Biogeography of the Cicadas (Hemiptera: Cicadidae) of North America, North of Mexico
Abstract
:1. Introduction
2. Results and Discussion
| Subfamily | Tribe | Genus | Species | Distribution |
|---|---|---|---|---|
| Cicadinae | Cryptotympanini | Cacama | C. californica Davis | CA, NV |
| C. collinaplaga Sanborn & Heath | TX | |||
| C. crepitans (Van Duzee) | CA | |||
| C. moorei Sanborn & Heath | AZ, NV | |||
| C. valvata (Uhler) | AZ, CA, CO, KS, NV, NM, OK, TX, UT | |||
| C. variegata Davis | TX | |||
| Diceroprocta | D. apache (Davis) | AZ, CA, CO, NV, UT | ||
| D. arizona (Davis) | AZ | |||
| D. aurantiaca Davis | NM, TX | |||
| D. averyi Davis | TX | |||
| D. azteca (Kirkaldy) | KS, OK, TX | |||
| D. bequaerti (Davis) | AL, AR, LA, MS, OK, TX | |||
| D. bibbyi Davis | TX | |||
| D. biconica (Walker) | FL | |||
| D. canescens Davis | TX | |||
| D. cinctifera cinctifera (Uhler) | NM, TX | |||
| D. cinctifera limpia Davis | TX | |||
| D. cinctifera viridicosta Davis | TX | |||
| D. delicata (Osborn) | LA, TX | |||
| D. eugraphica (Davis) | AZ, CO, KS, NM, OK, TX | |||
| D. knighti (Davis) | AZ | |||
| D. lata Davis | TX | |||
| D. marevagans Davis | TX | |||
| D. olympusa (Walker) | AL, FL, GA, MS, NC, SC | |||
| D. semicincta (Davis) | AZ, NM | |||
| Diceroprocta | D. swalei swalei (Distant) | AZ | ||
| D. swalei davisi Davis | AZ | |||
| D. texana (Davis) | TX, NM | |||
| D. viridifascia (Walker) | AL, FL, GA, LA, MS, NC, SC, VA | |||
| D. vitripennis (Say) | AL, AR, IL, IN, KS, KY, LA, MI, MS, MO, NE, OK, TN, TX, WI | |||
| Tibicen (eastern species) | T. auriferus (Say) | AR, KS, MO, NE, NM, OK, TX | ||
