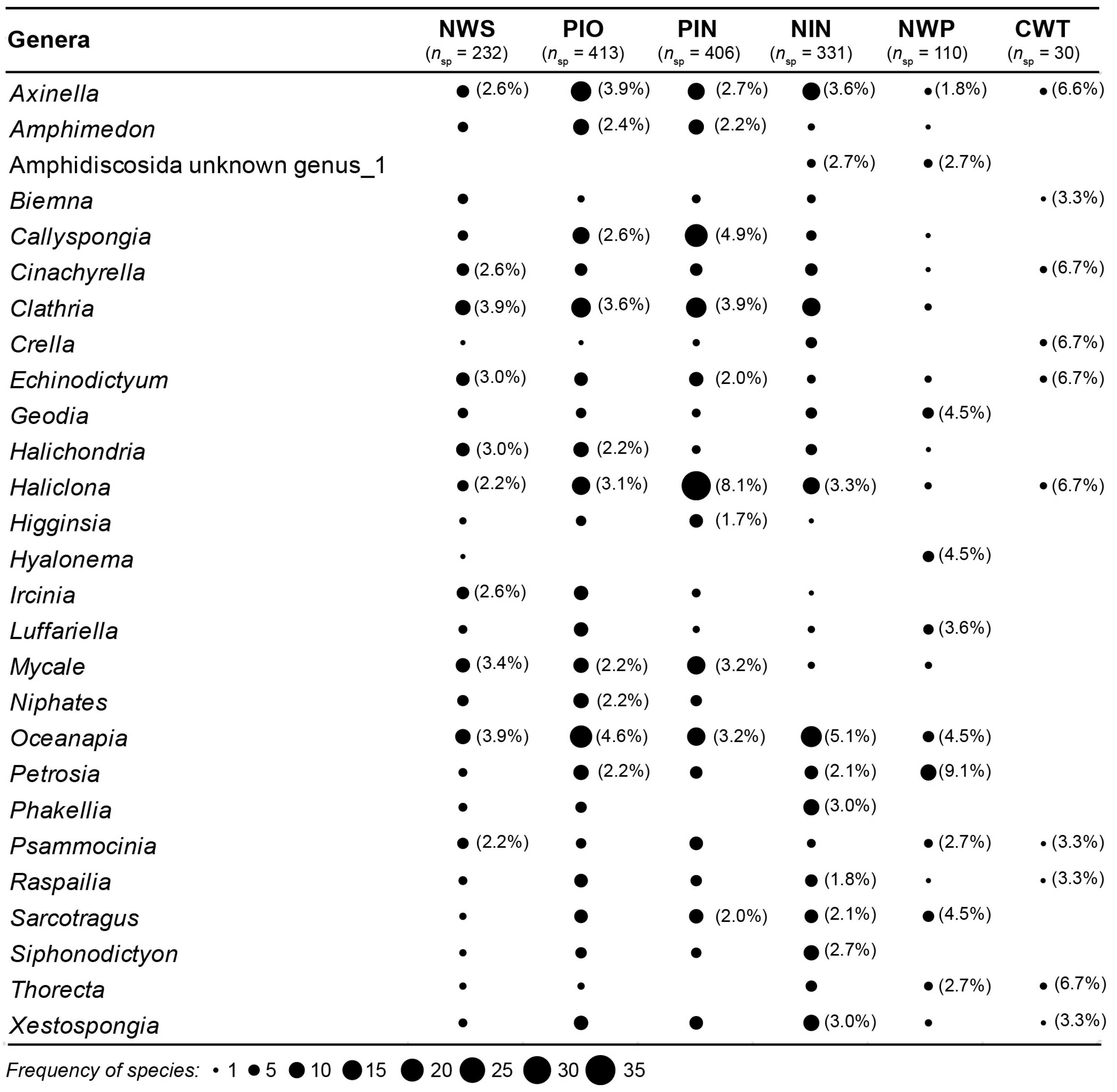
3.1. Species Composition
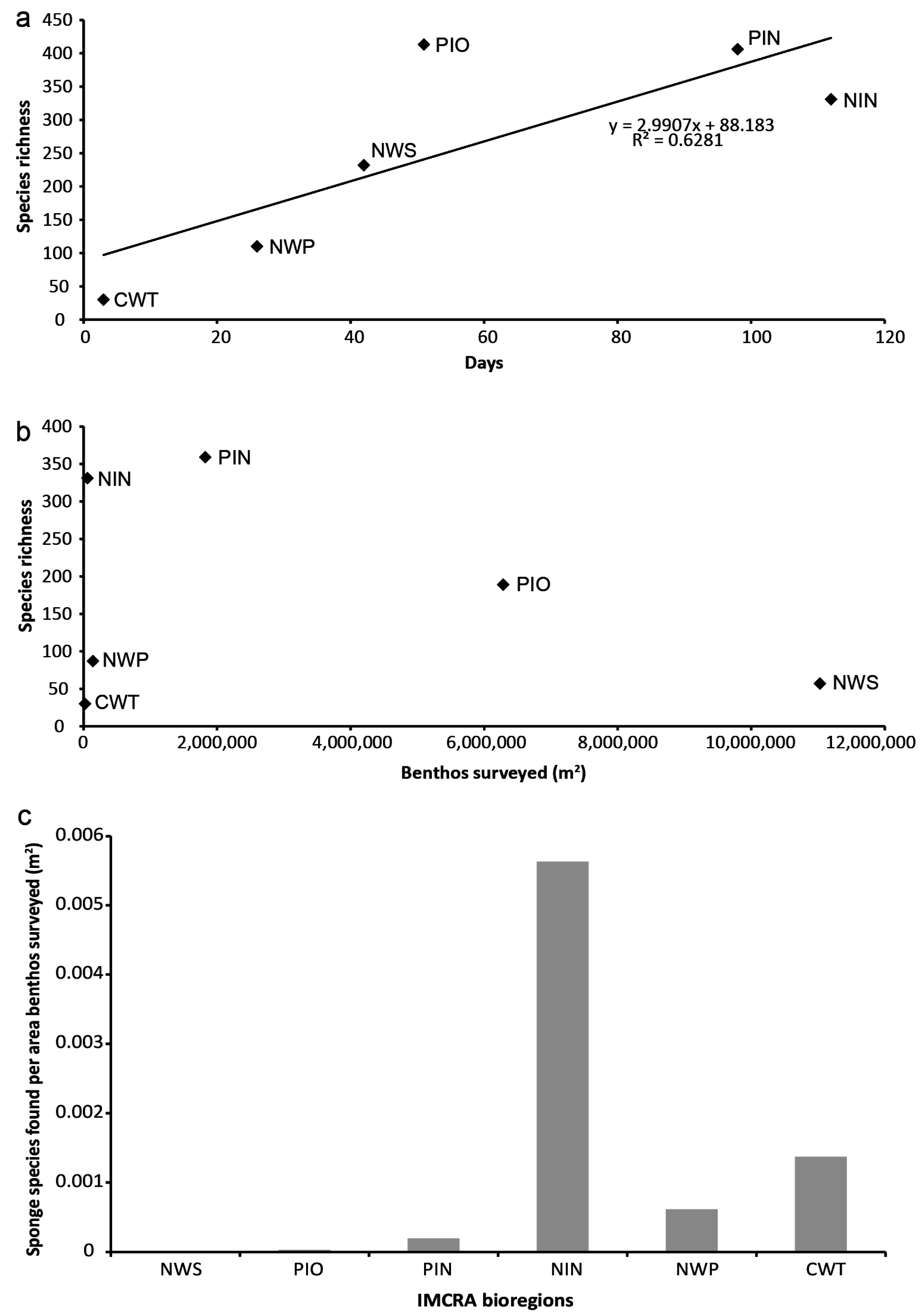
3.2. Species Richness and Collection Effort
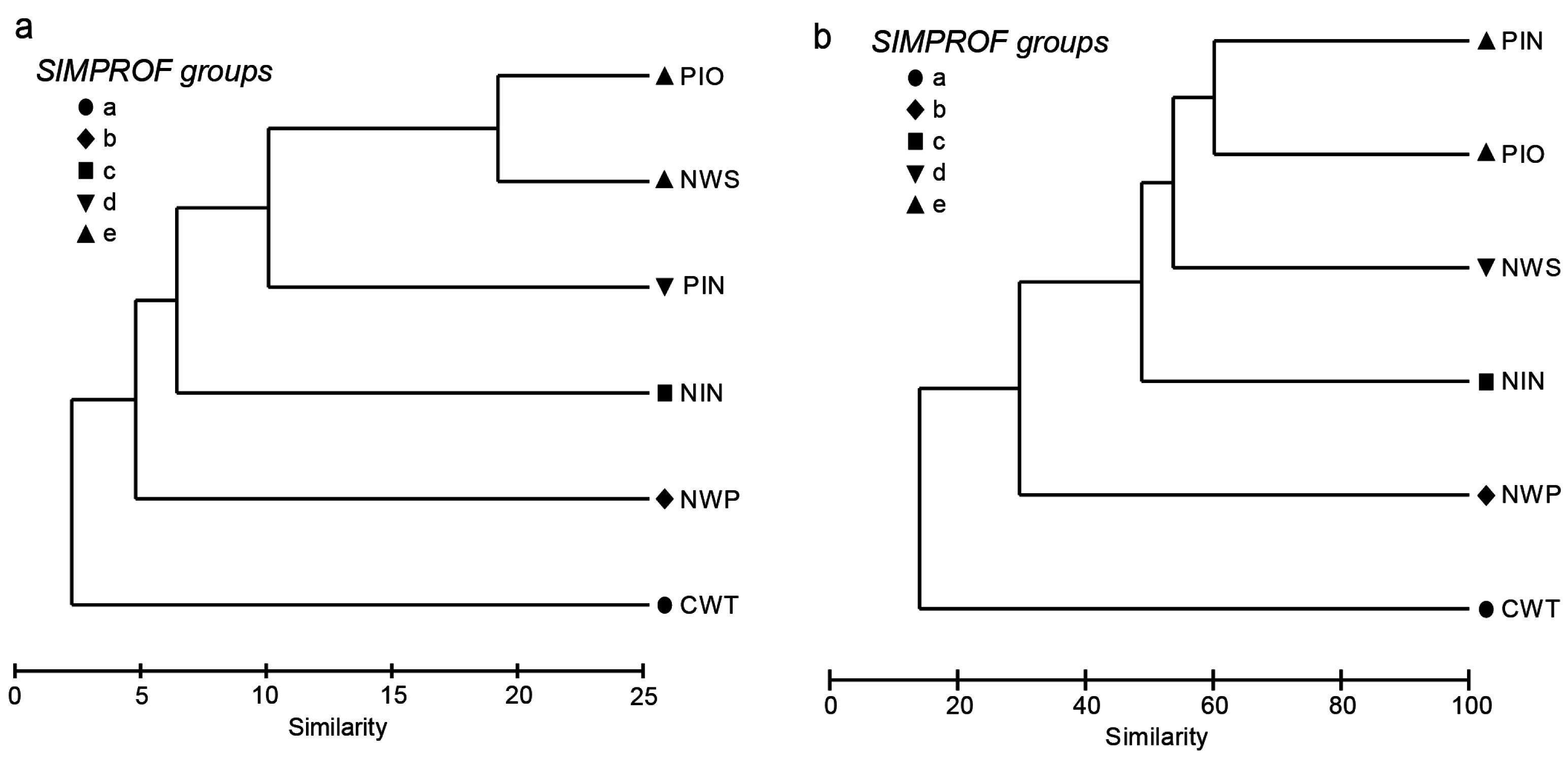
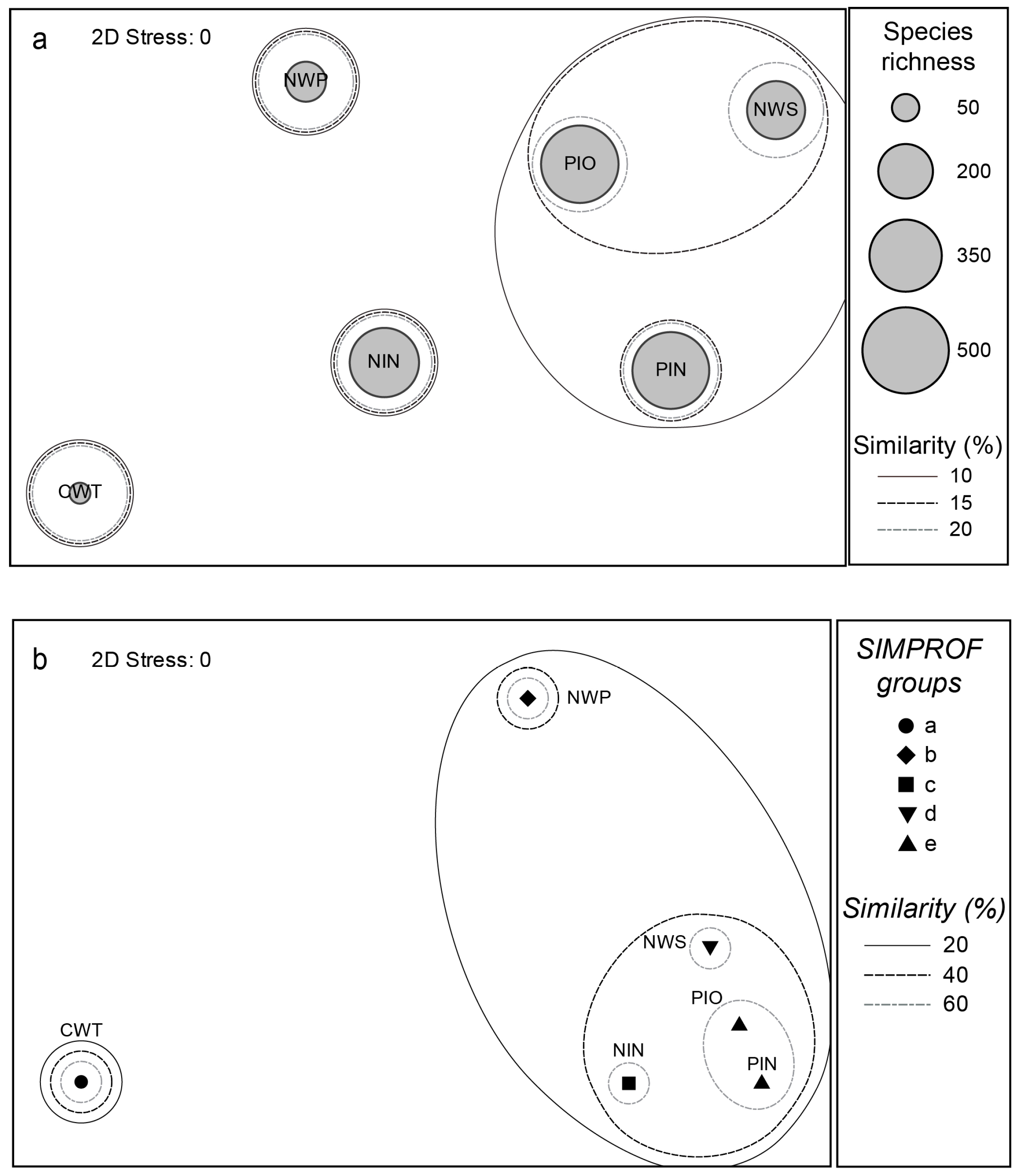
3.3. Community Classification between Bioregions
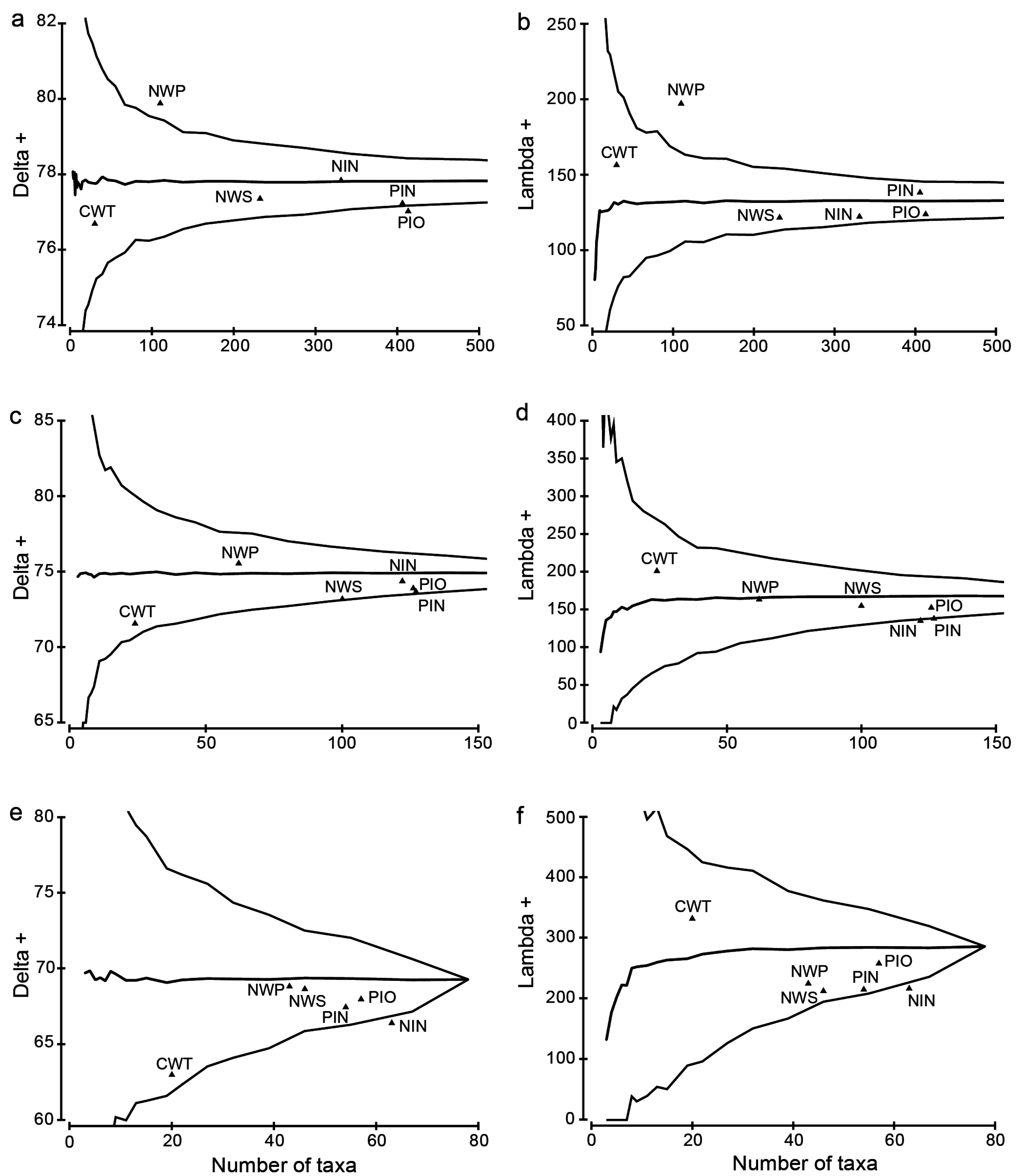
3.4. Taxonomic Distinctness Analyses
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Heyward, A.; Fromont, J.; Schönberg, C.Z.; Colquhoun, J.; Radford, B.; Gomez, O. The sponge gardens of Ningaloo Reef, Western Australia. Open Mar. Biol. J. 2010, 4, 3–11. [Google Scholar]
- Wörheide, G.; Vargas, S.; Lüter, C.; Reitner, J. Precious coral and rock sponge gardens on the deep aphotic fore-reef of Osprey Reef (Coral Sea, Australia). Coral Reefs 2011, 30, 901. [Google Scholar] [CrossRef]
- Schönberg, C.H.L.; Fromont, J. Sponge gardens of Ningaloo Reef (Carnarvon Shelf, Western Australia) are biodiversity hotspots. Hydrobiologia 2012, 687, 143–161. [Google Scholar] [CrossRef]
- Przeslawski, R.; Alvarez, B.; Battershill, C.; Smith, T. Sponge biodiversity and ecology of the Van Diemen Rise and eastern Joseph Bonaparte Gulf, northern Australia. Hydrobiologia 2014, 730, 1–16. [Google Scholar] [CrossRef]
- Wulff, J.L. Ecological interactions of marine sponges. Can. J. Zool. 2006, 84, 146–166. [Google Scholar] [CrossRef]