| T. canicularis (Harris) | AR, CT, DC, IL, IN, IA, KS, ME, MB, MD, MA, MI, MN, MO, NE, NB, NH, NJ, NY, NC, ND, NS, OH, ON, PA, PE, QC, RI, SC, SD, TN, VT, VA, WV, WI | |||
| T. davisi davisi (Smith & Grossbeck) | AL, DE, DC, FL, GA, LA, MD, MA, MS, NJ, NY, NC, PA, SC, TN, TX, VA, WV | |||
| T. davisi hardeni Davis | AR, MS | |||
| T. latifasciatus (Davis) | MD, NJ, NC, VA | |||
| T. linnei (Smith & Grossbeck) | AL, AR, CT, DE, DC, FL, GA, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, NE, NJ, NY, NC, OH, ON, PA, SC, TN, VT, VA, WV, WI | |||
| T. lyricen lyricen (Degeer) | AL, AR, CT, DE, DC, FL, GA, IL, IN, IA, KS, KY, LA, MD, MA, MI, MS, MO, NE, NH, NJ, NY, NC, OH, OK, ON, PA, RI, SC, TN, TX, VA, WV, WI | |||
| T. lyricen engelhardti (Davis) | AL, CT, DE, DC, FL, GA, IN, KY, MD, MA, MS, NJ, NY, NC, OH, PA, RI, SC, TN, VA, WV | |||
| Tibicen | T. lyricen virescens Davis | FL, GA, NC, SC | ||
| T. pruinosus pruinosus (Say) | AL, AR, CO, FL, GA, IL, IN, IA, KS, KY, LA, MA, MD, MI, MN, MS, MO, NE, NJ, NY, NC, OH, OK, PA, SC, SD, TN, TX, VA, WV, WI | |||
| T. pruinosus fulvus Beamer | KS, OK | |||
| T. robinsonianus Davis | AL, AR, DC, FL, GA, IN, KS, MD, MS, MO, NC, OH, PA, TN, TX, VA | |||
| T. similaris (Smith & Grossbeck) | AL, FL, GA, LA, MS, NC, SC | |||
| T. superbus (Fitch) | AR, KS, LA, MO, NM, OK, TX | |||
| T. tibicen tibicen (Linnaeus) | AL, AR, CT, DE, DC, FL, GA, IL, IN, IA, KS, KY, LA, MD, MA, MI, MS, MO, NE, NJ, NY, NC, OH, OK, PA, RI, SC, SD, TN, TX, VT, VA, WV, WI | |||
| T. tibicen australis (Davis) | AL, FL, GA, MS, NC, SC, TN, VA | |||
| T. winnemanna (Davis) | AL, AR, DE, DC, GA, IN, KY, LA, MD, MS, NC, OH, OK, PA, SC, TN, TX, VA, WV | |||
| Tibicen (western species) | T. bifidus (Davis) | AZ, CO, KS, NM, OK, TX, UT | ||
| T. chiricahua Davis | AZ, NM | |||
| T. chisosensis Davis | TX | |||
| T. duryi Davis | AZ, CO, NM, OK, TX, UT | |||
| T. inauditus Davis | AZ, CO, NM, OK, TX | |||
| T. longiperculus Davis | AZ | |||
| T. parallelus Davis | AZ | |||
| T. simplex Davis | AZ | |||
| T. texanus Metcalf | NM, OK, TX | |||
| T. townsendii (Uhler) | AZ, NM, TX | |||
| Tibicen (large species) | T. auletes (Germar) | AL, AR, CT, DE, DC, FL, GA, IL, IN, IA, KS, KY, LA, MD, MA, MI, MS, MO, NE, NJ, NY, NC, OH, OK, PA, SC, TN, TX, VA, WV, WI | ||
| T. cultriformis (Davis) | AZ, NM | |||
| T. dealbatus (Davis) | CO, IA, KS, MT, NE, NM, ND, OK, SD, TX, WY | |||
| T. dorsatus (Say) | AR, CO, ID, IL, IA, KS, MO, MT, NE, NM, OK, SD, TX, WY | |||
| T. figuratus (Walker) | AL, AR, FL, GA, LA, MS, NC, SC, TN, TX, VA | |||
| T. pronotalis pronotalis Davis | IA, MO, NE, ND, OK, SD | |||
| T. pronotalis walkeri Metcalf | AL, AR, FL, GA, IL, IN, IA, KS, KY, LA, MD, MI, MN, MS, MO, NE, NC, ND, OH, OK, SD, TN, TX, VA, WV, WI, WY | |||
| T. resh (Haldeman) | AR, KS, LA, MS, NE, OK, SC, TN, TX | |||
| T. resonans (Walker) | AL, FL, GA, LA, MS, NC, SC, TN, TX, VA | |||
| T. tremulus Cole | CO, KS, NE, OK, TX | |||
| Cornuplura | C. nigroalbata Davis | AZ | ||
| Fidicinini | Beameria | B. ansercollis Sanborn & Heath | UT | |
| B. venosa (Uhler) | AZ, AR, CO, IA, KS, MO, NE, NM, OK, TX, UT | |||
| B. wheeleri Davis | AZ, NM | |||
| Pacarina | P. puella Davis | AZ, LA, OK, TX | ||
| P. shoemakeri Sanborn & Heath | AZ, CO, NM, TX | |||
| Hyantiini | Quesada | Q. gigas (Olivier) | TX | |
| Cicadini | Neocicada | N. chisos (Davis) | TX | |