- Wulff, J.L. Assessing and monitoring coral reef sponges: Why and how? Bull. Mar. Sci. 2001, 69, 831–846. [Google Scholar]
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Richter, C.; Wunsch, M.; Rasheed, M.; Kötter, I.; Badran, M.I. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature 2001, 413, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Carmona, M.C.; Velásquez, Z.; Puig, A.; Cruzado, A.; López, A.; Young, C.M. Siliceous sponges as a silicon sink: An overlooked aspect of the benthopelagic coupling in the marine silicon cycle. Limnol. Oceanogr. 2005, 50, 799–809. [Google Scholar] [CrossRef]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient fluxes through sponges. Biology, budgets, and ecological implications. In Advances in Marine Biology; Elsevier Ltd.: London, UK, 2012; Volume 62, pp. 113–182. [Google Scholar]
- De Goeij, J.M.; van Oevelen, D.; Vermeij, M.J.A.; Osinga, R.; Middelburg, J.J.; de Goeij, A.F.P.M.; Admiraal, W. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 2013, 342, 108–110. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, J.M.; van Den Berg, H.; van Oostveen, M.M.; Epping, E.H.G.; van Duyl, F.C. Major bulk dissolved organic carbon (DOC) removal by encrusting coral reef cavity sponges. Mar. Ecol. Prog. Ser. 2008, 357, 139–151. [Google Scholar] [CrossRef]