| N. hieroglyphica hieroglyphica (Say) | AL, AR, DE, FL, GA, IL, IN, KS, KY, LA, MD, MS, MO, NJ, NY, NC, OH, OK, SC, TN, TX, VA | |||
| N. hieroglyphica johannis (Walker) | AL, FL, GA, NC, SC | |||
| Cicadettinae | Taphurini | Magicicada | M. cassinii (Fisher) | GA, IA, IL, IN, KS, KY, MD, MO, NC, NE, NJ, NY, OH, OK, PA, TN, TX, VA, WI, WV |
| M. neotredecim Marshall & Cooley | AR, IA, IL, IN, KY, MO, TN | |||
| M. septendecim (Linnaeus) | CT, DC, DE, GA, IA, IL, IN, KS, KY, MA, MD, MI, MO, NC, NE, NJ, NY, OH, PA, RI, SC, TN, VA, WI, WV | |||
| M. septendecula Alexander & Moore | GA, IA, IL, IN, KS, KY, MO, NC, NJ, NY, OH, PA, TN, VA, WV | |||
| M. tredecassini Alexander & Moore | AL, AR, GA, IA, IL, IN, KY, MD, MO, MS, NC, OK, SC, TN, VA | |||
| M. tredecim (Walsh & Riley) | AL, AR, GA, IL, IN, KY, LA, MD, MO, MS, NC, OK, SC, TN, VA | |||
| M. tredecula Alexander & Moore | AL, AR, GA, IA, IL, IN, KY, LA, MO, MS, NC, OK, SC, TN, VA | |||
| Cicadettini | Cicadetta | C. calliope calliope (Walker) | AL, AR, CO, FL, GA, IL, IN, IA, KS, KY, LA, MD, MS, MO, NE, NC, OH, OK, SC, SD, TN, TX, VA | |
| C. calliope floridensis (Davis) | AL, FL, GA | |||
| C. camerona (Davis) | TX | |||
| C. kansa (Davis) | CO, KS, LA, NE, NM, OK, TX | |||
| C. texana (Davis) | TX | |||
| Tibicininae | Platypediini | Platypedia | P. affinis Davis | UT |
| P. aperta Davis | CA | |||
| P. areolata (Uhler) | BC, CA, ID, MT, NV, OR, UT, WA, WY | |||
| P. balli Davis | AZ | |||
| Platypedia | P. barbata Davis | CA | ||
| P. bernardinoensis Davis | AZ, CA, CO, NM | |||
| P. falcata Davis | AZ, CA, OR, TX, WA | |||
| P. intermedia Van Duzee | CA, NV | |||
| P. laticapitata Davis | CA | |||
| P. mariposa Davis | CA | |||
| P. middlekauffi Simon | CA | |||