- Keesing, J.K.; Strzelecki, J.; Fromont, J.; Thomson, D. Sponges as important sources of nitrate on an oligotrophic continental shelf. Limnol. Oceanogr. 2013, 58, 1947–1958. [Google Scholar] [CrossRef]
- Becerro, M. Quantitative trends in sponge ecology research. Mar. Ecol. 2008, 29, 167–177. [Google Scholar] [CrossRef]
- Przeslawski, R.; Ahyong, S.; Byrne, M.; Wörheide, G.; Hutchings, P. Beyond corals and fish: The effects of climate change on noncoral benthic invertebrates of tropical reefs. Glob. Chang. Biol. 2008, 14, 2773–2795. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleuddin, A.; Fenton, M. Biology of neglected groups: Porifera (sponges). Can. J. Zool. Res. 2006, 82, 143–356. [Google Scholar]
- Bell, J.J.; Mcgrath, E.; Biggerstaff, A.; Bates, T.; Cárdenas, C.A.; Bennett, H. Global conservation status of sponges. Conserv. Biol. 2015, 29, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Przeslawski, R.; Alvarez, B.; Kool, J.; Bridge, T.; Caley, M.J.; Nichol, S. Implications of sponge biodiversity patterns for the management of a marine reserve in Northern Australia. PLoS ONE 2015, 10, e0141813. [Google Scholar] [CrossRef] [PubMed]
- Al-Zibdah, M.K.; Damhoureyeh, S.A.; Badran, M.I. Temporal variations in coral reef health at a coastal industrial site on the Gulf of Aqaba Red Sea. Oceanologia 2007, 49, 565–578. [Google Scholar]