| P. minor Uhler | CA, OR | |||
| P. mohavensis mohavensis Davis | AZ, CA, CO, NV, NM, UT | |||
| P. mohavensis rufescens Davis | AZ, CA, CO, NV, NM, UT | |||
| P. putnami putnami (Uhler) | AZ, BC, CA, CO, ID, MT, NE, NV, NM, OR, SD, UT, WA, WY | |||
| P. putnami keddiensis Davis | CA | |||
| P. putnami lutea Davis | AZ, CA, CO, ID, MT, NV, NM, OR, SD, UT, WA, WY | |||
| P. putnami occidentalis Davis | CA | |||
| P. rufipes Davis | CA | |||
| P. scotti Davis | CA | |||
| P. similis Davis | CA, OR | |||
| P. sylvesteri Simons | CA | |||
| P. tomentosa Davis | CA | |||
| P. usingeri Simons | CA | |||
| P. vanduzeei Davis | CA | |||
| Neoplatypedia | N. ampliata (Van Duzee) | CA, OR | ||
| N. constricta Davis | AZ, CA, CO, ID, NV, NM OR, UT | |||
| Tibicinini | Clidophleps | C. beameri Davis | CA | |
| C. blaisdelli (Uhler) | AZ, CA | |||
| Clidophleps | C. distanti distanti (Van Duzee) | CA, NV | ||
| C. distanti truncata (Van Duzee) | CA | |||
| C. rotundifrons (Davis) | AZ, CA | |||
| C. tenuis Davis | CA, NV | |||
| C. vagans Davis | AZ, CA, NV | |||
| C. wrighti Davis | CA | |||
| Okanagana | O. annulata Davis | CA, CO | ||
| O. arboraria Wymore | CA | |||
| O. arctostaphylae Van Duzee | CA | |||
| O. aurora Davis | CA, NV | |||
| O. balli Davis | IL, IA, KS, MN, NE, ND, SD, WI | |||
| O. bella Davis | AB, AZ, BC, CA, CO, ID, MT, NV, NM, OR, SD, UT, WA, WY | |||
| O. canadensis (Provancher) | AB, BC, CA, CO, ID, ME, MB, MI, MN, MT, NB, NH, NY, NT, OH, ON, OR, PA, QC, SK, SD, UT, VT, WI | |||
| O. canescens Van Duzee | CA, WA | |||
| O. catalina Davis | CA | |||
| O. cruentifera (Uhler) | CA, ID, NV, OR, UT, WY | |||
| O. ferrugomaculata Davis | CA, CO, ID, OR, WA | |||