- Mirams, A.G.K.; Treml, E.A.; Shields, J.L.; Liggins, L.; Riginos, C. Vicariance and dispersal across an intermittent barrier: Population genetic structure of marine animals across the Torres Strait land bridge. Coral Reefs 2011, 30, 937–949. [Google Scholar] [CrossRef]
- Pitcher, C.R.; Ellis, N.; Venables, W.N.; Wassenberg, T.J.; Burridge, C.Y.; Smith, G.P.; Browne, M.; Pantus, F.; Poiner, I.R.; Doherty, P.J.; et al. Effects of trawling on sessile megabenthos in the Great Barrier Reef and evaluation of the efficacy of management strategies. ICES J. Mar. Sci. 2016, 73, i115–i126. [Google Scholar] [CrossRef]
- Gilliam, D.S.; Walker, B.K.; Saelens, S.J.; Fahy, D.P.; Kosmynin, V.N. Recovery of injured giant barrel sponges, Xestospongia muta, offshore southeast Florida. Proc. 11th Int. Coral Reef Symp. 2008, 55, 7–11. [Google Scholar]
- Makowski, C.; Keyes, P. Using the benthic ecological assessment for marginal reefs (BEAMR) method to quantify nearshore reef conditions in the southeast Gulf of Mexico. J. Coast. Res. 2011, 27, 428–440. [Google Scholar] [CrossRef]
- Lang, J.; Marks, K.; Kramer, P.; Ginsburg, R. AGRRA Protocols Version 5.4. Available online: http://www.agrra.org/method/AGRRA-V5.4_2010.pdf (accessed on 10 August 2012).