| O. formosa Davis | NV, UT | |||
| O. fratercula Davis | AB, BC, CA, CO, ID, MT, NV, OR, WA, WY | |||
| O. fumipennis Davis | AZ, CO, NM, UT | |||
| O. georgi Heath & Sanborn | AZ | |||
| O. gibbera Davis | CA, CO, ID, MT, NV, NM, OR, UT, WA | |||
| O. hesperia (Uhler) | AZ, CA, CO, KS, MT, NE NV, NM, OK, SD, TX, UT, WY | |||
| Okanagana | O. hirsuta Davis | CA | ||
| O. lurida Davis | BC, ID, OR, WA | |||
| O. luteobasalis Davis | AB, BC, CA, CO, ID, MB, MT, NV, ND, OR, UT, WA, WY | |||
| O. magnifica Davis | AZ, CA, CO, NV, NM, OR, UT, WY | |||
| O. mariposa mariposa Davis | AZ, CA, NM, OR, UT | |||
| O. mariposa oregonensis Davis | OR | |||
| O. napa Davis | CA, ID, WA | |||
| O. nigriviridis Davis | CA | |||
| O. nigrodorsata Davis | CA, NV, WA | |||
| O. noveboracensis (Emmons) | NY, ON | |||
| O. occidentalis (Walker) | AZ, BC, AB, CA, CO, ID, MT, NV, NM, OR, UT, WA | |||
| O. opacipennis Davis | CA | |||
| O. oregona Davis | CA, ID, MT, OR, WA | |||
| O. orithyia Bliven | CA | |||
| O. ornata Van Duzee | BC, CA, ID, NV, OR | |||
| O. pallidula Davis | CA, NV, OR | |||
| O. pernix Bliven | CA | |||
| O. rhadine Bliven | CA | |||
| O. rimosa rimosa (Say) | AB, BC, CA, CT, ID, IL, IN, IA, ME, MB, MD, MA, MI, MN, MT, NV, NB, NH, NJ, NY, ND, OH, ON, OR, PA, QC, SD, UT, VT, VA, WA, WI, WY | |||
| O. rimosa ohioensis Davis | OH | |||
| O. rubrovenosa Davis | AZ, CA, OR, UT | |||
| O. salicicola Bliven | CA | |||
| O. salicicola Bliven | CA | |||
| O. schaefferi Davis | AZ, CO, NV, NM, UT | |||
| Okanagana | O. sequoiae Bliven | CA | ||
| O. simulata Davis | CA, OR | |||
| O. sperata Van Duzee | CA, NV | |||
| O. striatipes (Haldeman) | AZ, CA, CO, ID, KS, MT, NE, NV, NM, OR, SD, UT, WY | |||