- Hooper, J.N.A.; Kennedy, J.A.; Quinn, R.J. Biodiversity “hotspots”, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera). Biodivers. Conserv. 2002, 11, 851–885. [Google Scholar] [CrossRef]
- Fromont, J.; Vanderklift, M.A.; Kendrick, G.A. Marine sponges of the Dampier Archipelago, Western Australia: Patterns of species distributions, abundance and diversity. Biodivers. Conserv. 2006, 15, 3731–3750. [Google Scholar] [CrossRef]
- Currie, D.R.; Dixon, C.D.; Roberts, S.D.; Hooper, G.E.; Sorokin, S.J.; Ward, T.M. Relative importance of environmental gradients and historical trawling effort in determining the composition and distribution of benthic macro-biota in a large inverse estuary. Fish. Res. 2011, 107, 184–195. [Google Scholar] [CrossRef]
- Fromont, J.; Althaus, F.; McEnnulty, F.R.; Williams, A.; Salotti, M.; Gomez, O.; Gowlett-Holmes, K. Living on the edge: The sponge fauna of Australia’s southwestern and northwestern deep continental margin. Hydrobiologia 2012, 687, 127–142. [Google Scholar] [CrossRef]
- Sorokin, S.; Fromont, J.; Currie, D. Demosponge biodiversity in the Benthic Protection Zone of the Great Australian Bight. Trans. R. Soc. South Aust. 2007, 131, 192–204. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; de Voogd, N.J.; de Glasby, B.A.; Hajdu, E.; Pisera, A.; Manconi, R.; Schönberg, C.H.L.; Janussen, D.; et al. World Porifera Database. Available online: http://www.marinespecies.org/porifera (accessed on 13 July 2015).
- Poppe, J.; Sutcliffe, P.; Hooper, J.N.A.; Wörheide, G.; Erpenbeck, D. CO I barcoding reveals new clades and radiation patterns of indo-pacific sponges of the family Irciniidae (Demospongiae: Dictyoceratida). PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, J.N.A.; Hall, K.A.; Ekins, M.; Erpenbeck, D.; Wörheide, G.; Jolley-Rogers, G. Managing and sharing the escalating number of sponge “unknowns”: The Sponge Maps project. Integr. Comp. Biol. 2013, 53, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.; Ekins, M. Collation and validation of museum collection databases related to the distribution of marine sponges in Northern Australia. Tech. Rep. Queensl. Museum 2004, 2, 1–225. [Google Scholar]
- Semeniuk, V. Coastal forms and quaternary processes along the arid Pilbara coast of northwestern Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996, 123, 49–84. [Google Scholar] [CrossRef]
- Semeniuk, V. The Pilbara coast: A riverine coastal plain in a tropical arid setting, northwestern Australia. Sediment. Geol. 1993, 83, 235–256. [Google Scholar] [CrossRef]
- Wilson, B. The Biogeography of the Australian North West Shelf: Environmental Change and Life’s Response; Elsevier: Burlington, NJ, USA, 2013. [Google Scholar]
- Sainsbury, K.; Campbell, R.; Whitelaw, A. Effects of Trawling on the Marine Habitat on the North West Shelf of Australia and Implications for Sustainable Fisheries Management; Geoscience Australia: Symonston, Australia, 1993; pp. 137–145.
- Daume, S.; Fromont, J.; Hart, A. Management of Bioeroding Sponges in Wild Stocks of Pinctada Maxima in Western Australia; Final Fisheries Report No. 196, 2009; Western Australian Fisheries and Marine Research Laboratories: Perth, Australia, 2009.
- Ball, A.; Bernie, K.; Feng, A.; McCluskey, C.; Stanwix, G.; Willcock, T.; Wokker, N. Energy in Australia 2014; Australian Government: Canberra, Australia, 2014.
- Atlas of Living Australia. Available online: http://www.ala.org.au (accessed on 1 March 2013).
- Short, A.D. Sediment transport around Australia—Sources, mechanisms, rates, and barrier forms. J. Coast. Res. 2010, 263, 395–402. [Google Scholar] [CrossRef]
- Short, A.D. Australian beach systems—Nature and distribution. J. Coast. Res. 2006, 221, 11–27. [Google Scholar] [CrossRef]
- Lafratta, A.; Fromont, J.; Speare, P.; Schönberg, C.H.L. Coral bleaching in turbid waters of north-western Australia. Mar. Freshw. Res. 2016, MF15314. [Google Scholar] [CrossRef]
- Bancroft, K.P.; Long, S. Modelling Suggests Connectivity between the Ningaloo Reef and Coral Reefs of the Pilbara. In Discovering Ningaloo: Latest findings and their implications for management; Waples, K., Hollander, E., Eds.; Ningaloo Research Coordinating Committee: Kensington, WA, USA, 2008; pp. 61–64. [Google Scholar]
- Fromont, J.; Sampey, A. Kimberley Marine Biota. Historical Data: Sponges (Porifera). Rec. West. Aust. Museum 2014, 84, 69–100. [Google Scholar] [CrossRef]
- Sampey, A.; Bryce, C.; Osborne, S.; Miles, A. Kimberley marine biota. Historical data: Introduction and methods. Records of the Western Australian Museum Supplement. Rec. West. Aust. Museum Suppl. 2014, 84, 19–43. [Google Scholar] [CrossRef]
- Interim Marine and Coastal Regionalisation for Australia Technical Group. Interim Marine and Coastal Regionalisation for Australia: An Ecosystem-Based Classification for Marine and Coastal Environments. Version 3.3; Environment Australia, Department of the Environment: Canberra, Australia, 1998; pp. 1–27.
- Commonwealth of Australia. A Guide to the Integrated Marine and Coastal Regionalisation of Australia: IMCRA Version 4.0.; Australian Government: Canberra, Australia, 2006.
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Morrow, C.; Cardenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Schönberg, C.H.L. Happy relationships of marine sponges with sediments–a review and some observations from Australia. J. Mar. Biol. Assoc. UK 2015, 96, 493–514. [Google Scholar] [CrossRef]
- Zar, J. Biostatistical Analysis; Northern Illinois University: DeKalb, IL, USA, 1999. [Google Scholar]
- Clarke, K.R.; Gorley, R. Primer Version 7: User Manual/ Tutorial; PRIMER-E: Plymouth, UK, 2015. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical proprieties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. Taxonomic distinctness and environmental assessment. J. Appl. Ecol. 1998, 35, 532–543. [Google Scholar] [CrossRef]
- Izsak, C.; Price, A. Measuring β-diversity using a taxonomic similarity index, and its relation to spatial scale. Mar. Ecol. Prog. Ser. 2001, 215, 69–77. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; Kennedy, J.A. Small-scale patterns of sponge biodiversity (Porifera) on Sunshine Coasts reefs, eastern Australia. Invertebr. Syst. 2002, 16, 637–653. [Google Scholar] [CrossRef]
- Sutcliffe, P.R.; Hooper, J.N.A.; Pitcher, C.R. The most common sponges on the Great Barrier Reef seabed, Australia, include species new to science ( Phylum Porifera ). Zootaxa 2010, 2616, 1–30. [Google Scholar]
- Connolly, S.R.; Bellwood, D.R.; Hughes, T.P. Indo-Pacific biodiversity of coral reefs: Deviations from a mid-domain model. Ecology 2003, 84, 2178–2190. [Google Scholar] [CrossRef]
- Malcolm, H.A.; Jordan, A.; Smith, S.D.A. Biogeographical and cross-shelf patterns of reef fish assemblages in a transition zone. Mar. Biodivers. 2010, 40, 181–193. [Google Scholar] [CrossRef]
- Anderson, R.; Bolton, J.; Smit, A.; da Silva Neto, D. The seaweeds of Angola: The transition between tropical and temperate marine floras on the west coast of southern Africa. Afr. J. Mar. Sci. 2012, 34, 1–13. [Google Scholar] [CrossRef]
- Gratwicke, B.; Speight, M.R. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish Biol. 2005, 66, 650–667. [Google Scholar] [CrossRef]
- Kovalenko, K.E.; Thomaz, S.M.; Warfe, D.M. Habitat complexity: Approaches and future directions. Hydrobiologia 2012, 685, 1–17. [Google Scholar] [CrossRef]
- Western Australia, Department of conservation and land management. Indicative Management Plan for the proposed Dampier Archipelago Marine Park and Cape Preston Marine Management Area; Marine Parks and Reserves Authority: Kensington, Australia, 2005.
- Li, J.; Potter, A.; Huang, Z.; Daniel, J.; Heap, A. Predicting Seabed Mud Content Across the Australian Margin: Comparison of Statistical and Mathematical Techniques Using A Simulation Experiment; Geoscience Australia: Canberra, Australia, 2011.
- Hooper, J.; van Soest, R. Systema Porifera: A Guide to the Classification of Sponges; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Fromont, J.; Vanderklift, M.A. Porifera (sponges) of Mermaid, Scott and Seringapatam Reefs, north Western Australia. Rec. West. Aust. Museum 2009, 77, 89–103. [Google Scholar] [CrossRef]
- Fromont, J. Porifera (Sponges) in the Dampier Archipelago: Taxonomic Affinities and Biogeography. The Marine Flora and Fauna of Dampier, Western Australia; Western Australian Museum: Perth, Australia, 2003; pp. 405–417.
- Kelly, M.; Hooper, J.N.A.; Paul, V.; Pauley, G.; van Soest, R. Taxonomic inventory of sponges (Porifera) of the Mariana Islands. Micronesia 2002, 6, 100–120. [Google Scholar]
- Domingos, C.; Moraes, F.; Muricy, G. Four new species of Plakinidae (Porifera: Homoscleromorpha) from Brazil. Zootaxa 2013, 3718, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Barraza, J.A.; Carballo, J. First record of Plakortis Schulze (Porifera: Homosclerophorida) from the Northeast Pacific coast, with the description of Plakortis albicans sp. nov. Zootaxa 2005, 868, 1–12. [Google Scholar]
- Poloczanska, E.; Babcock, R.C.; Butler, A.; Hobday, A.; Hoegh-Guldberg, O.; Kunz, T.; Matear, R.; Milton, D.; Okey, T.; Richardson, A. Climate change and Australian marine life. Ocean. Mar. Biol. Ann. Rev. 2007, 45, 407. [Google Scholar]
- Reaka, M.; Rodgers, P.; Kudla, A. Patterns of biodiversity and endemism on Indo-West Pacific coral reefs. Proc. Nat. Acad. Sci. USA 2008, 105, 11474–11481. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.; Toonen, R.; Bowen, B. Endemism and dispersal: Comparative phylogeography of three surgeonfishes across the Hawaiian Archipelago. Mar. Biol. 2009, 156, 689–698. [Google Scholar]
- Maldonado, M. The ecology of the sponge larva. Can. J. Zool. 2006, 84, 175–194. [Google Scholar] [CrossRef]