| O. sugdeni Davis | UT | |||
| O. synodica synodica (Say) | AB, AZ, CA, CO, IA, KS, MO, MT, NE, NM, ND, OR, SD, TX, UT, WY | |||
| O. synodica nigra Davis | AZ, NM, UT | |||
| O. tanneri Davis | AZ, CO, UT | |||
| O. triangulata Davis | CA, NV | |||
| O. tristis tristis Van Duzee | AZ, CA, NV, OR, WA | |||
| O. tristis rubrobasalis Davis | CA, NV | |||
| O. uncinata Van Duzee | CA | |||
| O. utahensis Davis | AZ, BC, CA, CO, ID, MT, NV, NM, OR, UT, WA | |||
| O. vanduzeei Distant | BC, CA, ID, NV, OR, UT, WA | |||
| O. vandykei Van Duzee | CA, OR | |||
| O. venusta Davis | CA | |||
| O. villosa Davis | CA | |||
| O. viridis Davis | AR, LA, MS, TX | |||
| O. vocalis Bliven | CA | |||
| O. wymorei Davis | CA | |||
| O. yakimaensis Davis | WA | |||
| Okanagodes | O. gracilis gracilis Davis | AZ, CA, NV, UT | ||
| O. gracilis viridis Davis | AZ, CA | |||
| O. terlingua Davis | TX | |||
| Tibicinoides | T. cupreosparsa (Uhler) | CA | ||
| T. mercedita (Davis) | CA | |||
| T. minuta (Davis) | CA |
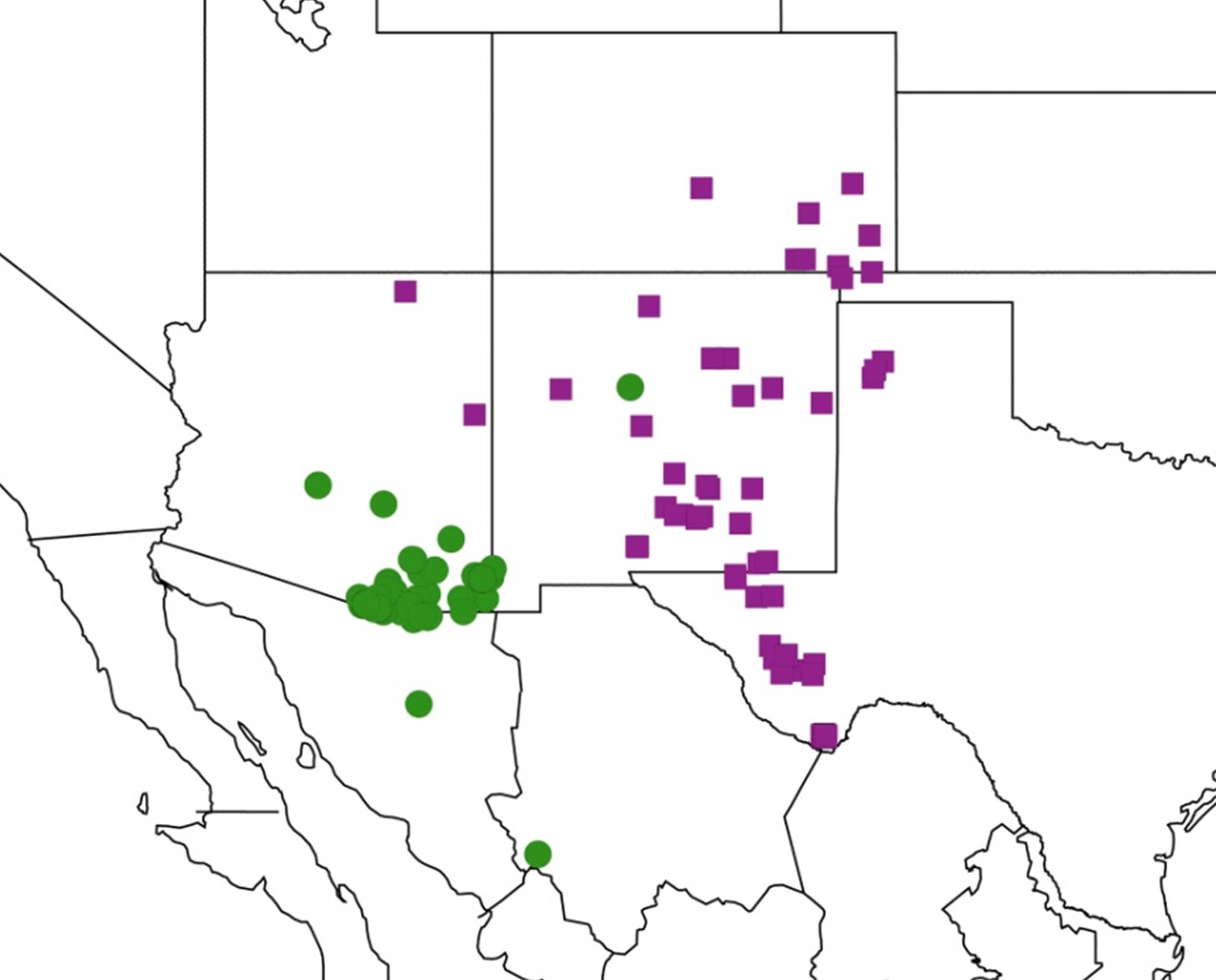
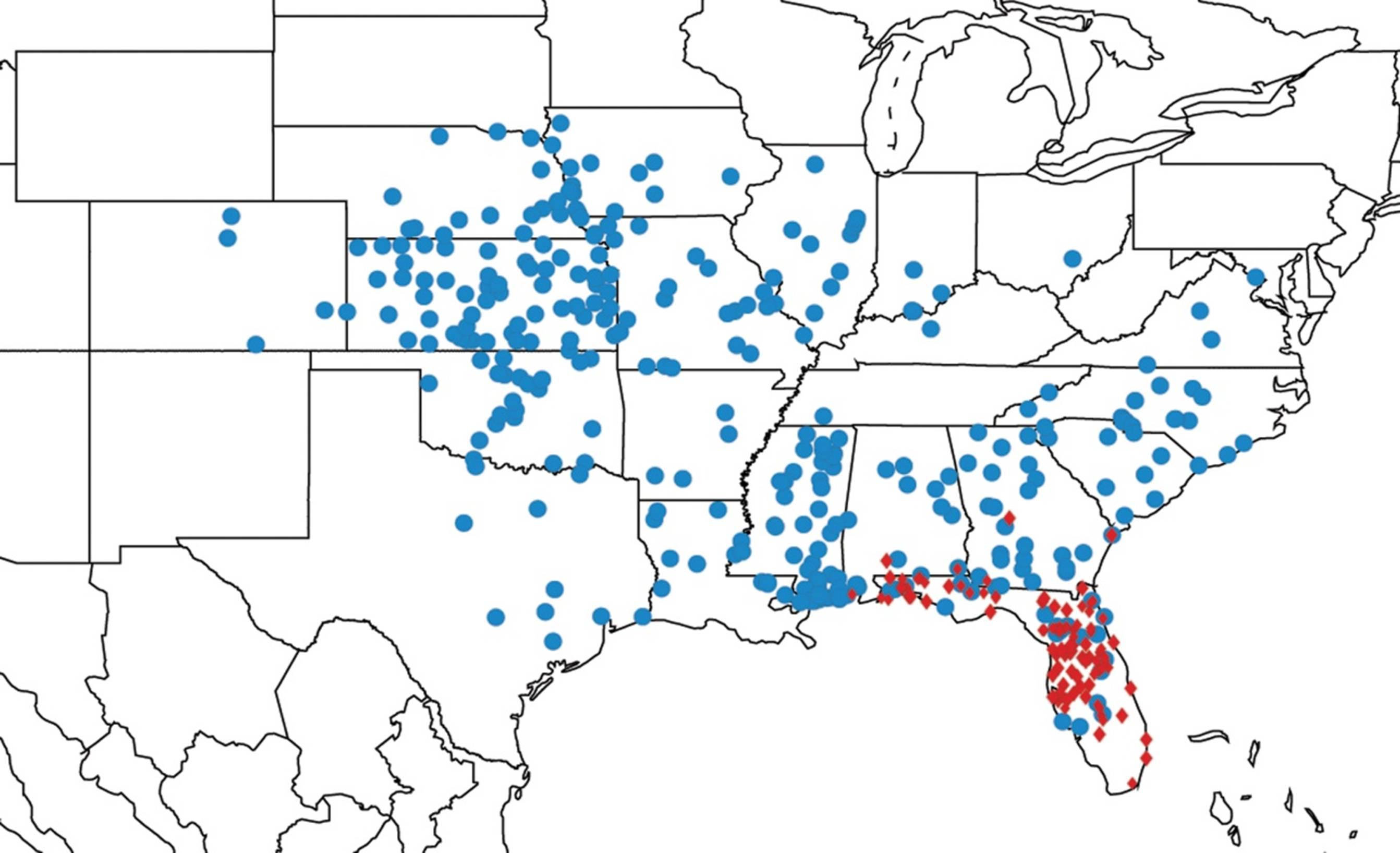
2.1. Biogeography of the Cacama Species

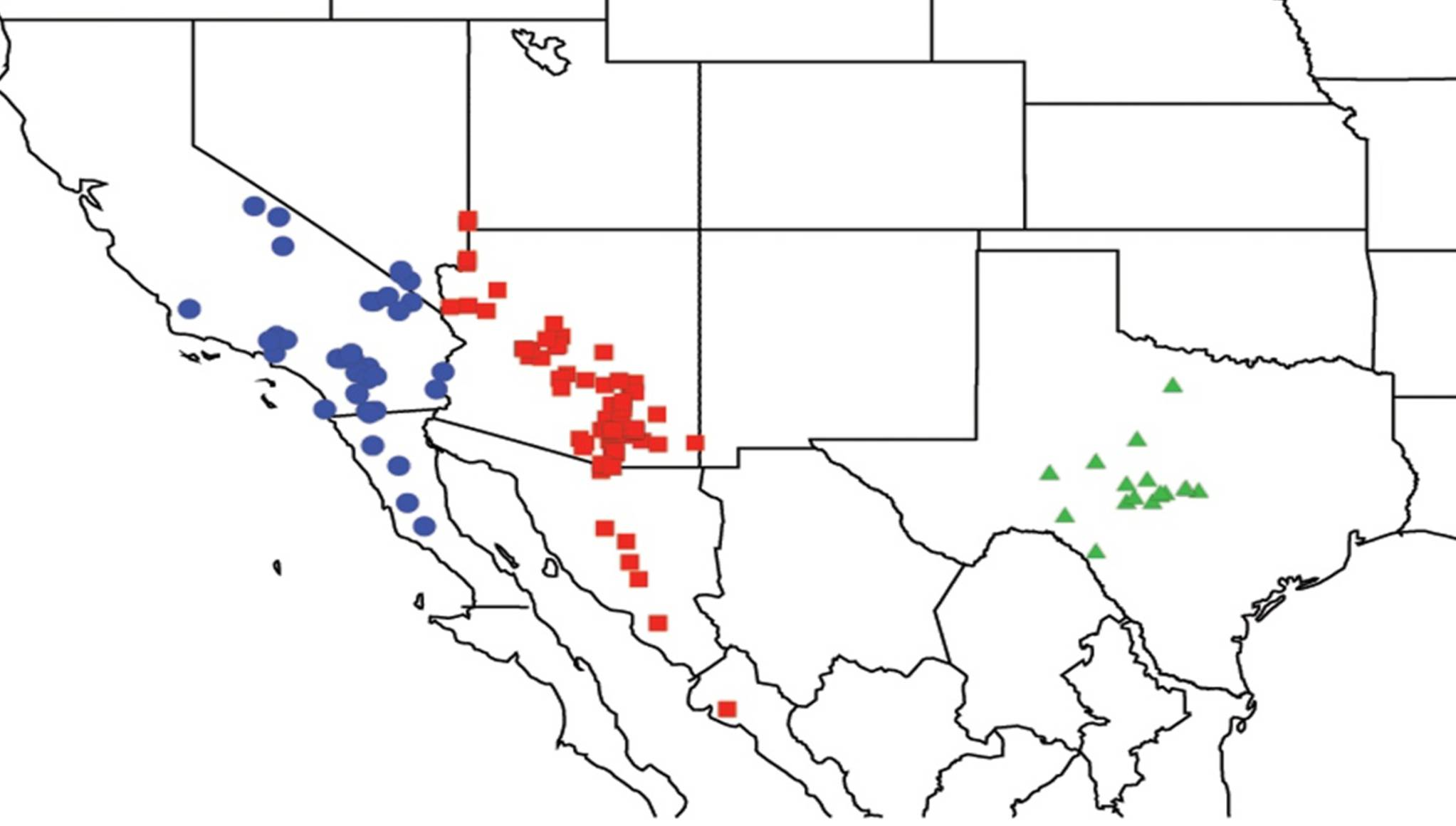
2.2. Biogeography of the Diceroprocta species


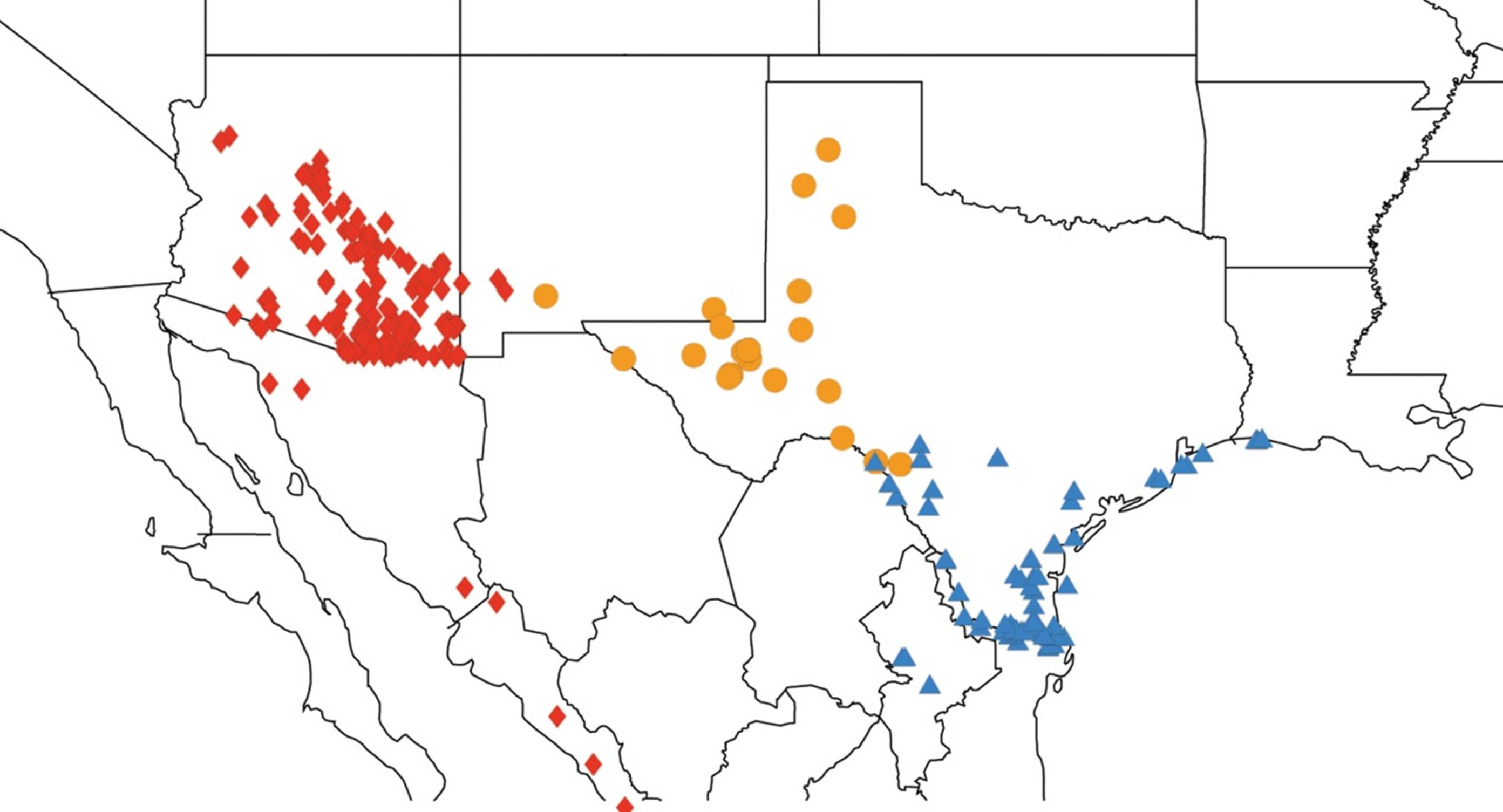
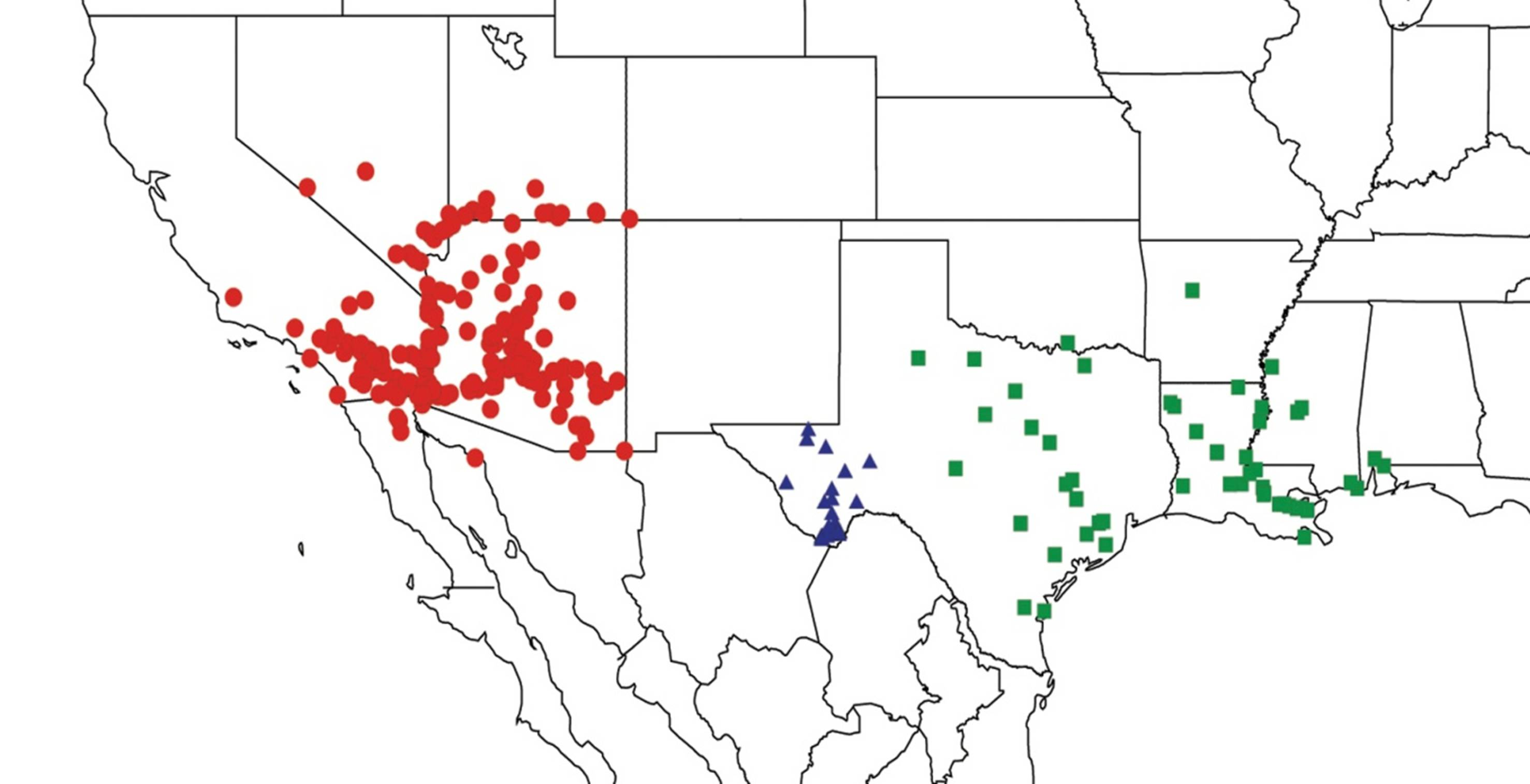
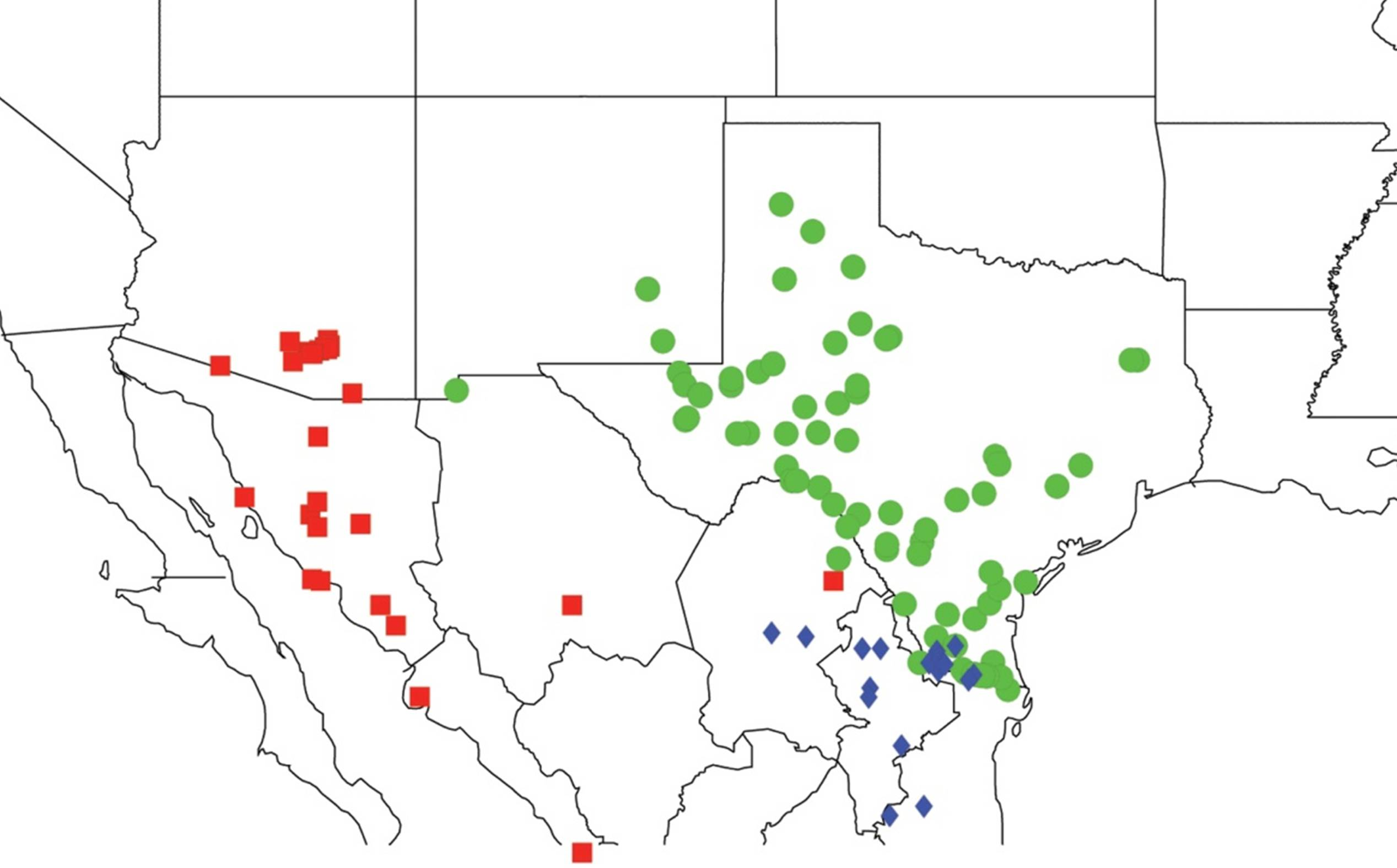
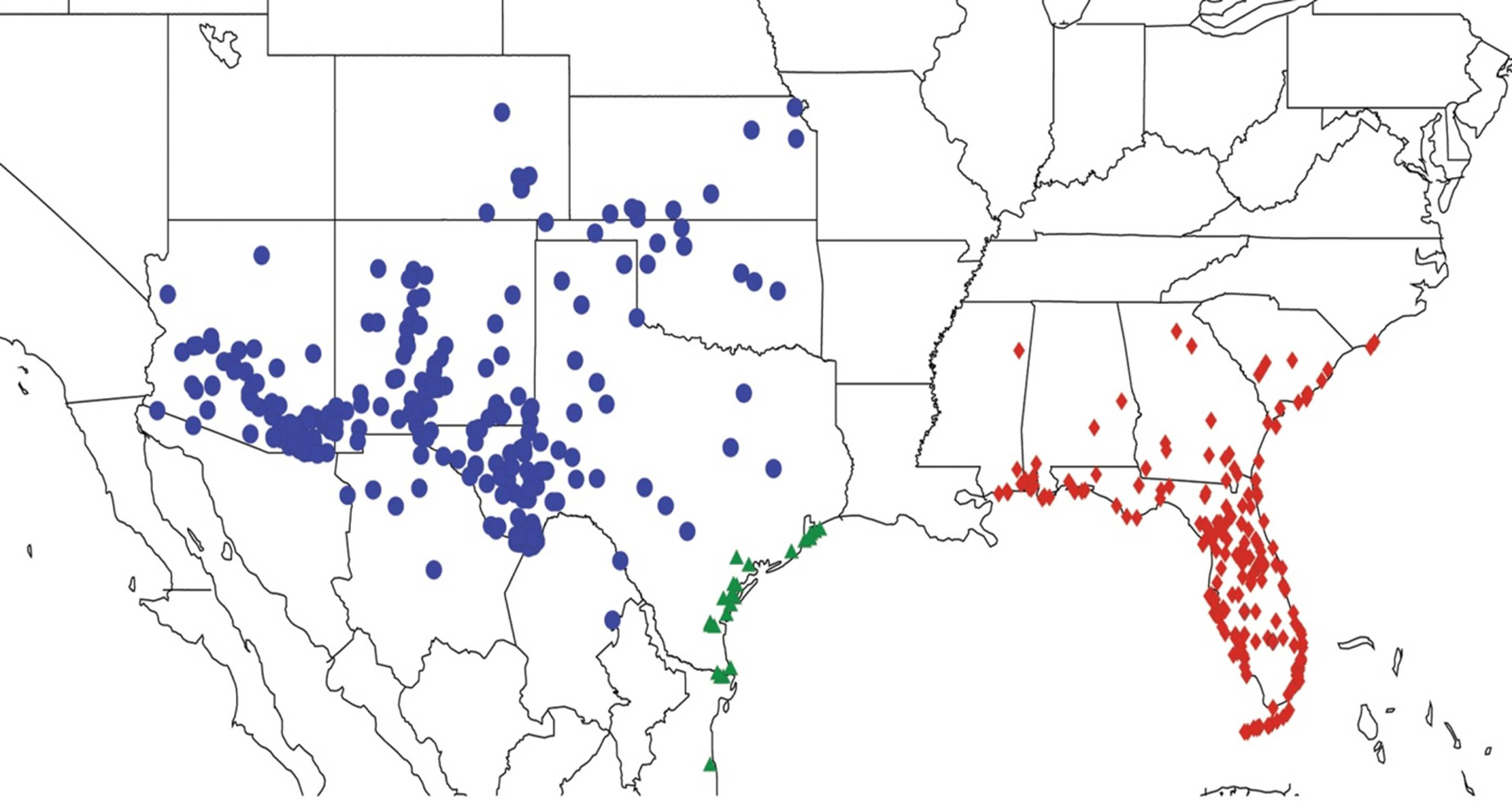
2.3. Biogeography of the Tibicen species
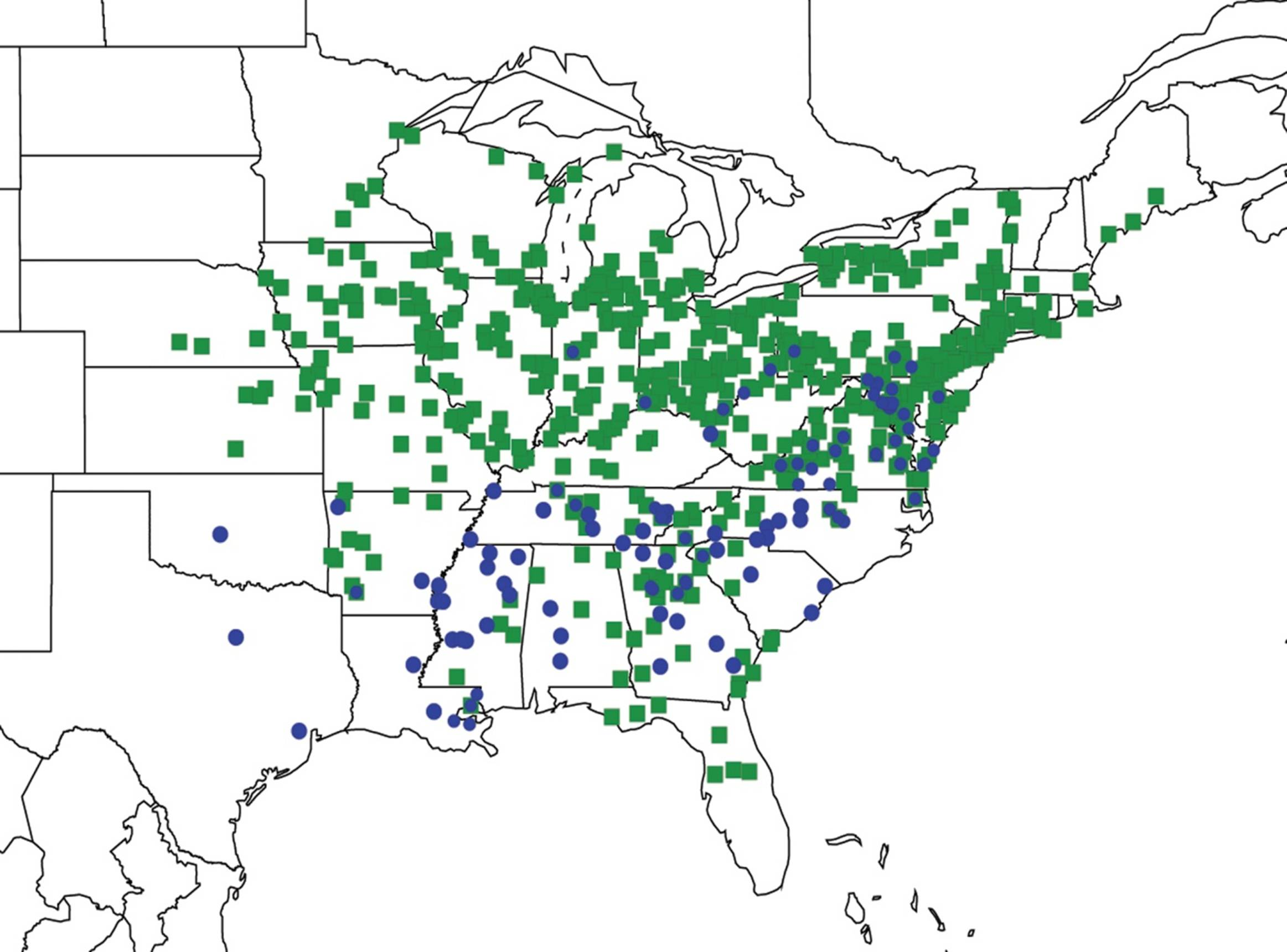
2.3.1. Biogeography of the Eastern Tibicen species




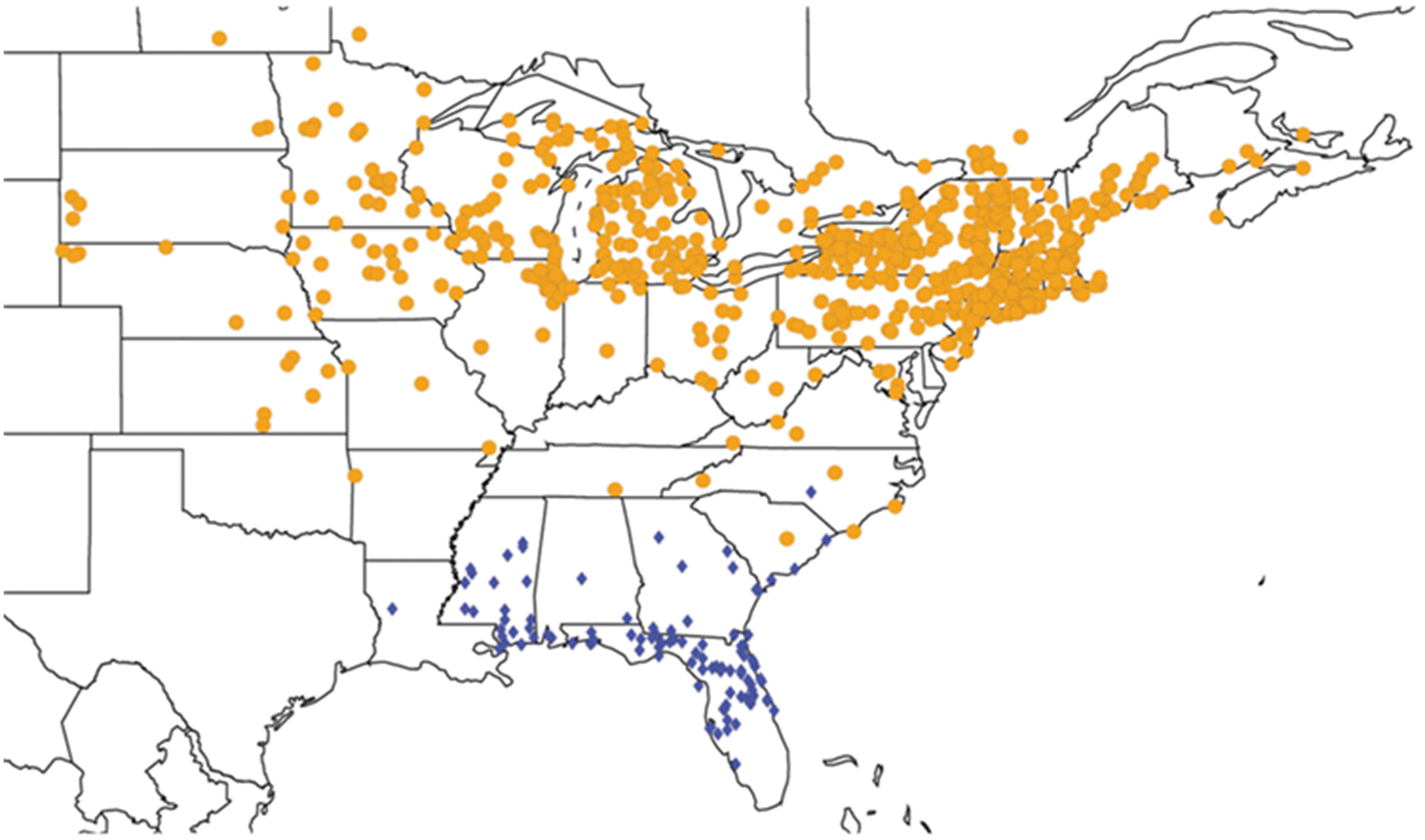
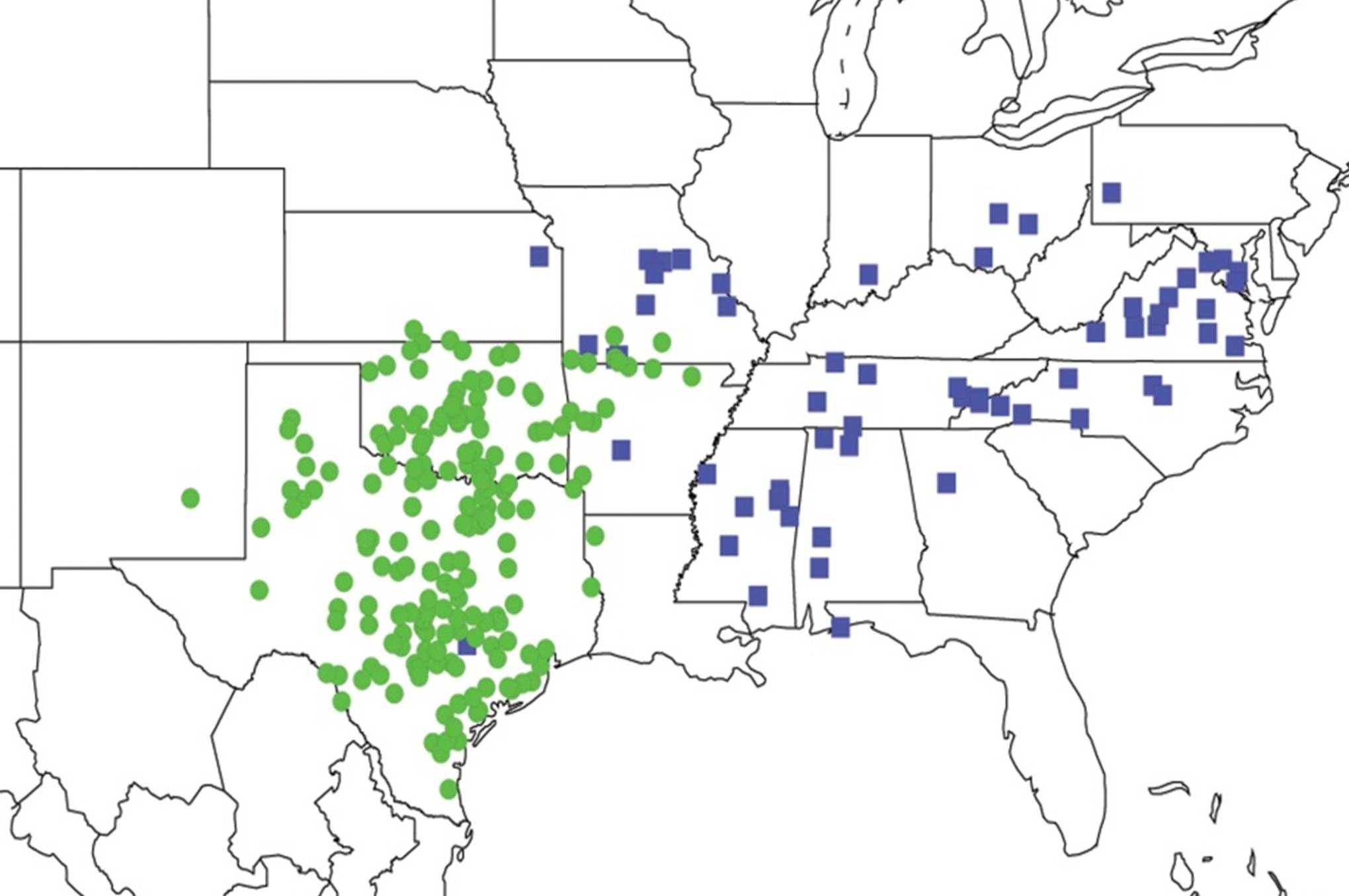
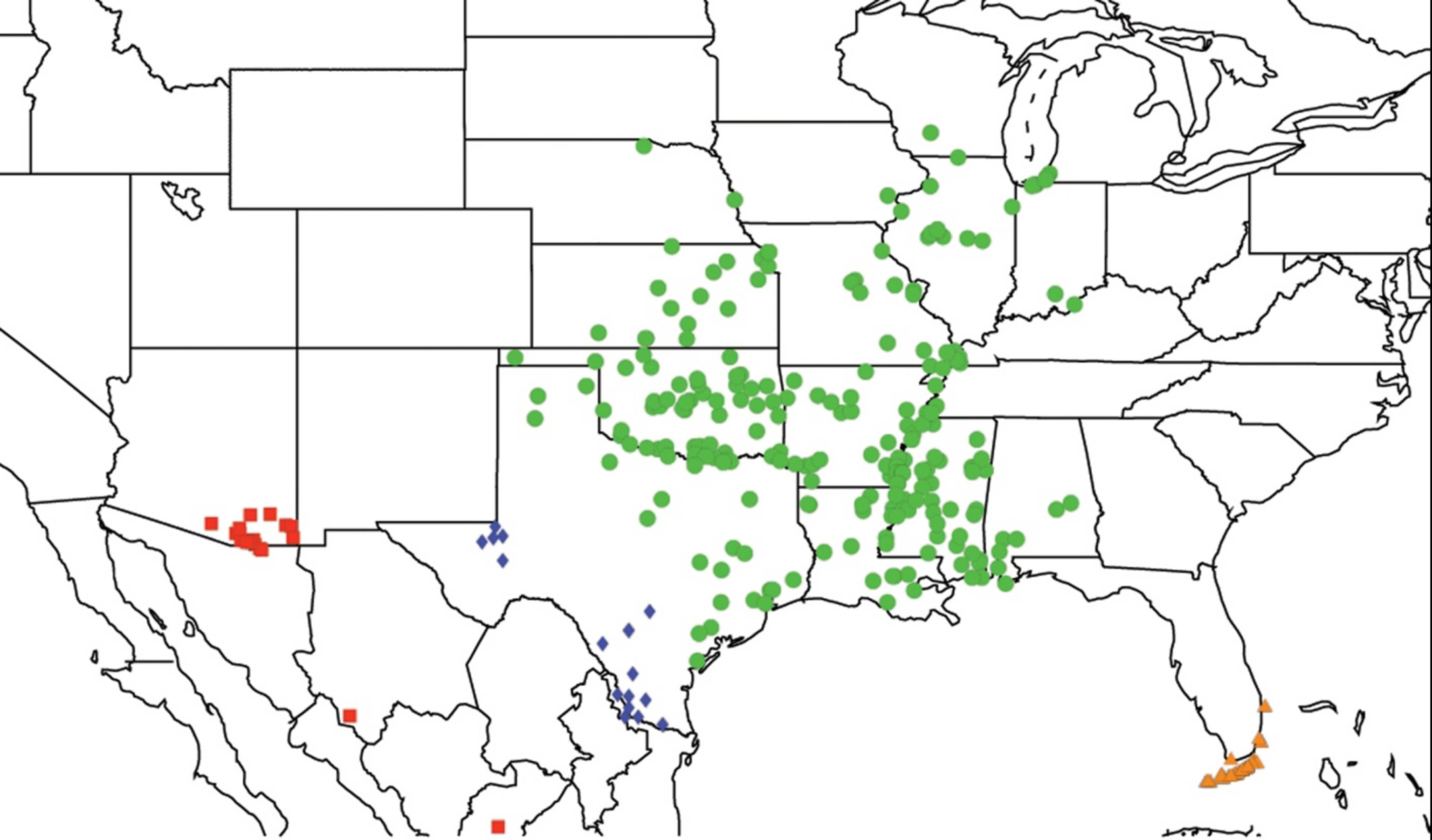
2.3.2. Biogeography of the Western Tibicen Species