- Abdul Wahab, M.A.; de Nys, R.; Whalan, S. Larval behaviour and settlement cues of a brooding coral reef sponge. Coral Reefs 2011, 30, 451–460. [Google Scholar] [CrossRef]
- Abdul Wahab, M.A.; Fromont, J.; Whalan, S.; Webster, N.; Andreakis, N. Combining morphometrics with molecular taxonomy: How different are similar foliose keratose sponges from the Australian tropics? Mol. Phylogenet. Evol. 2014, 73, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J. Revision of the family Raspailiidae (Porifera: Demospongiae), with description of Australian species. Invertebr. Taxon. 1991, 5, 1179–1481. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Kelly-Borges, M. Systematics and biogeography of the genus Ianthella (Demospongiae: Verongida: Ianthellidae) in the south-west Pacific. Beagle Rec. Mus. Art Gall. North. Territ. 1995, 12, 151–176. [Google Scholar]
- Andreakis, N.; Luter, H.M.; Webster, N.S. Cryptic speciation and phylogeographic relationships in the elephant ear sponge Ianthella basta (Porifera, Ianthellidae) from northern Australia. Zool. J. Linn. Soc. 2012, 166, 225–235. [Google Scholar] [CrossRef]
- Fisher, R.; O’Leary, R.; Low-Choy, S.; Mengersen, K.; Knowlton, N.; Brainard, R.; Caley, M.J. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 2015, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, H.; Fisher, R.; Davidson, C. The role of natural history collections in documenting species declines. Trends Ecol. Evol. 1998, 13, 27–30. [Google Scholar] [CrossRef]
- Ponder, W.; Carter, G.; Flemons, P.; Chapman, R.R. Evaluation of museum collection data for use in biodiversity assessment. Conserv. Biol. 2001, 15, 648–657. [Google Scholar] [CrossRef]
- Graham, C.; Ferrier, S.; Heuttman, F.; Moritz, C.; Peterson, A.T. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004, 19, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Lister, A. Natural history collections as sources of long-term datasets. Trends Ecol. Evol. 2011, 26, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; McClean, C.; Veron, J.; Hawkins, J.; Allen, G.; McAllister, D.; Mittermeier, C.; Schueler, F.; Spalding, M.; Wells, F. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1294. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.; Beckley, L. Priority areas for conservation of Western Australian coastal fishes: A comparison of hotspot, biogeographical and complementarity approaches. Biol. Conserv. 2005, 125, 399–410. [Google Scholar] [CrossRef]
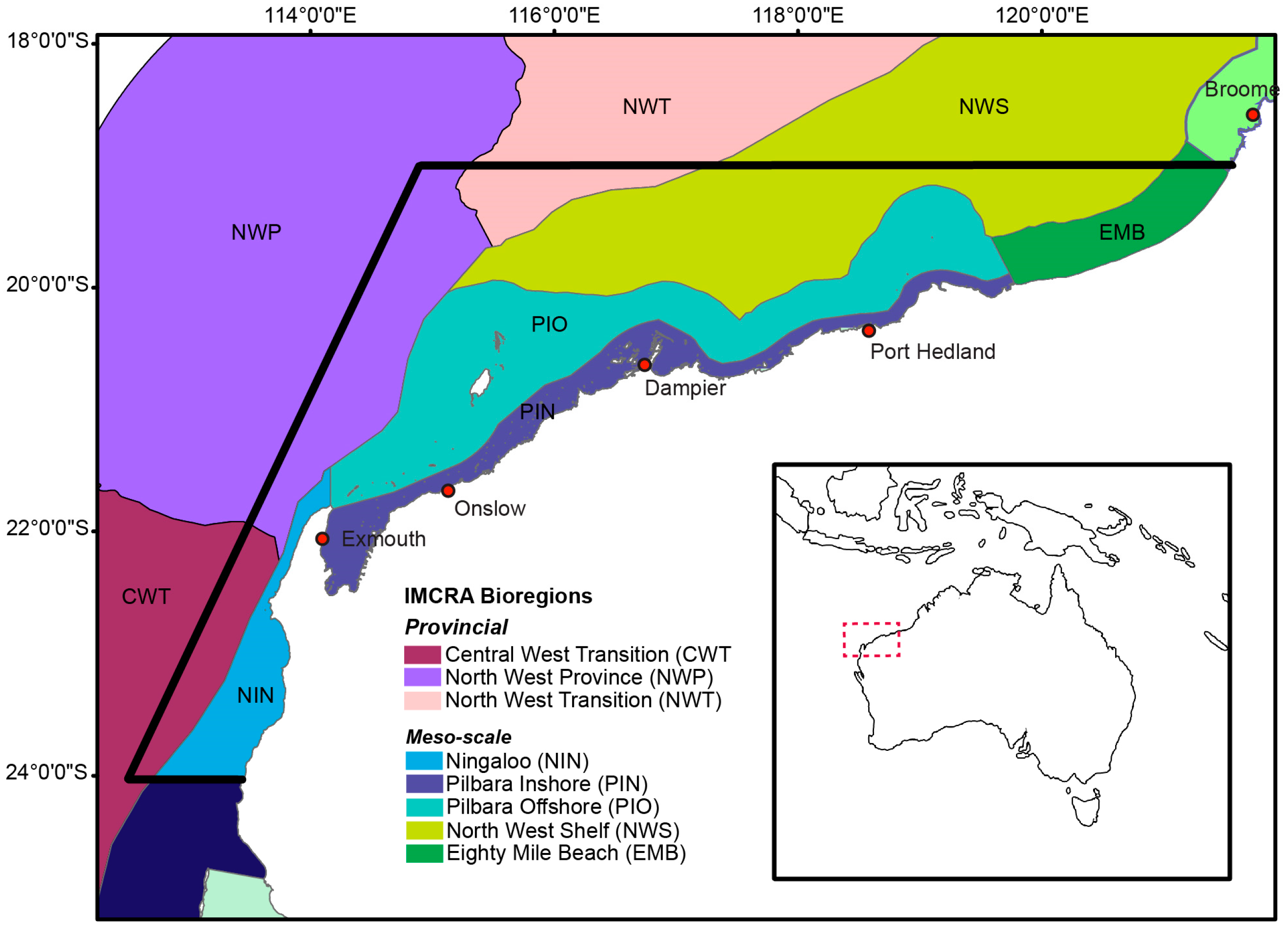
| Environmental Characteristics | ||||||
|---|---|---|---|---|---|---|
| Locality | Area (km2) | Depth (m) | Currents | Seafloor | Characteristics | |
| North West Shelf (NWS) | 238,759 | 30–200 | Indonesian Throughflow, Holloway Current | Largely flat shelf, some slope, terrace, to shelf edge | Strong tides, cyclonic storms, long-period swells, high primary production, low mud, with gravel, generally clear water | Tropical |
| North West Province (NWP) | 178,651 | Max. 5170 | Indonesian Throughflow, Leeuwin Current, Eastern Gyral Current | Slope, terraces, valley, trench, plateau | Bathyal, low primary production | Tropical |
| Pilbara Offshore (PIO) | 41,491 | 10–155 | Holloway Current | Largely inner shelf with terraces and some slope | Varied region, from high effective disturbance, high primary production, low mud, warm—to low primary production in north, low disturbance, rugose, less turbid than nearshore, outer limit of wave influence, natural inner shelf hydrodynamic boundary | Tropical |
| Pilbara Nearshore (PIN) | 13,861 | 10–30 | Holloway Current | Shallow inshore shelf with banks and shoals, fringing coral reefs around some islands | Low primary production, highly turbid water, large tidal range, innershelf, coastal | Tropical |
| Central West Transition (CWT) | 162,891 | Max. 5330 | Leeuwin Current, Leeuwin Undercurrent, Ningaloo Current | Slope and terrace | Sporadic productivity, high gravel, low mud, flat terrain | Tropical-temperate transition |
| Ningaloo (NIN) | 7339 | 0–200 | Leeuwin Current, Leeuwin Undercurrent, Ningaloo Current | Largely shelf, some slope and terrace, interrupted fringing reefs in south, continuous offshore reefs in north | Innershelf, coastal, reef flat,~300 km fringing reef, low mud, low gravel, low primary production, clear water, in south cold and flat terrain | Tropical-temperate transition |
| Number of Shared Species | ||||||
|---|---|---|---|---|---|---|
| Locality | NWS | PIO | PIN | NIN | NWP | CWT |
| North West Shelf (NWS) | 232 (5.447) | 104 | 44 | 18 | 9 | 1 |
| Pilbara Offshore (PIO) | 32.248 | 413 (6.023) | 93 | 64 | 34 | 6 |
| Pilbara Nearshore (PIN) | 13.793 | 22.710 | 406 (6.006) | 46 | 18 | 5 |
| Ningaloo (NIN) | 6.394 | 17.204 | 12.483 | 331 (5.802) | 25 | 19 |
| North West Province (NWP) | 5.263 | 13.001 | 6.977 | 11.338 | 110 (4.700) | 4 |
| Central West Transition (CWT) | 0.763 | 2.708 | 2.293 | 10.526 | 5.714 | 30 (3.401) |
| Locality | NWS | PIO | PIN | NIN | NWP |
|---|---|---|---|---|---|
| Sampling days | 0.179 | 0.294 * | 0.344 * | 0.821 * | 0.718 * |
| Collection stations | 0.090 | 0.133 | 0.385 * | 0.900 * | 0.787 * |
| Pairwise Group Comparisons | Average Group Dissimilarity | Genera Contributing Most to Defining Between-Group Dissimilarity (% Contribution) | No. Genera Accounting for Between-Group Dissimilarity (% Dissimilarity) |
|---|---|---|---|
| a v. b | 75.80% | Petrosia (3.9%), Sarcotragus (2.82%), Oceanapia (2.82%), Geodia (2.82%), Hyalonema (2.82%), Luffariella (2.52%), Amphidischosida unknown genus_1 (2.18%), Ectyoplasia (1.78%) | 8 (21.76%) |
| a v. c | 77.73% | Oceanapia (2.51%), Clathria (2.2%), Phakellia (1.93%), Siphonodictyon (1.83%), Sarcotragus (1.61%), Petrosia (1.61%), Theonella (1.49%), Polymastia (1.36%), Halichondria (1.36%), Geodia (1.36%), Xestospongia (1.32%), Axinella (1.25%), Calcarea unknown genus_1 (1.22%) | 13 (21.07%) |
| a v. d | 79.84% | Oceanapia (2.22%), Clathria (2.22%), Mycale (2.09%), Halichondria (1.96%), Ircinia (1.81%), Niphates (1.65%), Tethya (1.65%), Stelletta (1.65%), Spirastrella (1.48%), Spongia (1.48%), Callyspongia (1.48%), Amphimedon (1.48%) | 12 (21.18%) |
| a v. e | 83.93% | Oceanapia (2.05%), Clathria (2.03%), Callyspongia (2.01%), Mycale (1.7%), Haliclona (1.68%), Amphimedon (1.59%), Sarcotragus (1.41%), Petrosia (1.40%), Niphates (1.35%), Reniochalina (1.26%), Theonella (1.26%), Halichondria (1.22%), Dysidea (1.21%) | 13 (20.18%) |
| b v. c | 58.89% | Phakellia (2.05%), Siphonodictyon (1.94%), Crella (1.45%), Hyalonema (1.45%), Clathria (1.42%), Axinella (1.33%), Calcarea unknown genus_1 (1.29%), Pararhapoxya (1.29%), Coelosphaera (1.29%), Jaspis (1.29%), Ecionemia (1.29%), Haliclona (1.23%), Oceanapia (1.22%), Xestospongia (1.13%), Biemna (1.12%) | 15 (20.79%) |
| b v. d | 57.09% | Ircinia (1.94%), Niphates (1.77%), Biemna (1.59%), Spongia (1.59%), Phakellia (1.37%), Fasciospongia (1.37%), Thorectandra (1.37%), Amphidiscosida unknown genus_1 (1.37%), Halichondria (1.30%), Clathria (1.26%), Cinachyrella (1.15%), Petrosia (1.13%), Pericharax (1.12%), Reniochalina (1.12%), Higginsia (1.12%) | 15 (20.58%) |
| b v. e | 62.18% | Haliclona (1.86%), Callyspongia (1.65%), Niphates (1.49%), Clathria (1.43%), Reniochalina (1.39%), Higginsia (1.32%), Ircinia (1.30%), Axinella (1.28%), Hyalonema (1.27%), Carteriospongia (1.26%), Spheciospongia (1.25%), Spongia (1.25%), Siphonodictyon (1.20%), Amphimedon (1.18%), Mycale (1.08%) | 15 (20.21%) |
| c v. d | 43.51% | Niphates (1.57%), Calcarea unknown genus_1 (1.40%), Pararhaphoxya (1.40%), Ecionemia (1.40%), Agelas (1.22%), Chondrilla (1.22%), Fasciospongia (1.22%), Thorectandra (1.22%), Erylus (1.22%), Microscleroderma (1.22%), Siphonodictyon (1.11%), Ircinia (1.02%), Theonella (1.02%), Phakellia (1.00%), Xestospongia (1.00%), Pericharax (0.99%), Pipestela (0.99%) | 17 (20.22%) |
| c v. e | 35.08% | Niphates (1.92%), Carteriospongia (1.63%), Phakellia (1.50%), Pararhaphoxya (1.47%), Ecionemia (1.47%), Callyspongia (1.39%), Mycale (1.38%), Hyattella (1.27%), Amphimedon (1.22%), Fasciospongia (1.18%), Thorecta (1.12%), Taonura (1.04%), Zyzzya (1.04%), Guitarra (1.04%), Echinoclathria (1.04%), Aplysinidae unknown genus_1 (1.04%) | 16 (20.73%) |
| d v. e | 35.13% | Haliclona (2.00%), Callyspongia (1.55%), Phorbas (1.53%), Pseudoceratina (1.49%), Agelas (1.40%), Cymbastela (1.23%), Aplysina (1.23%), Theonella (1.18%), Rhaphoxya (1.16%), Pleraplysilla (1.16%), Fascaplysinopsis (1.16%), Homaxinella (1.16%), Cinachyra (1.16%), Aplysinella (1.16%), Arenosclera (1.12%), Topsentia (1.12%) | 16 (20.81%) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fromont, J.; Abdul Wahab, M.A.; Gomez, O.; Ekins, M.; Grol, M.; Hooper, J.N.A. Patterns of Sponge Biodiversity in the Pilbara, Northwestern Australia. Diversity 2016, 8, 21. https://doi.org/10.3390/d8040021
Fromont J, Abdul Wahab MA, Gomez O, Ekins M, Grol M, Hooper JNA. Patterns of Sponge Biodiversity in the Pilbara, Northwestern Australia. Diversity. 2016; 8(4):21. https://doi.org/10.3390/d8040021
Chicago/Turabian StyleFromont, Jane, Muhammad Azmi Abdul Wahab, Oliver Gomez, Merrick Ekins, Monique Grol, and John Norman Ashby Hooper. 2016. "Patterns of Sponge Biodiversity in the Pilbara, Northwestern Australia" Diversity 8, no. 4: 21. https://doi.org/10.3390/d8040021