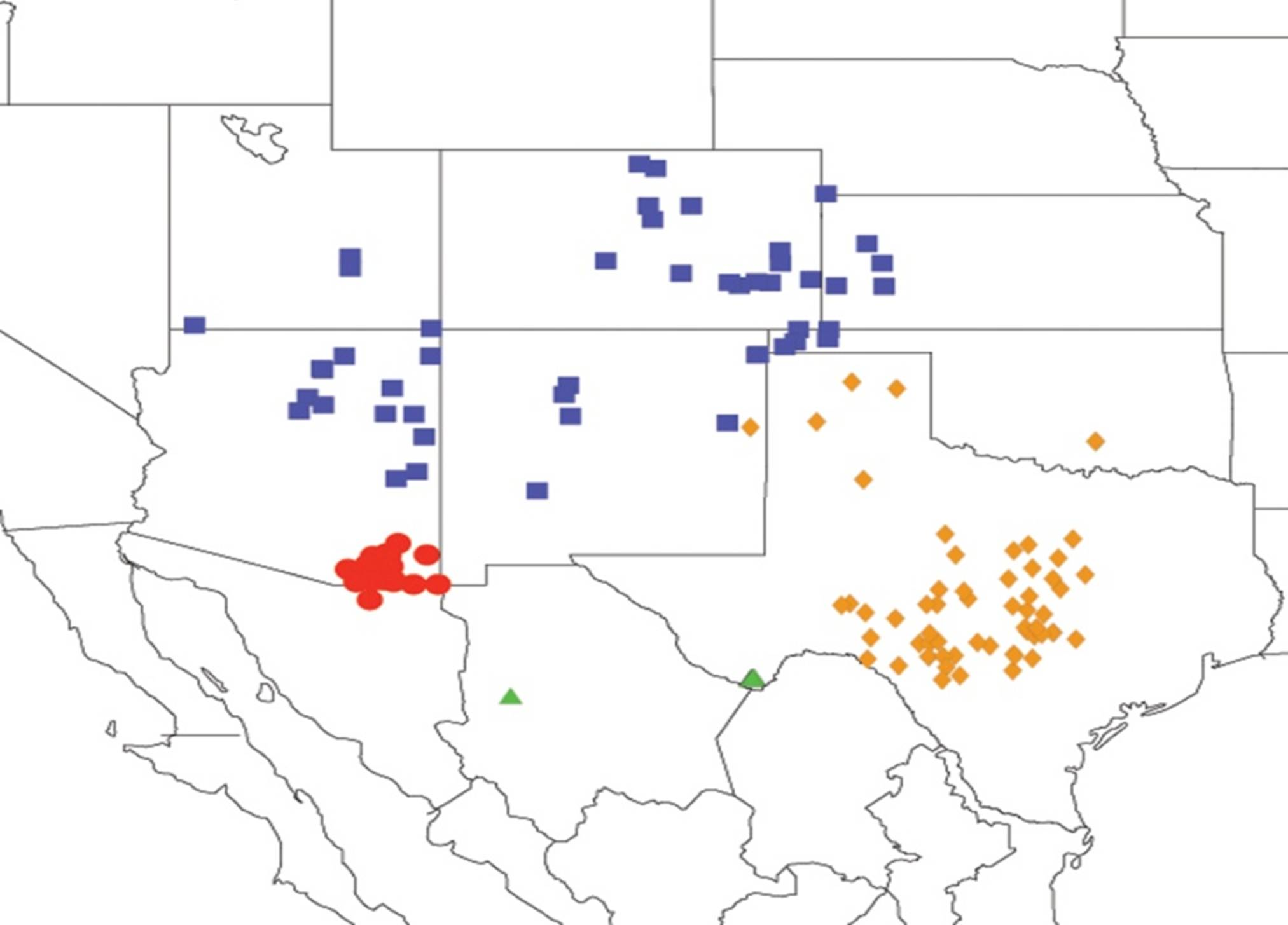
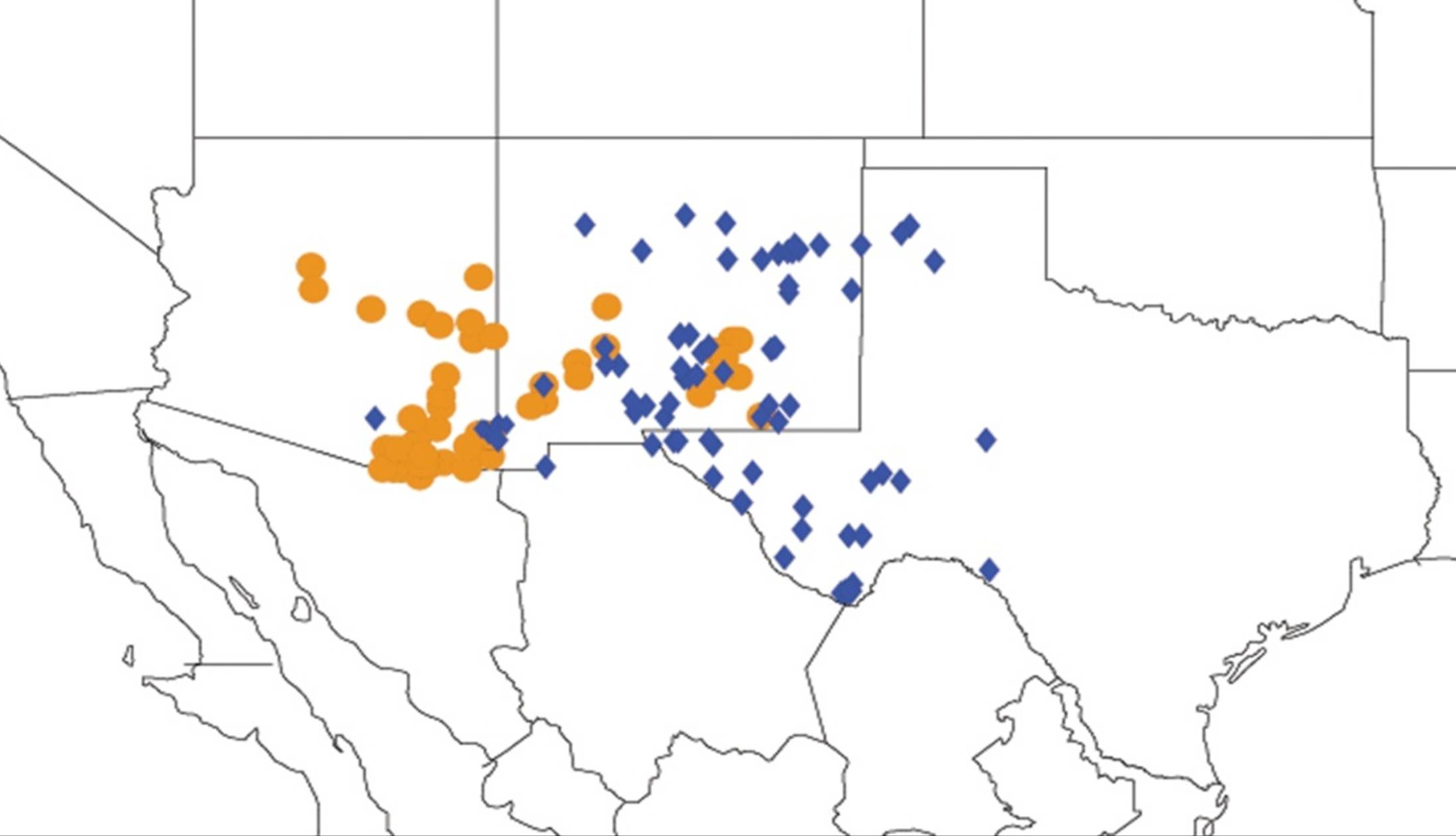

2.3.3. Biogeography of the large Tibicen species
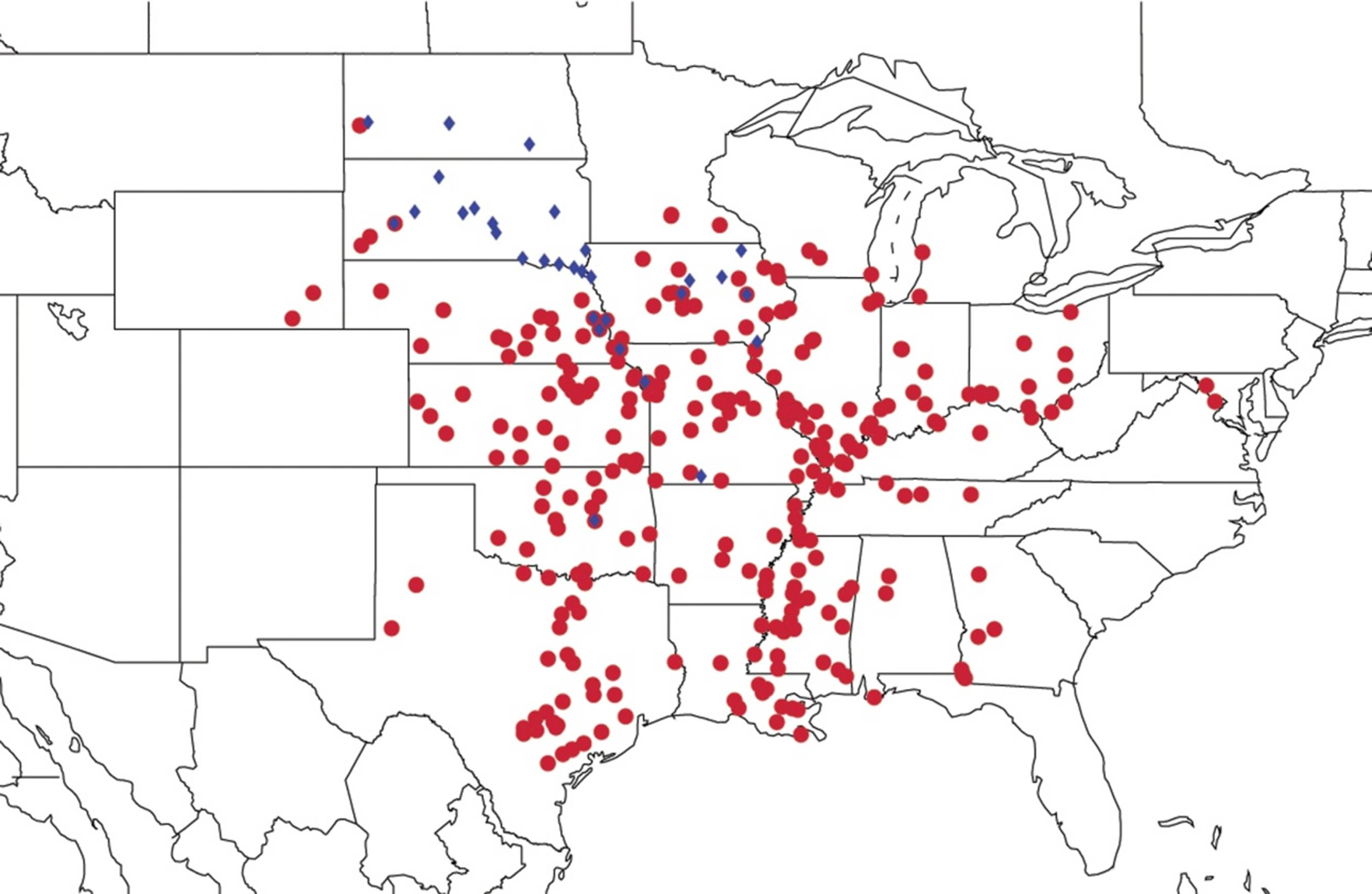


2.4. Biogeography of the Cornuplura species
2.5. Biogeography of the Beameria species
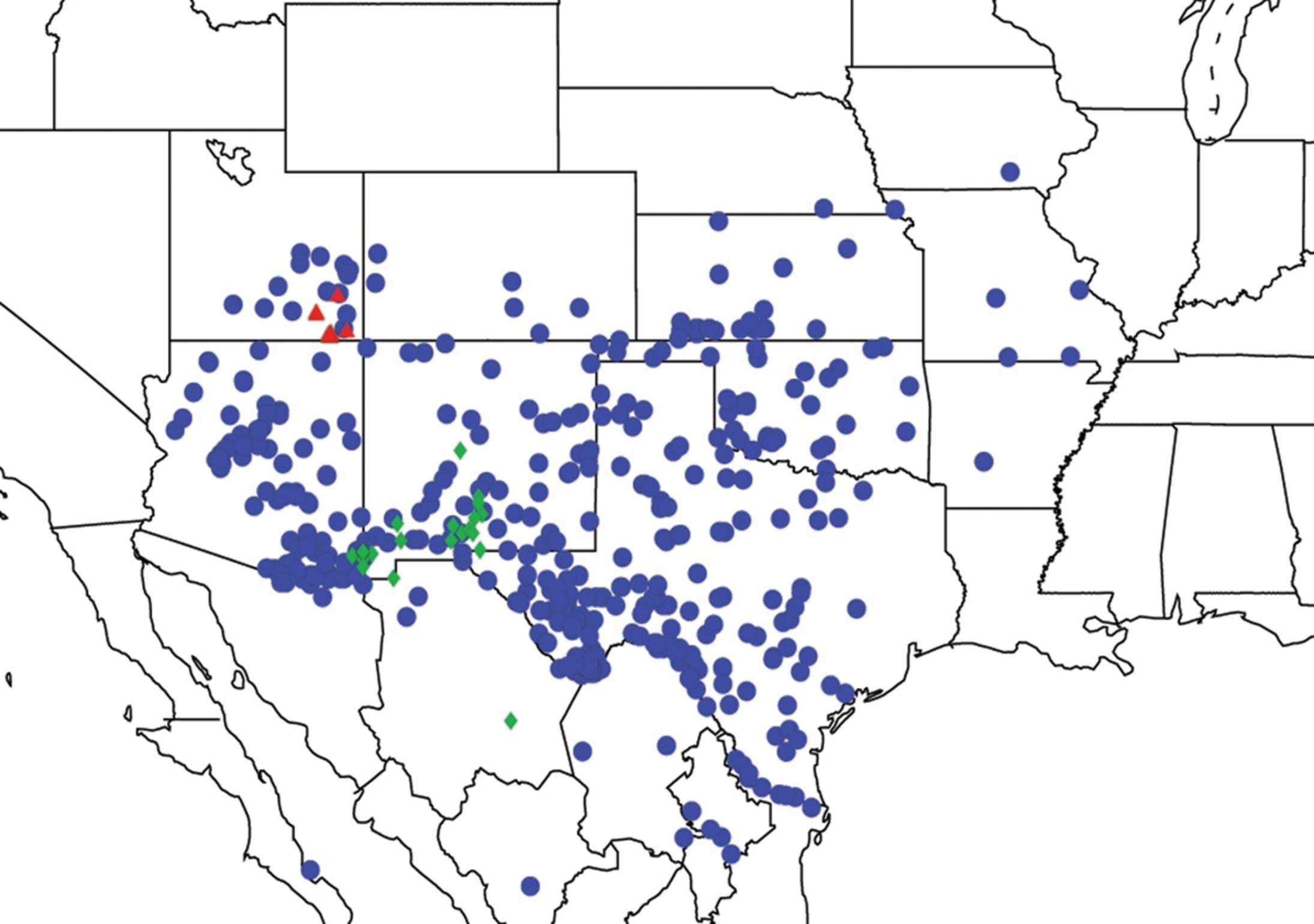
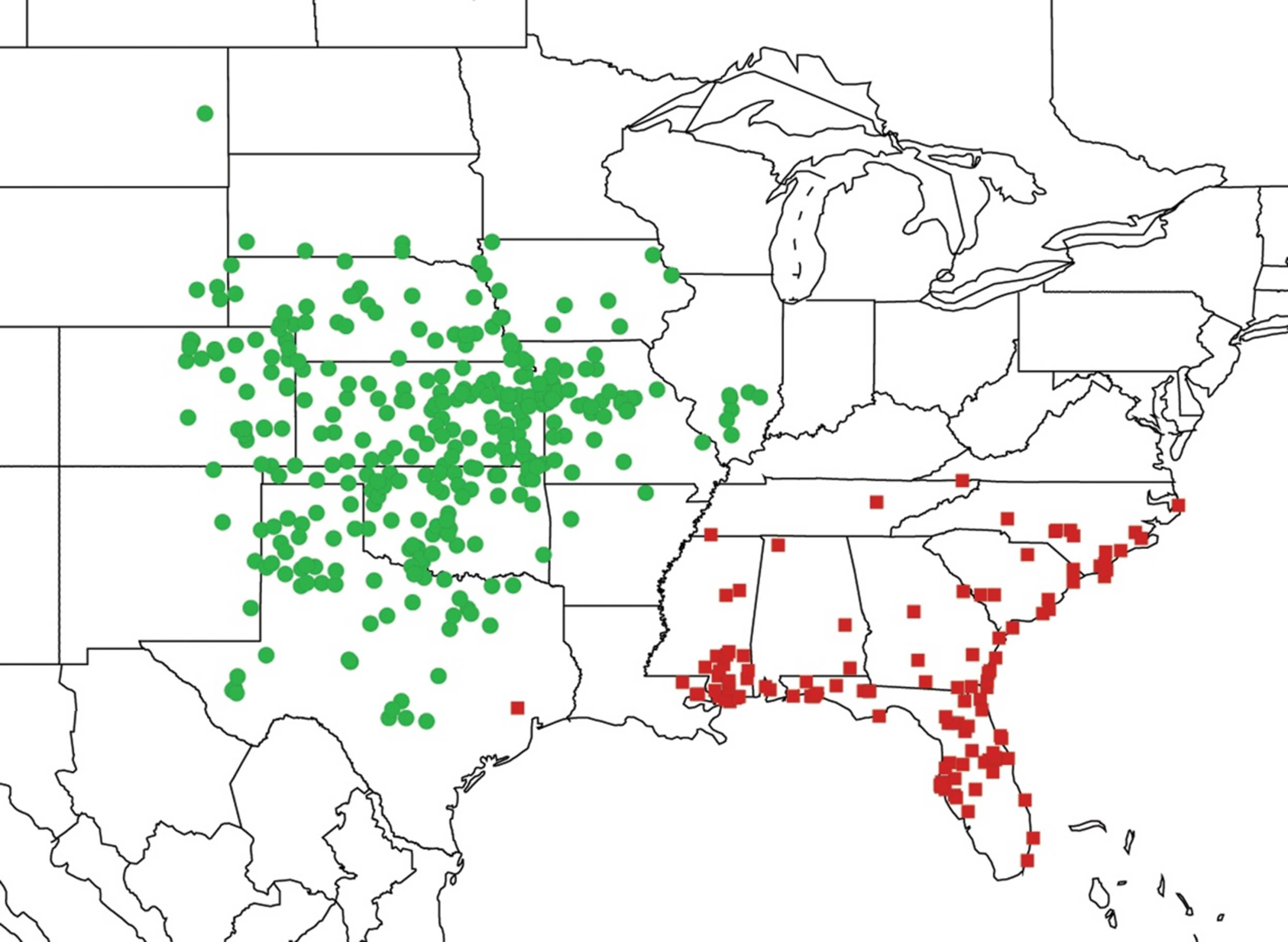
2.6. Biogeography of the Pacarina Species
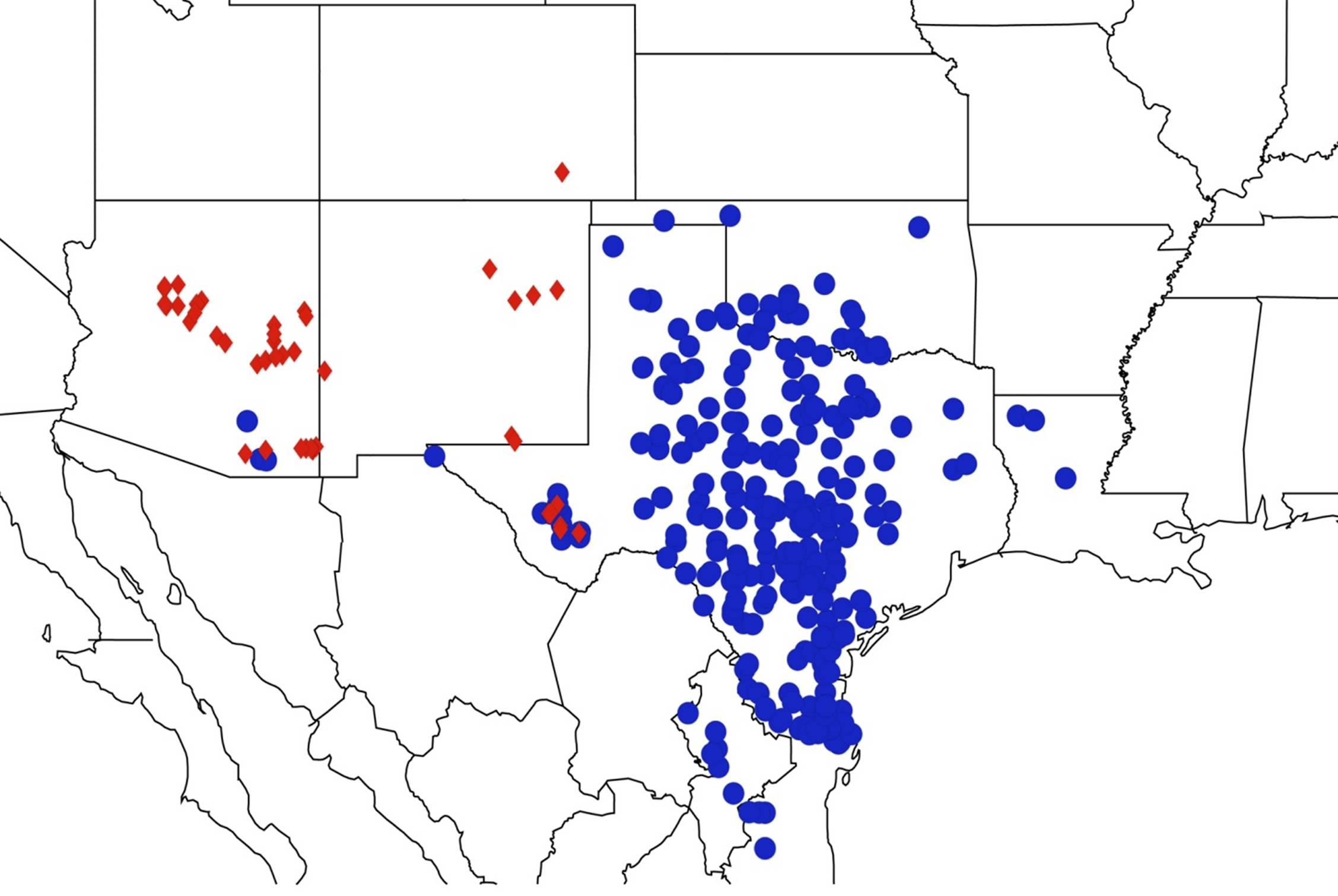
2.7. Biogeography of the Quesada Species
2.8. Biogeography of the Neocicada Species
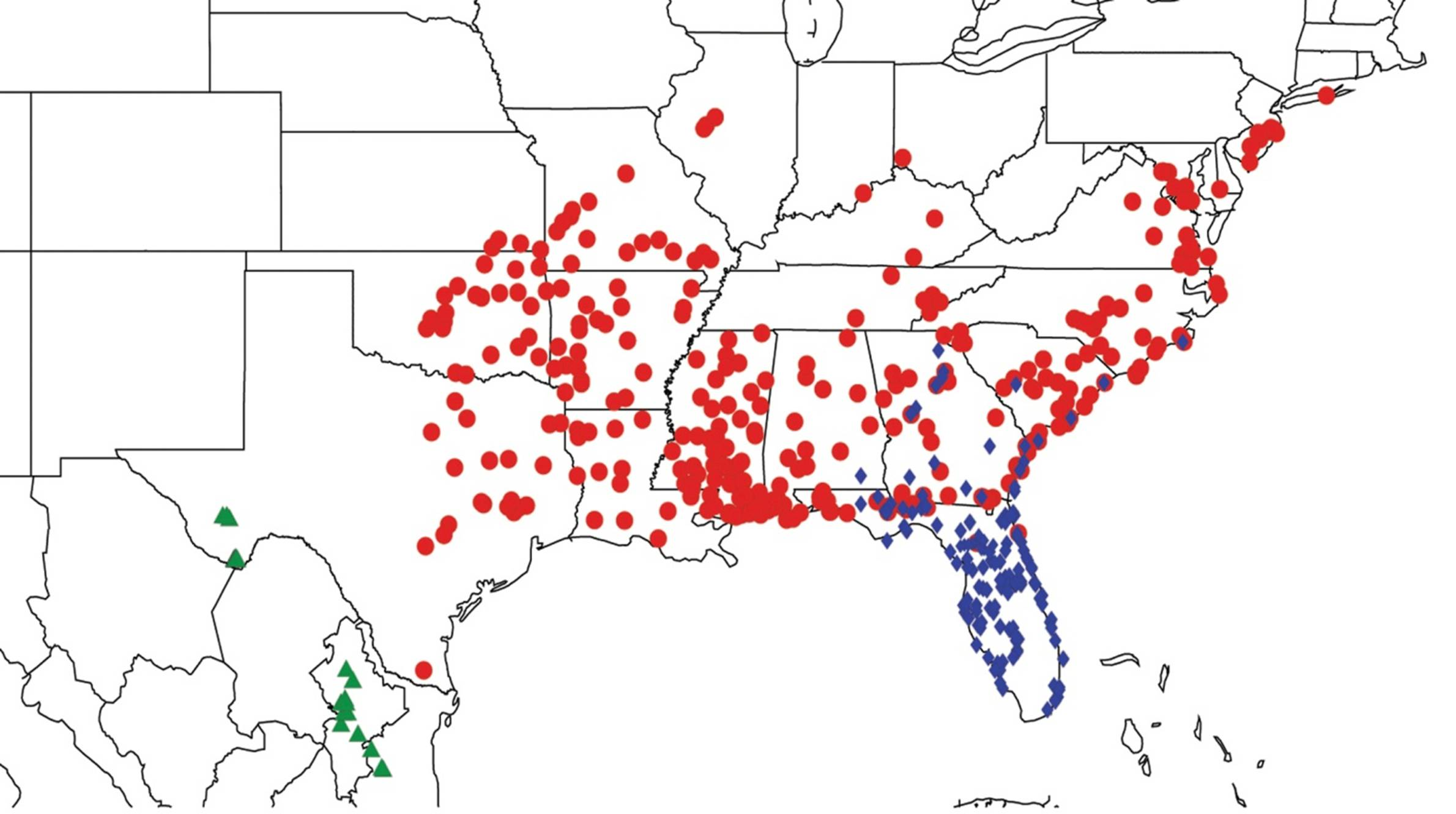
2.9. Biogeography of the Magicicada Species


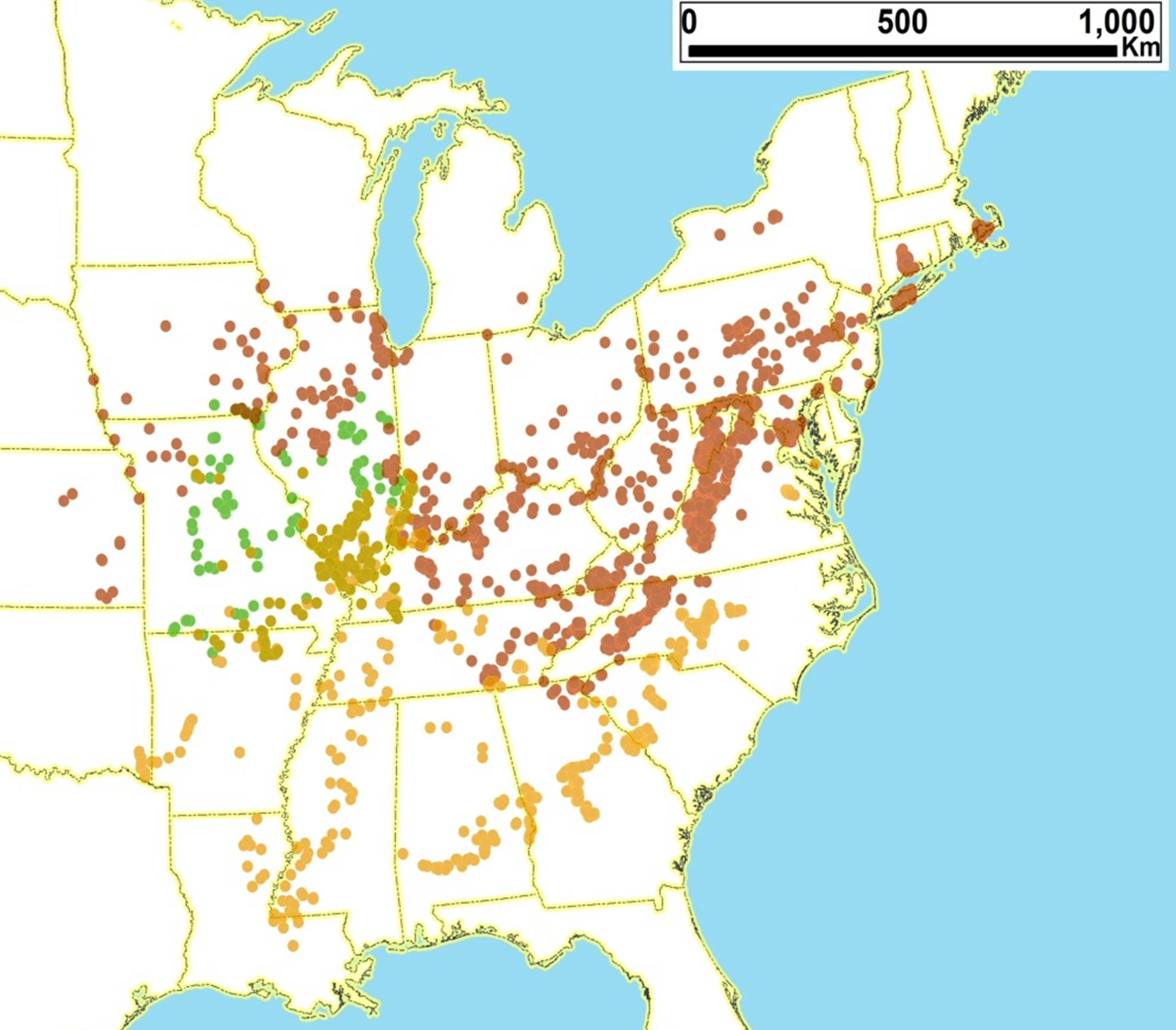
2.10. Biogeography of the Cicadetta Species

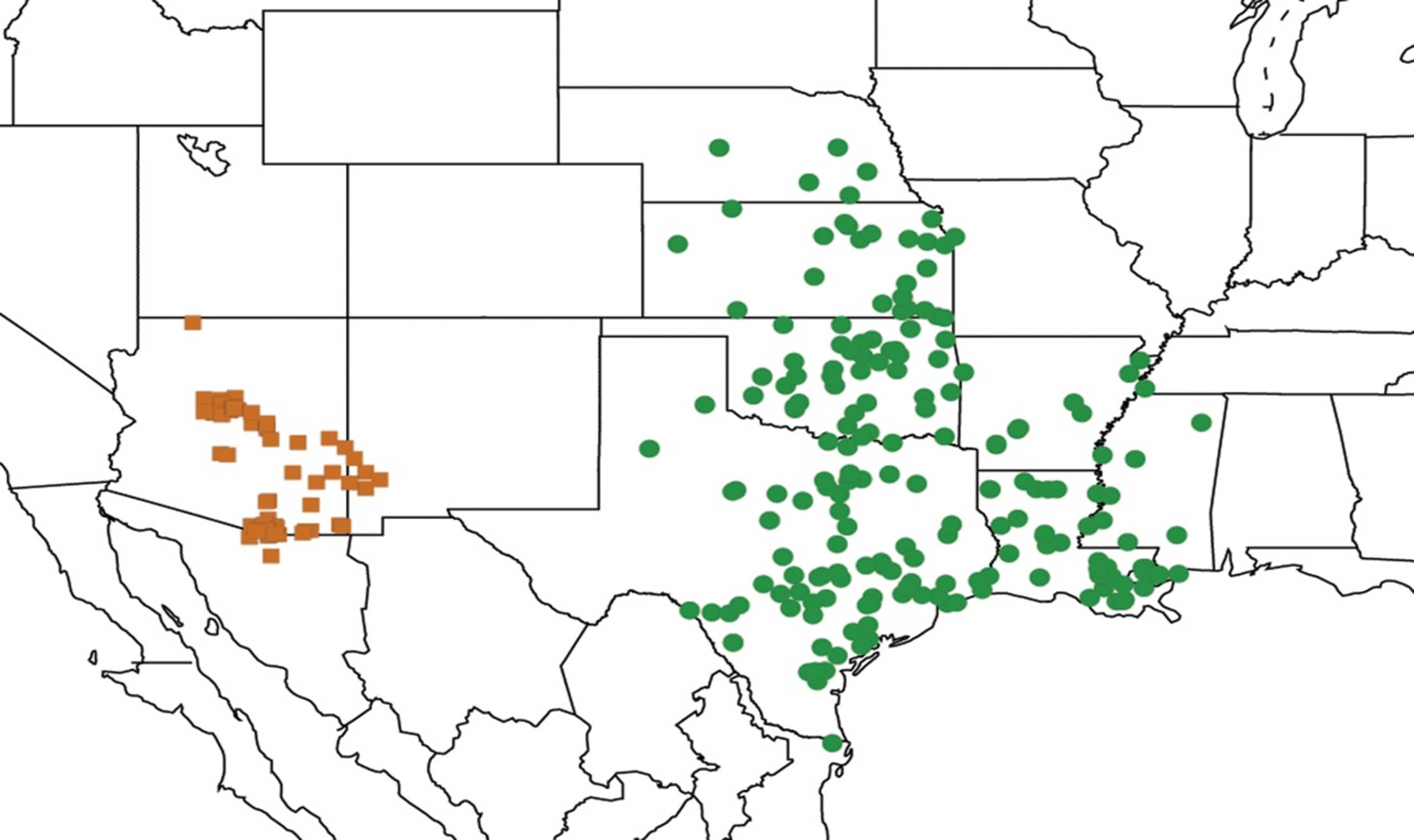
2.11. Biogeography of the Platypedia Species
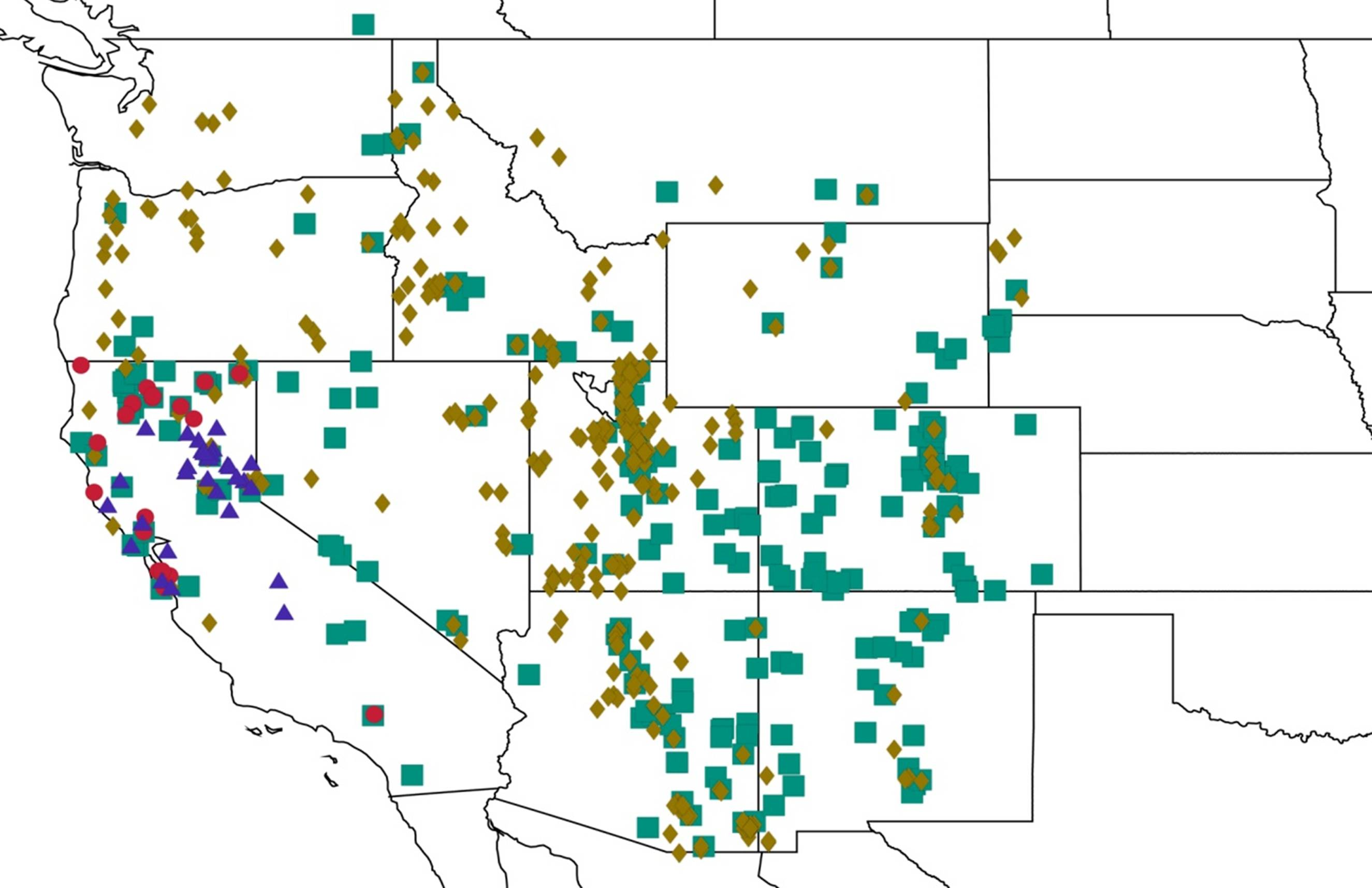





2.12. Biogeography of the Neoplatypedia Species
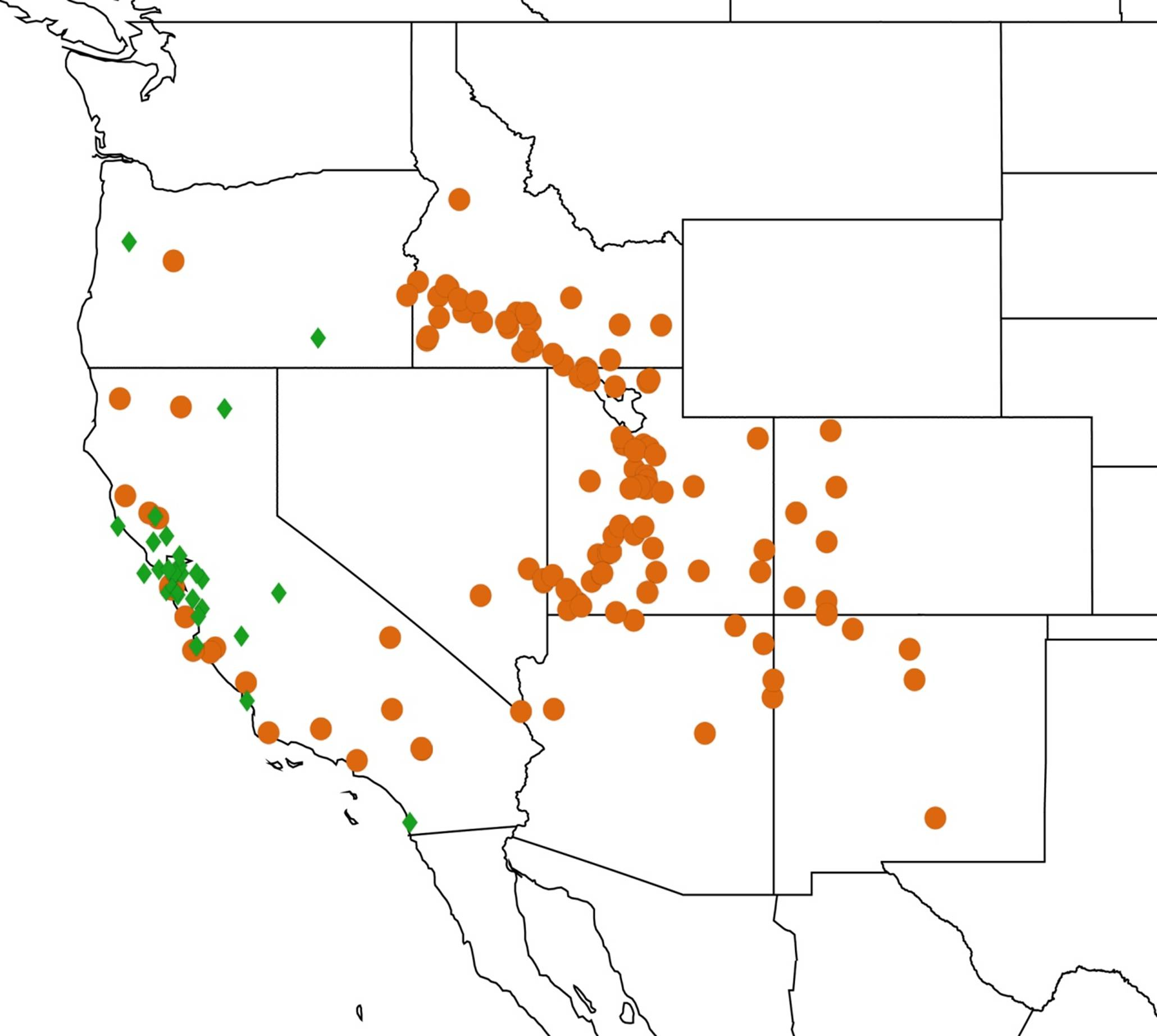
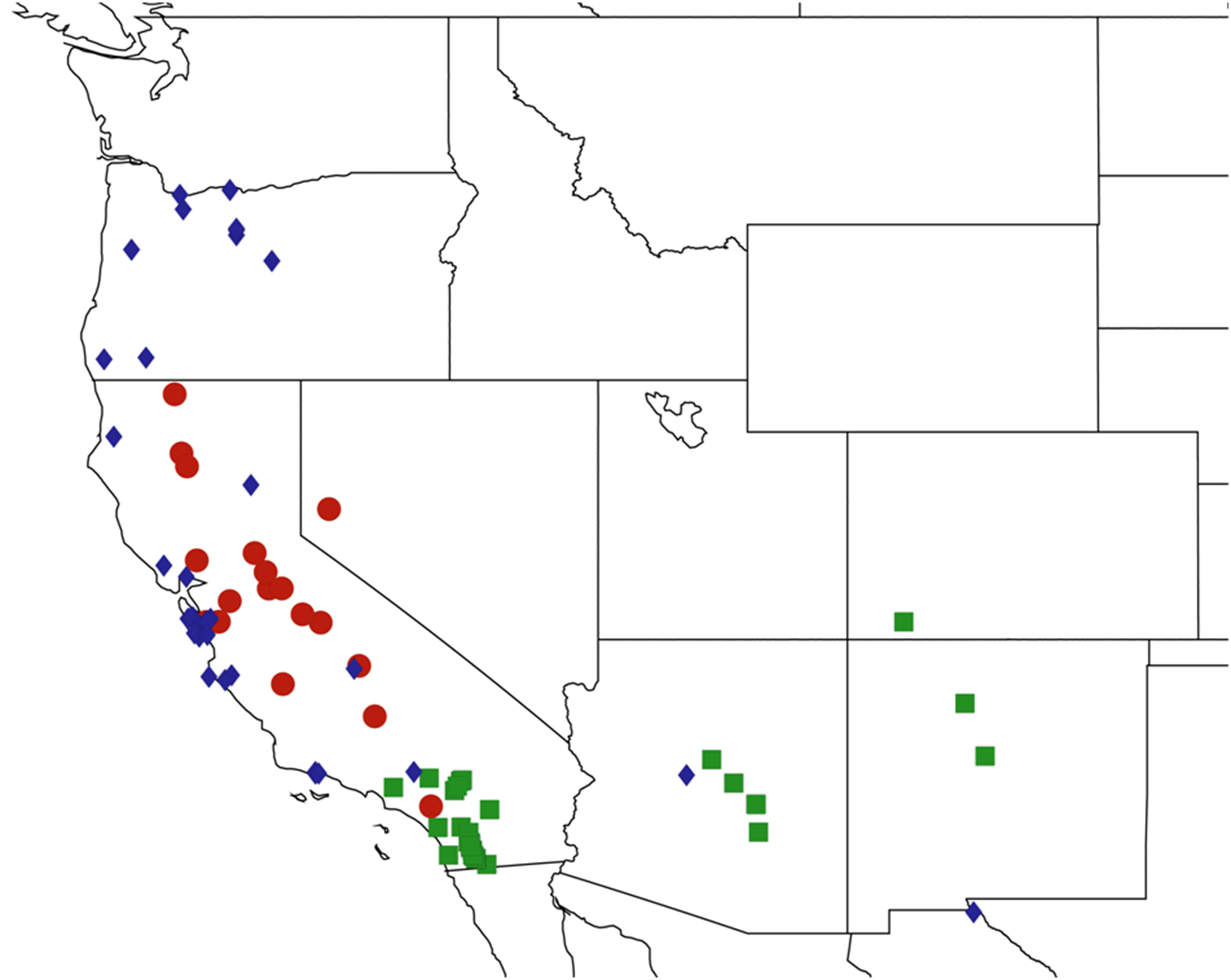
2.13. Biogeography of the Clidophleps Species

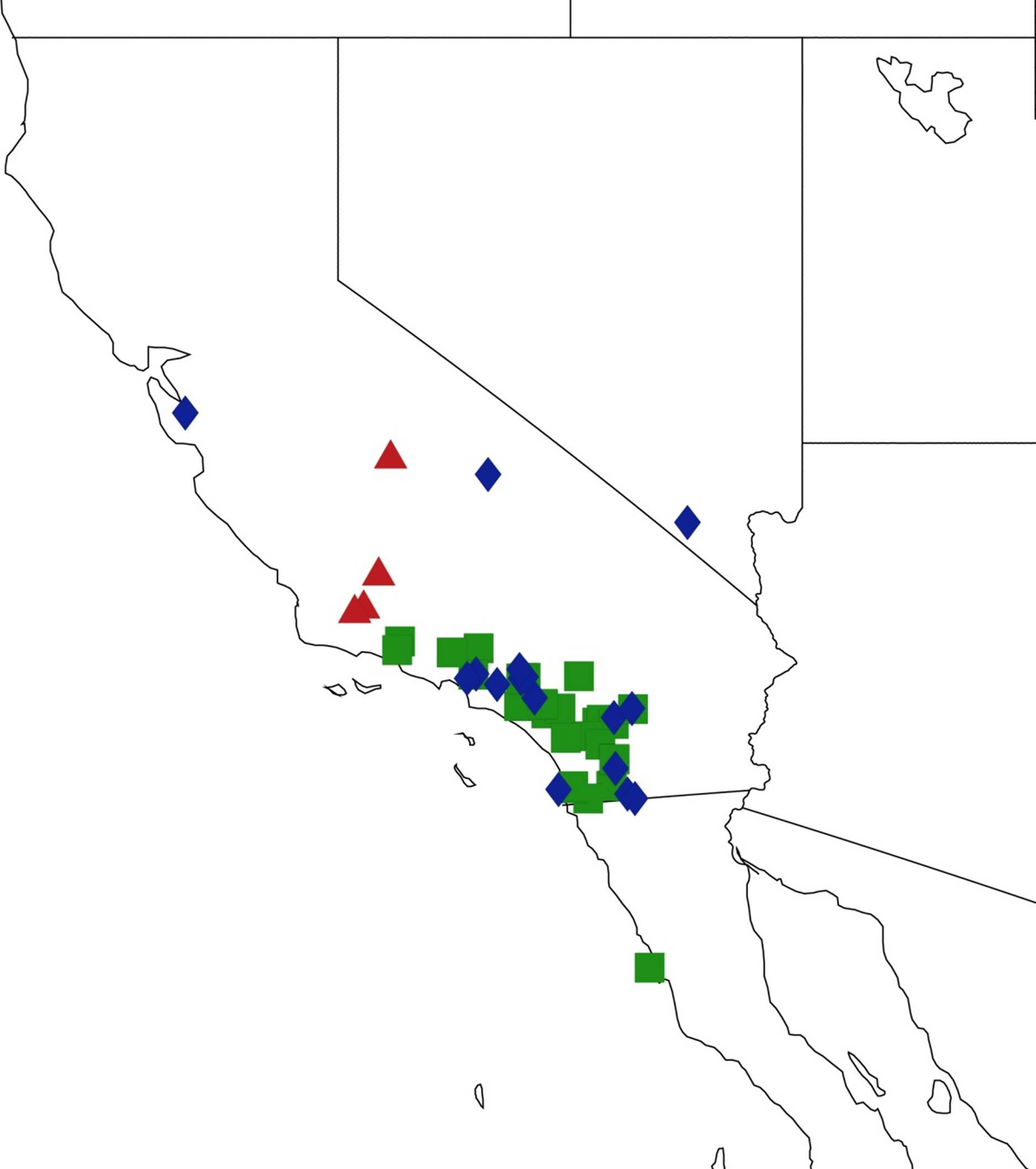
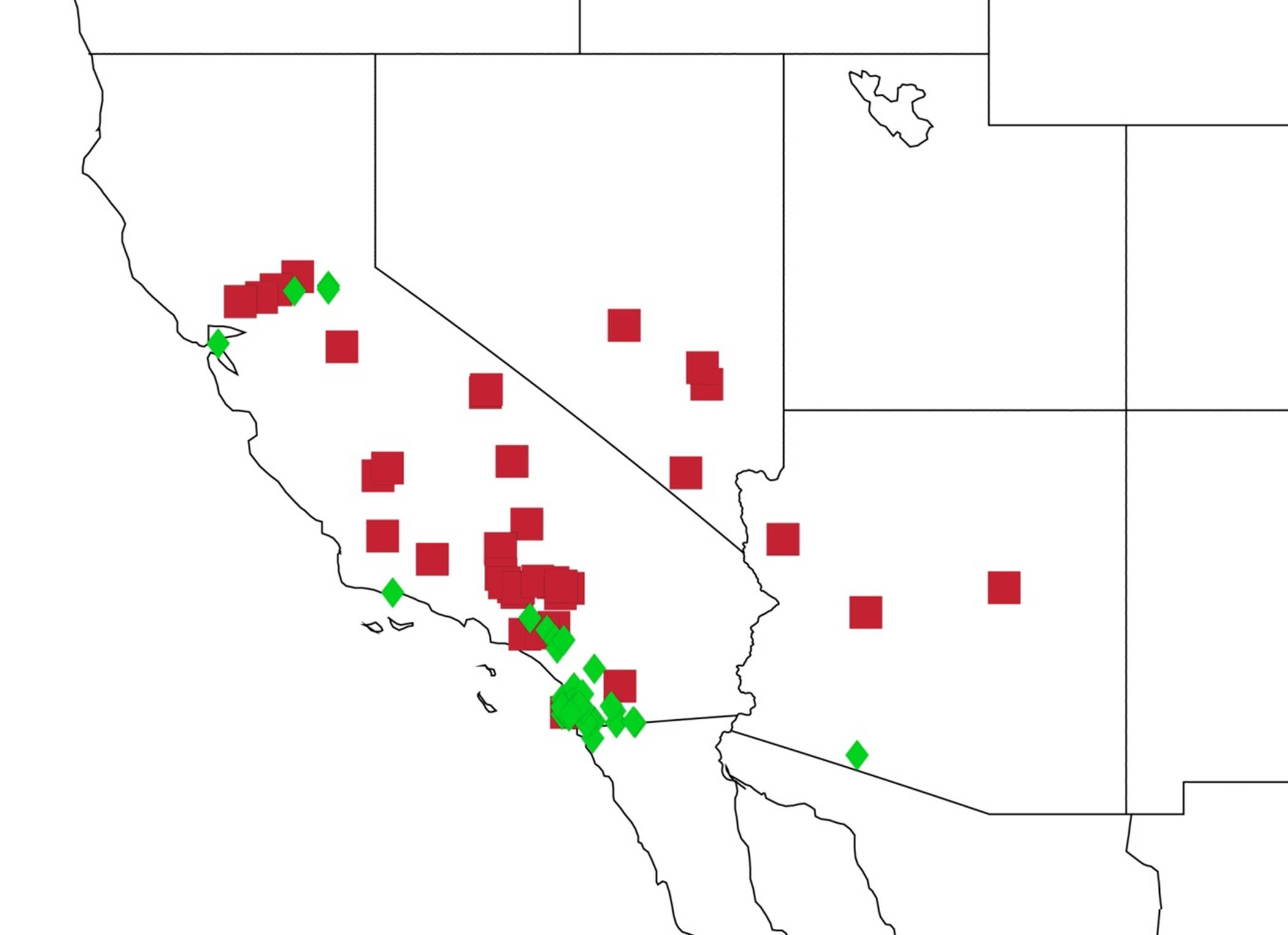
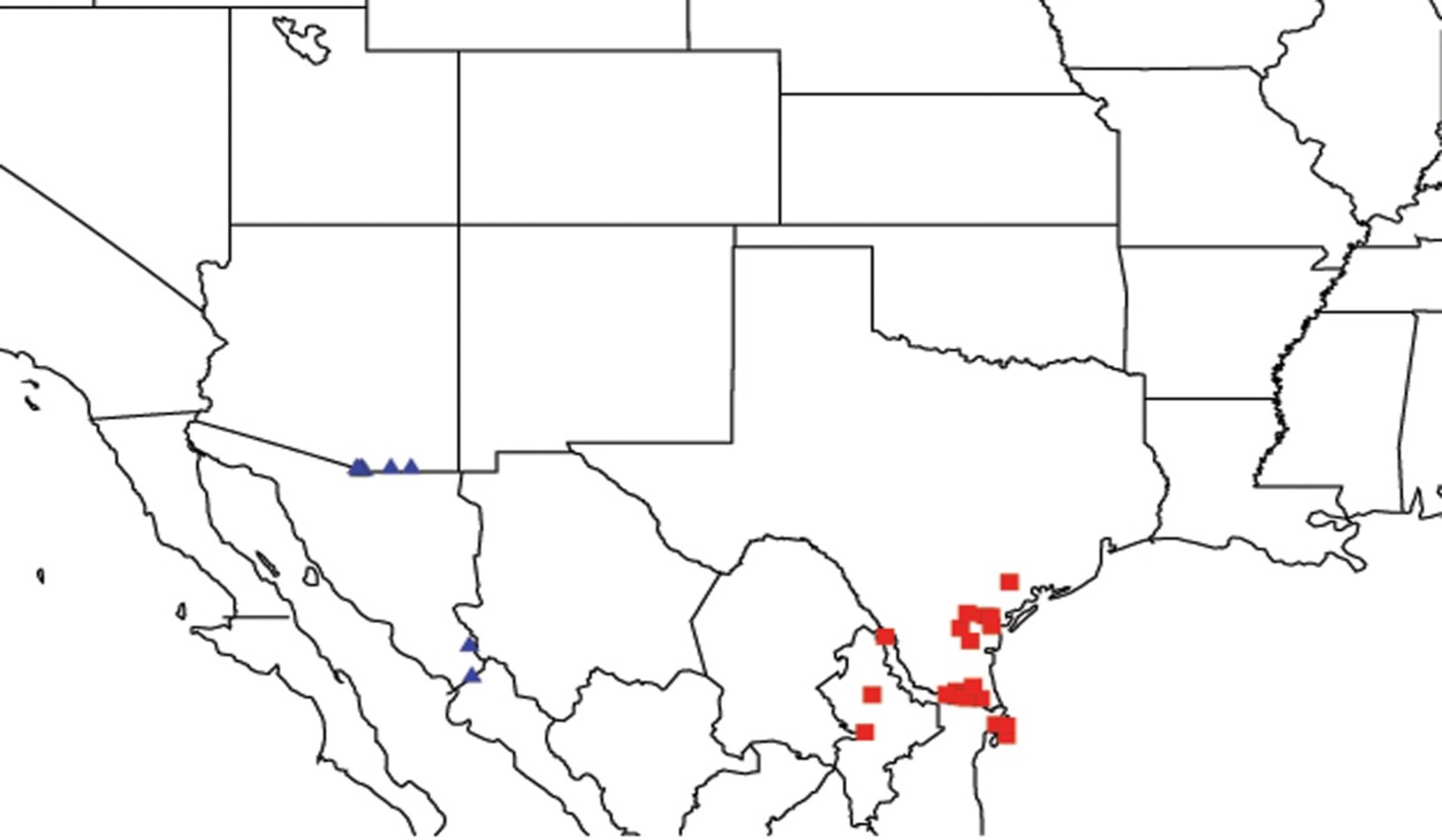
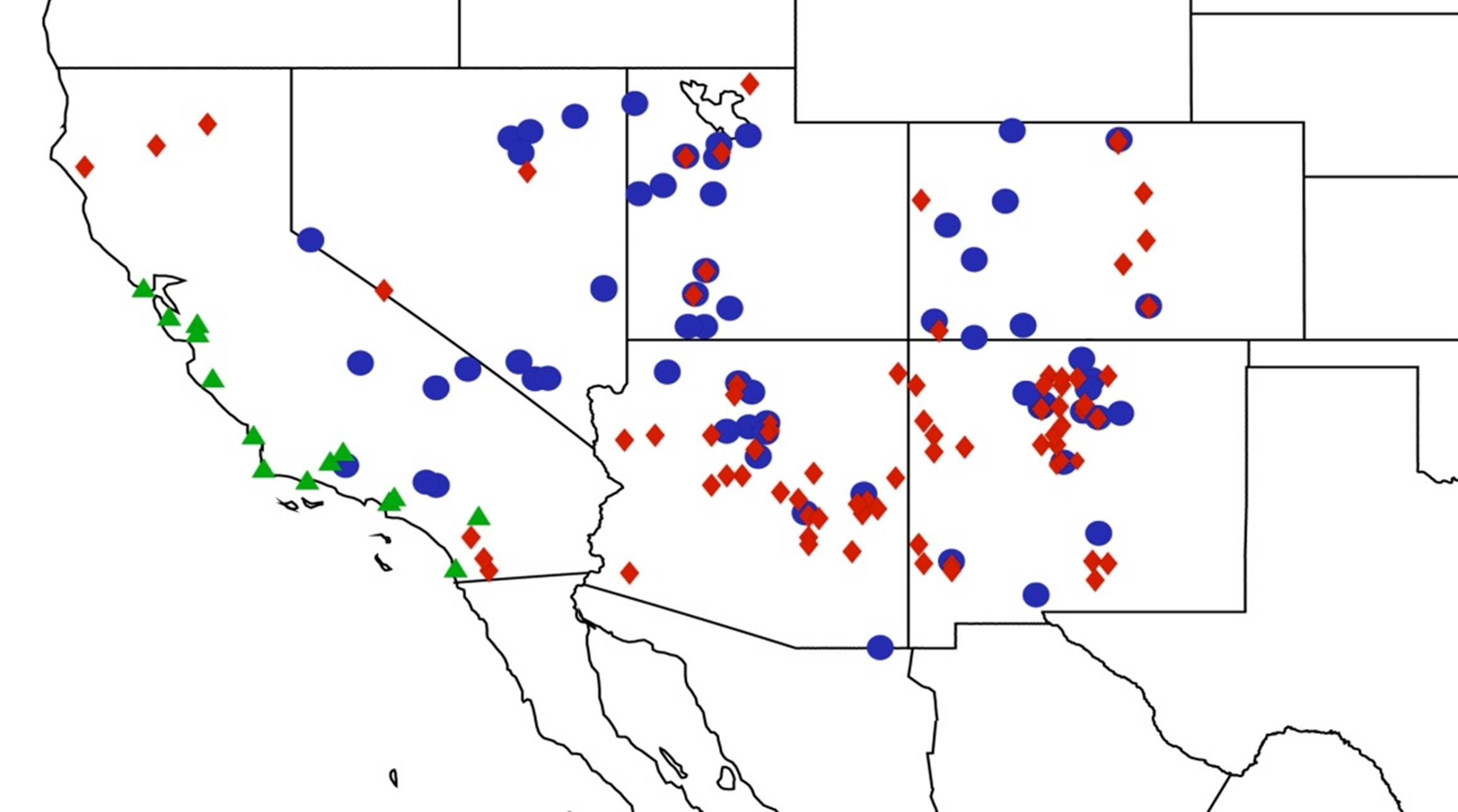
2.14. Biogeography of the Okanagana Species
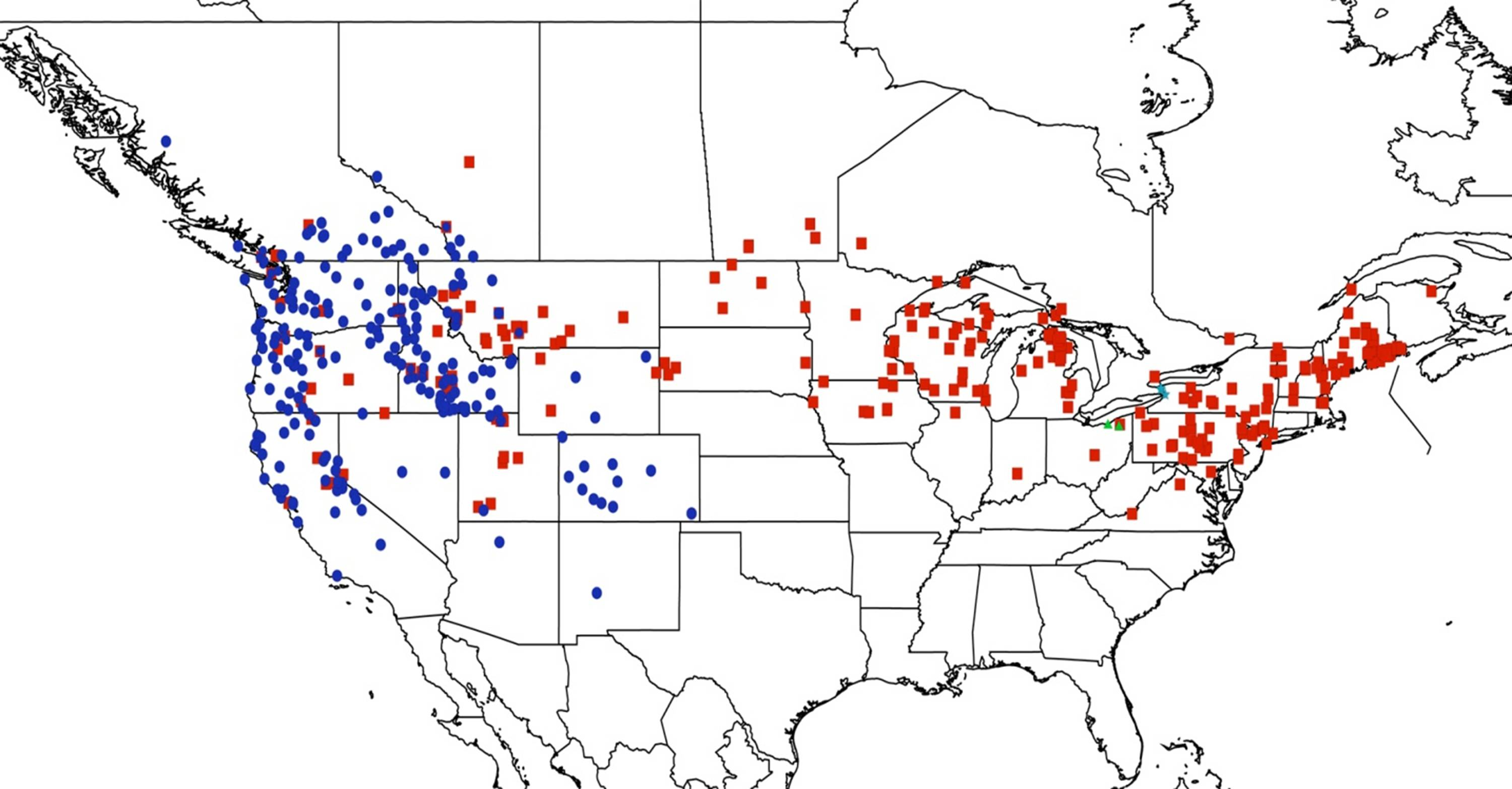
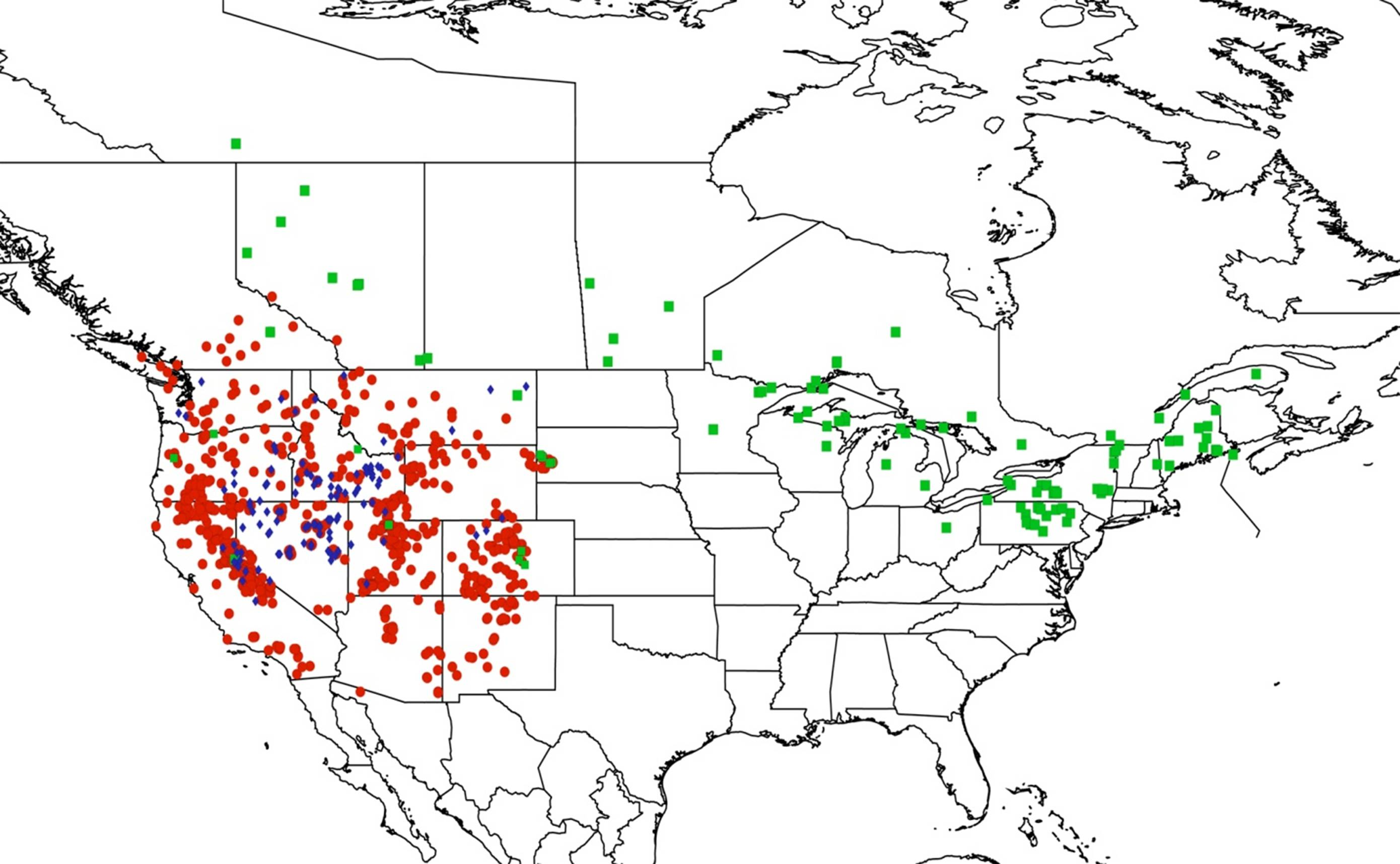

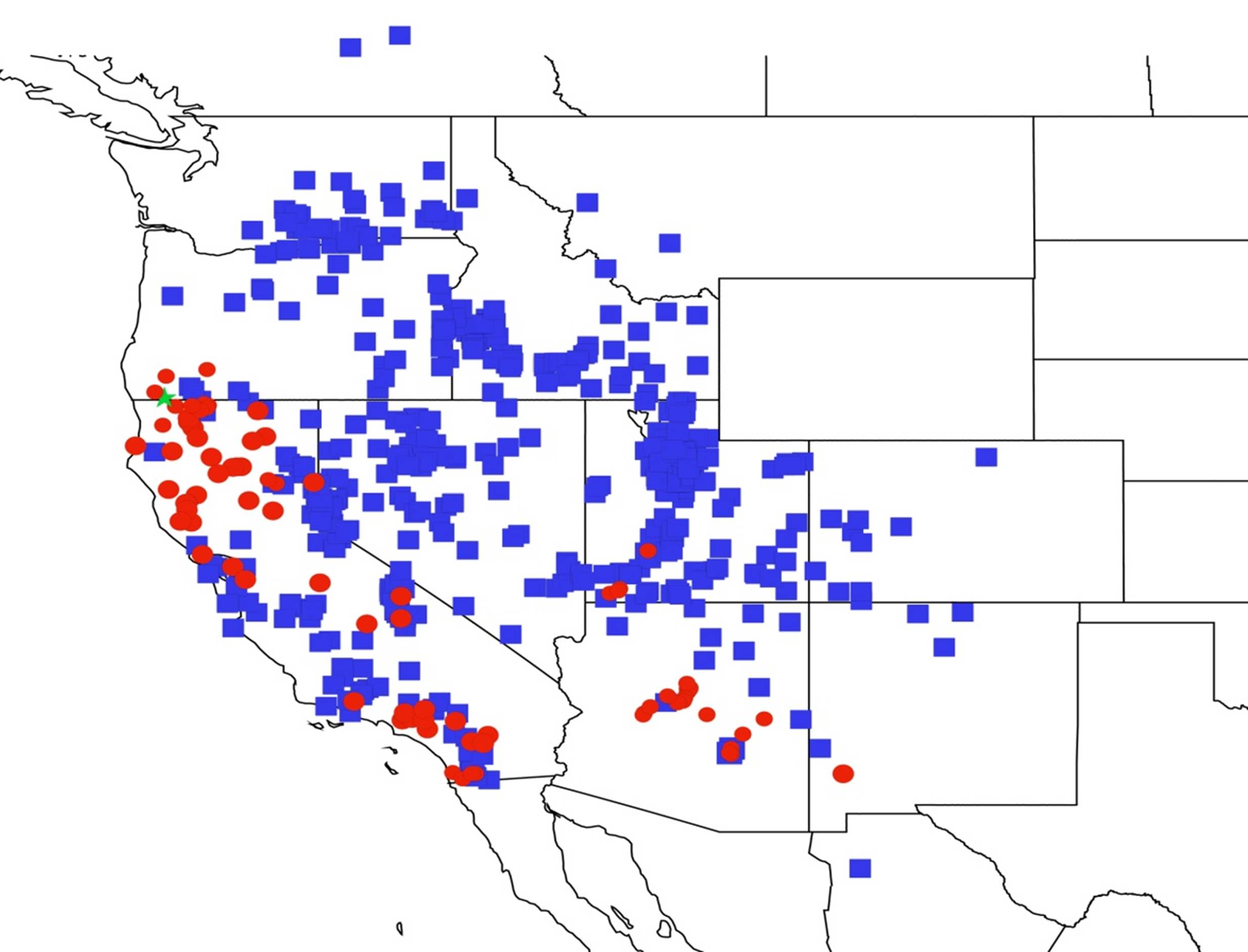
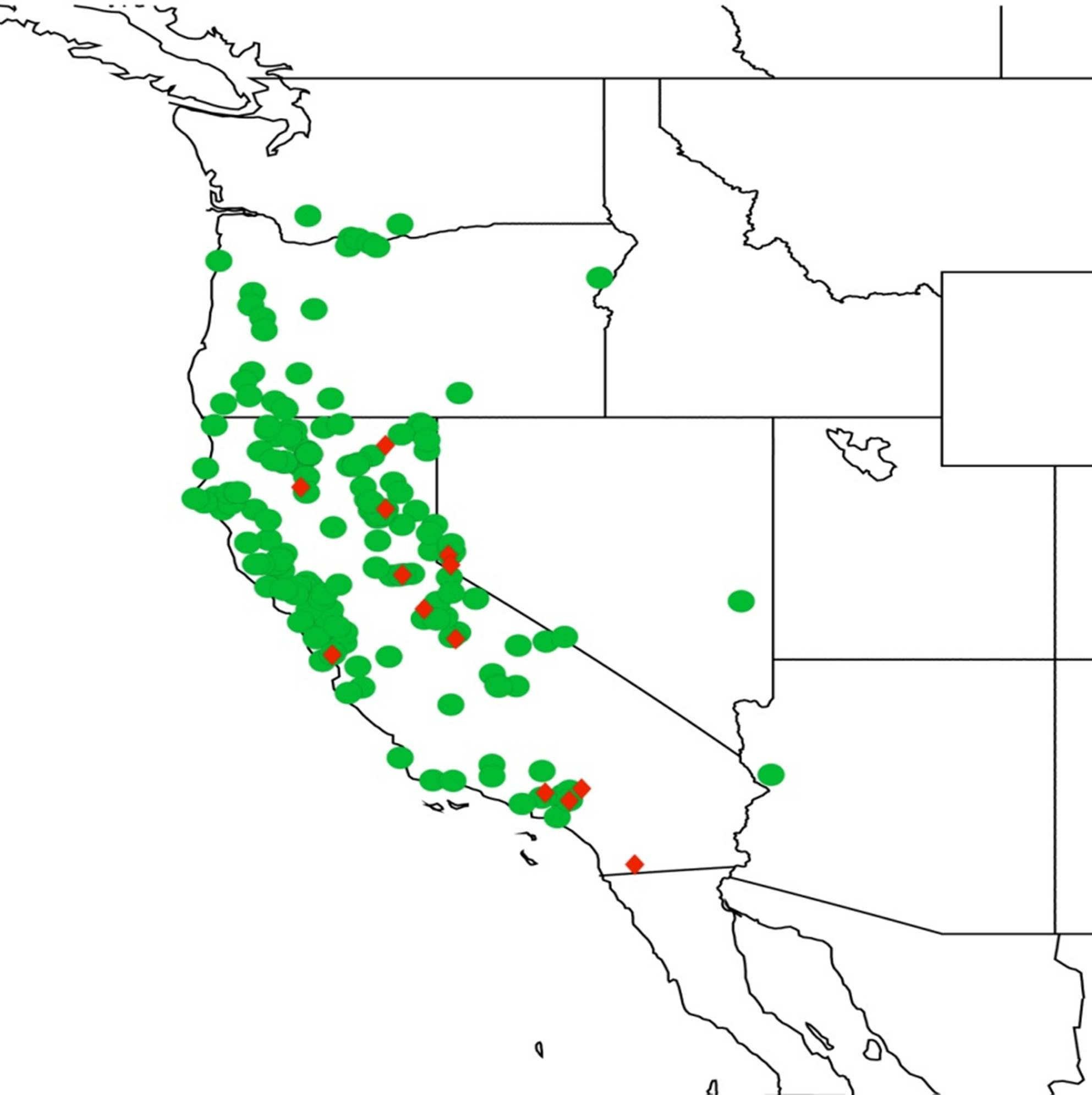
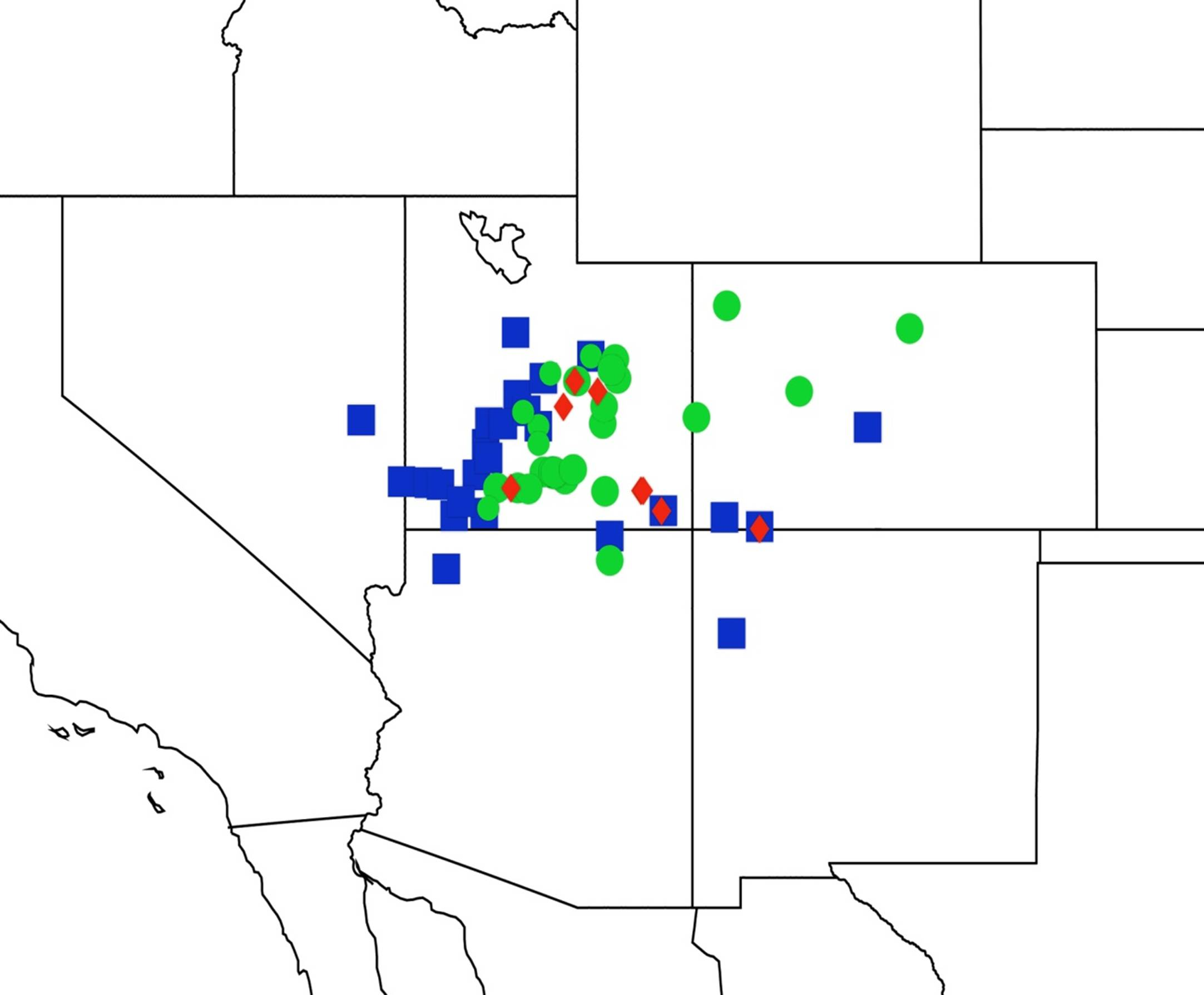
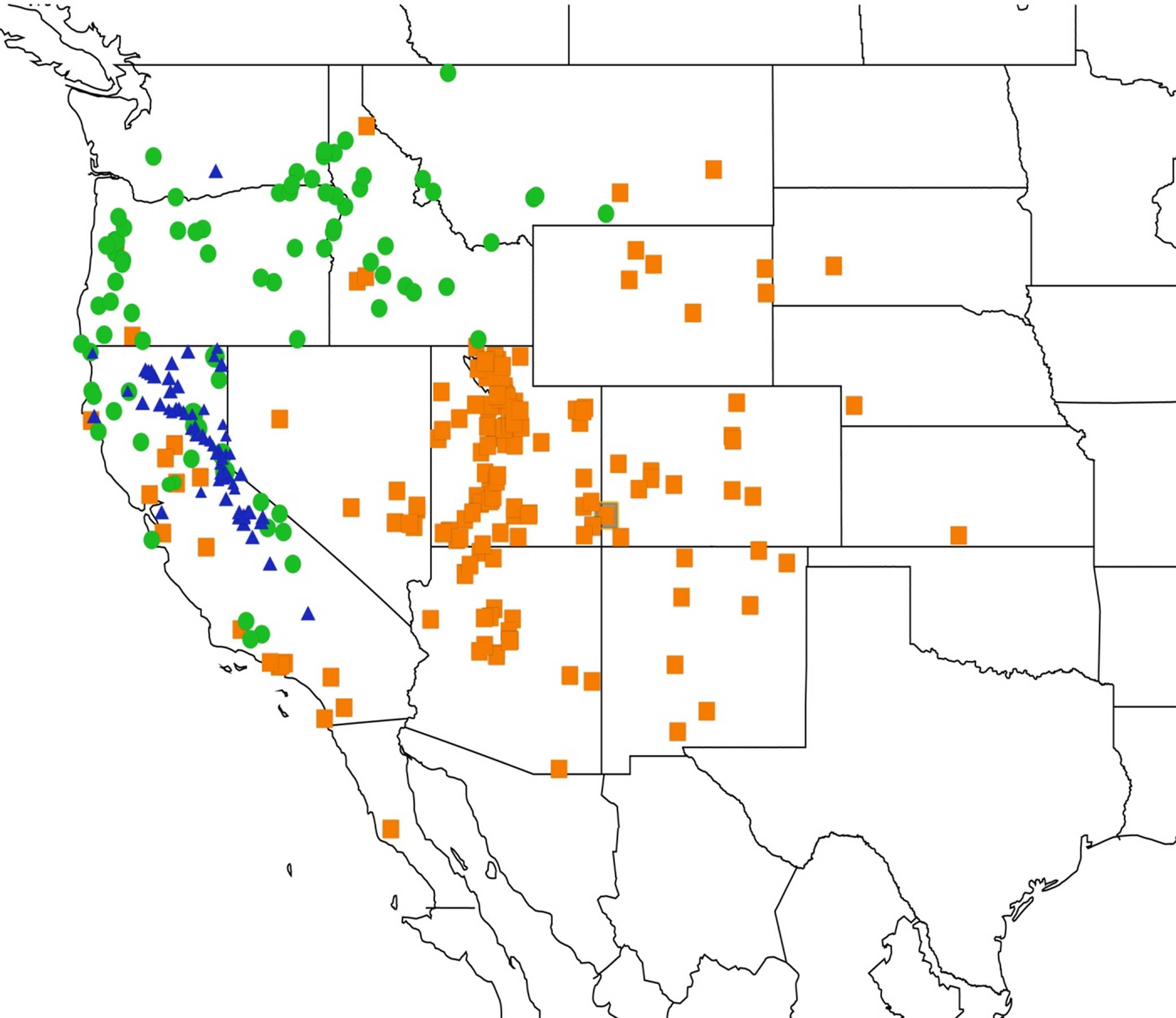
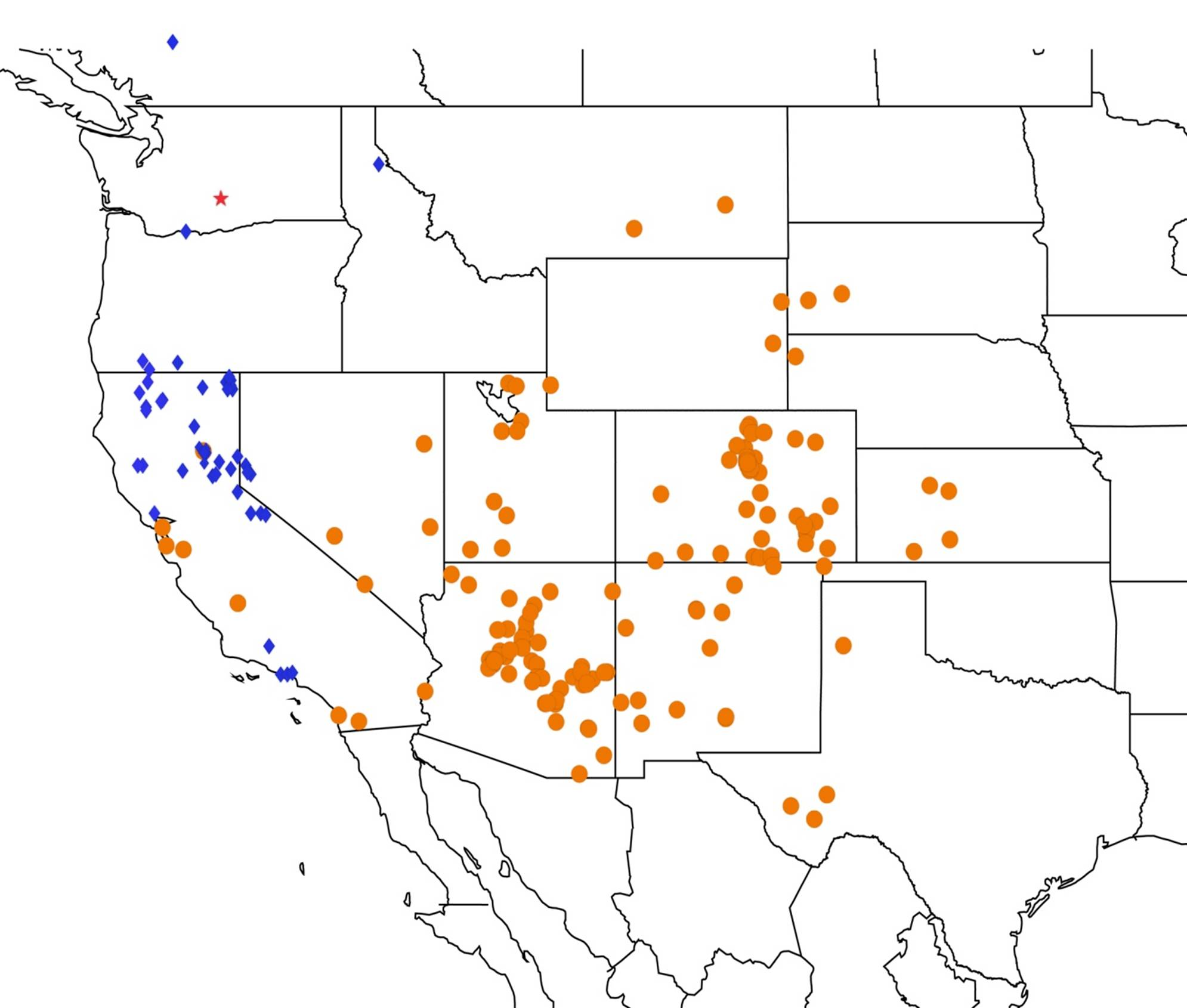
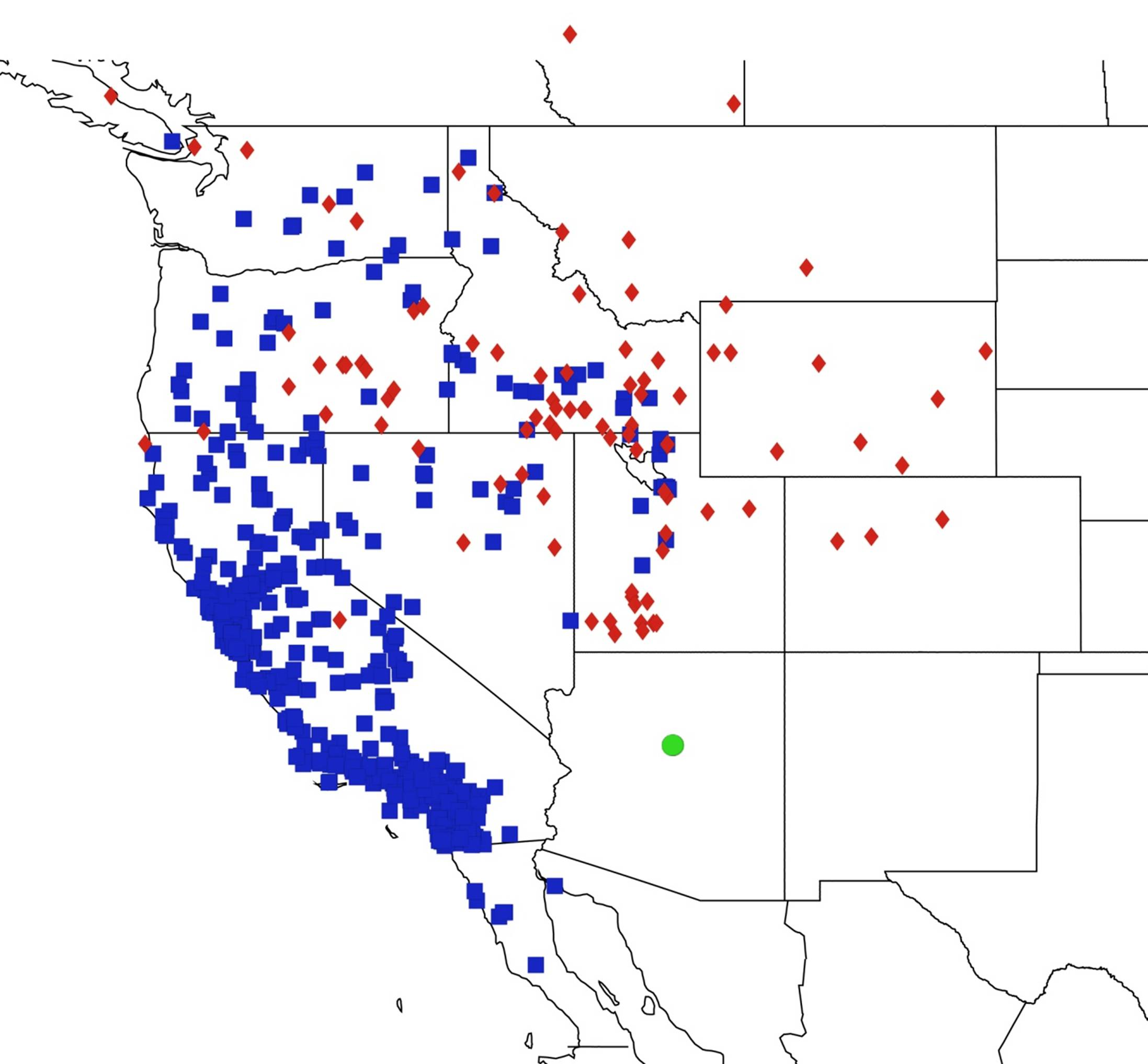
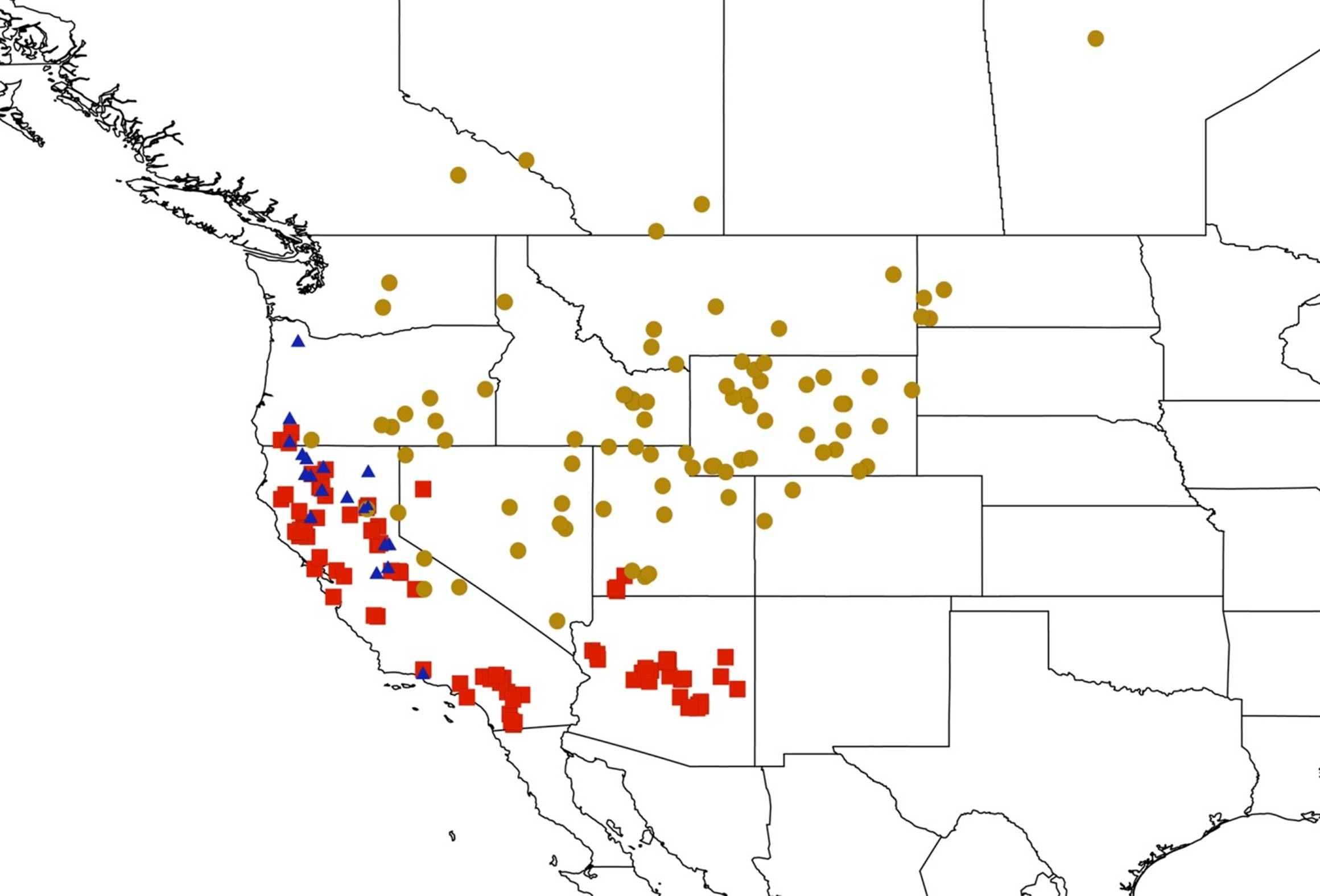
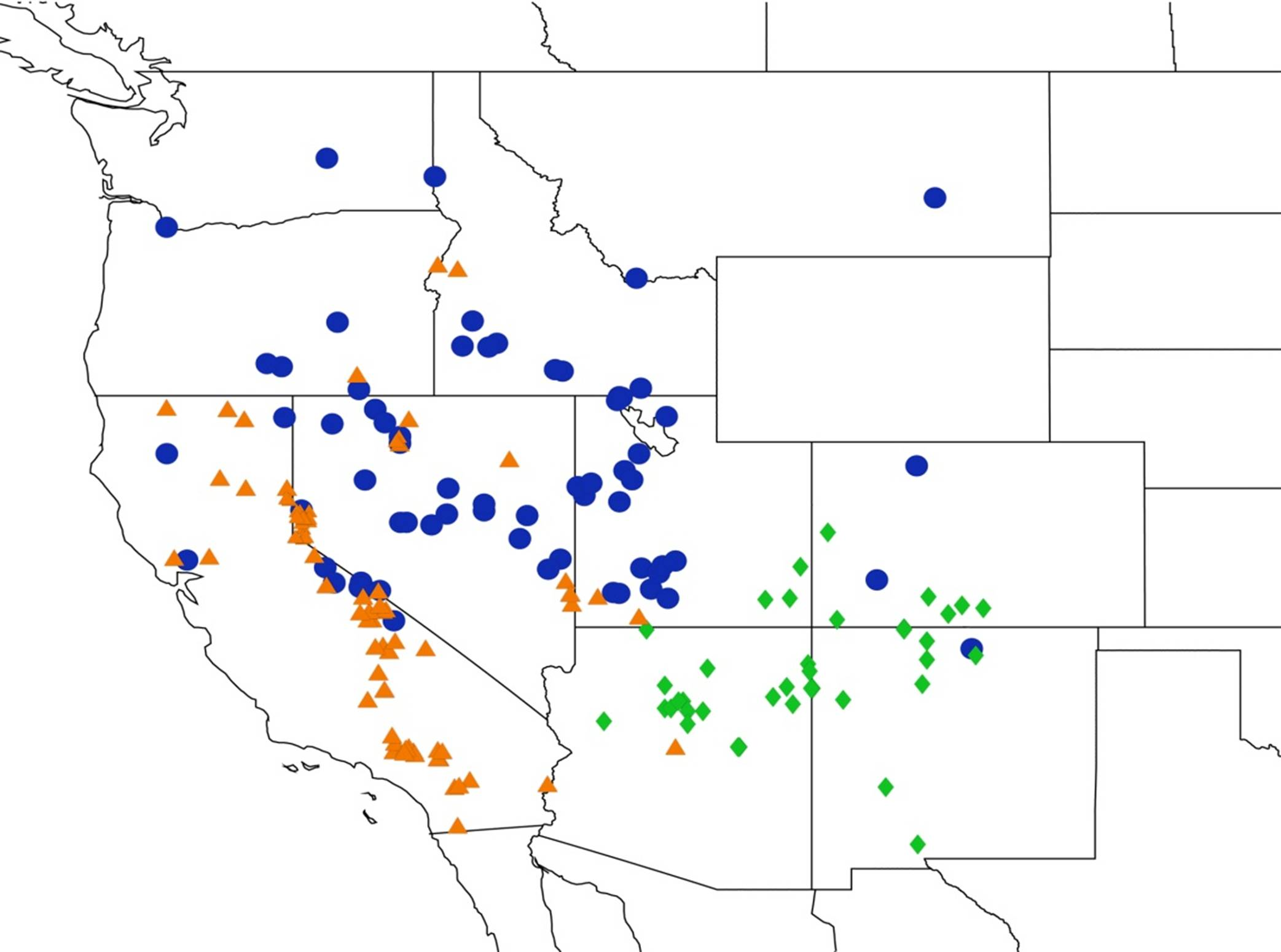

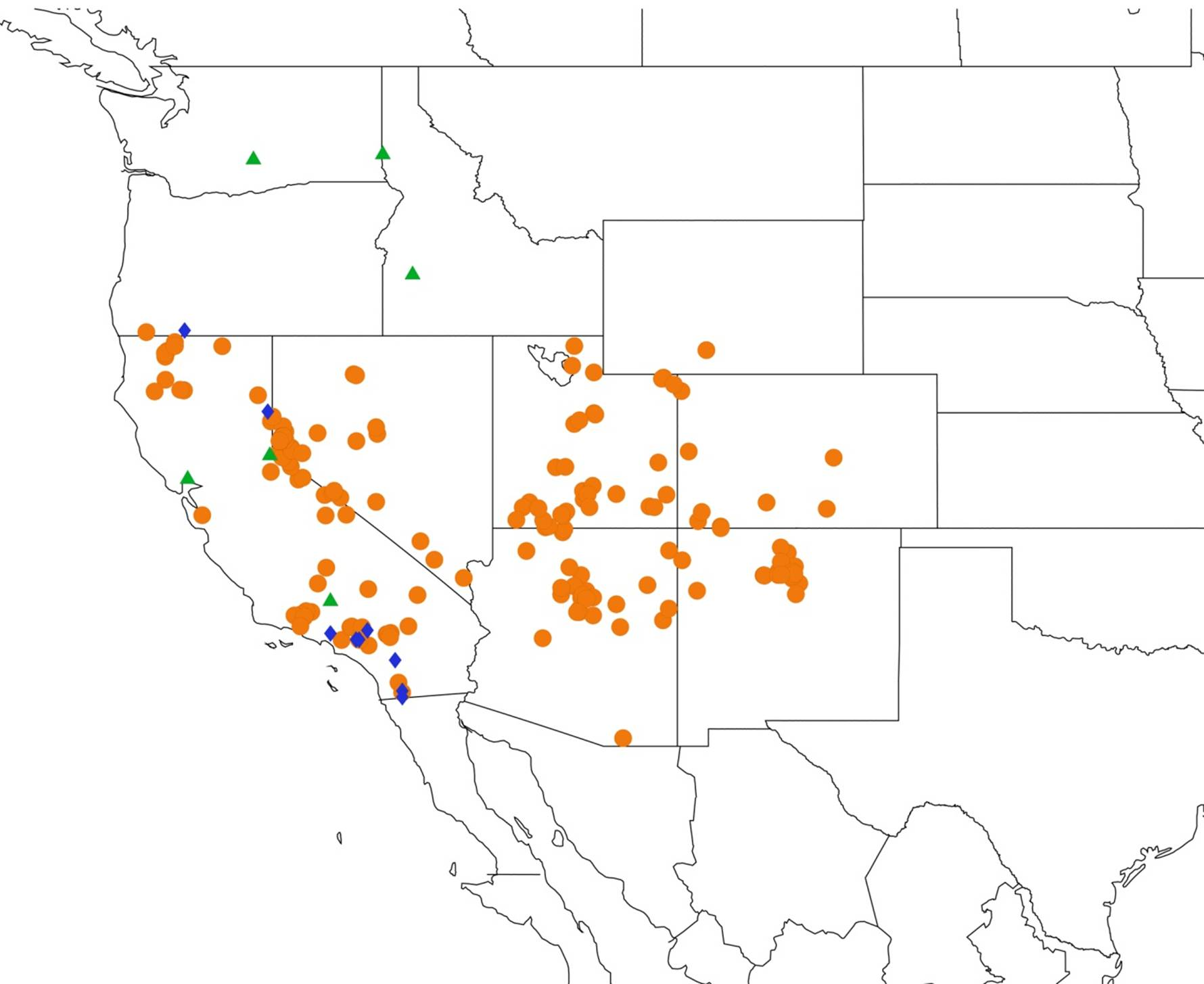
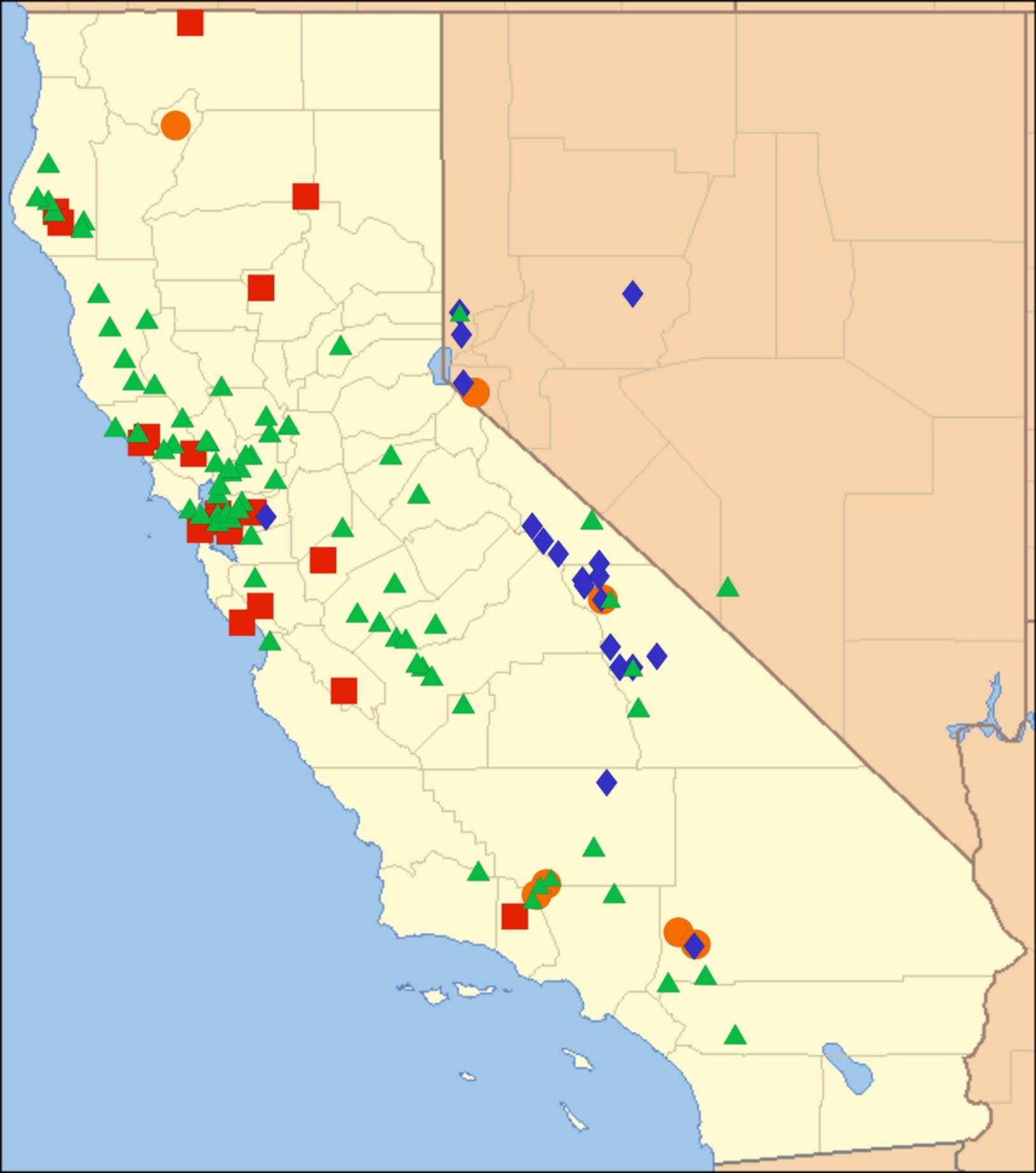
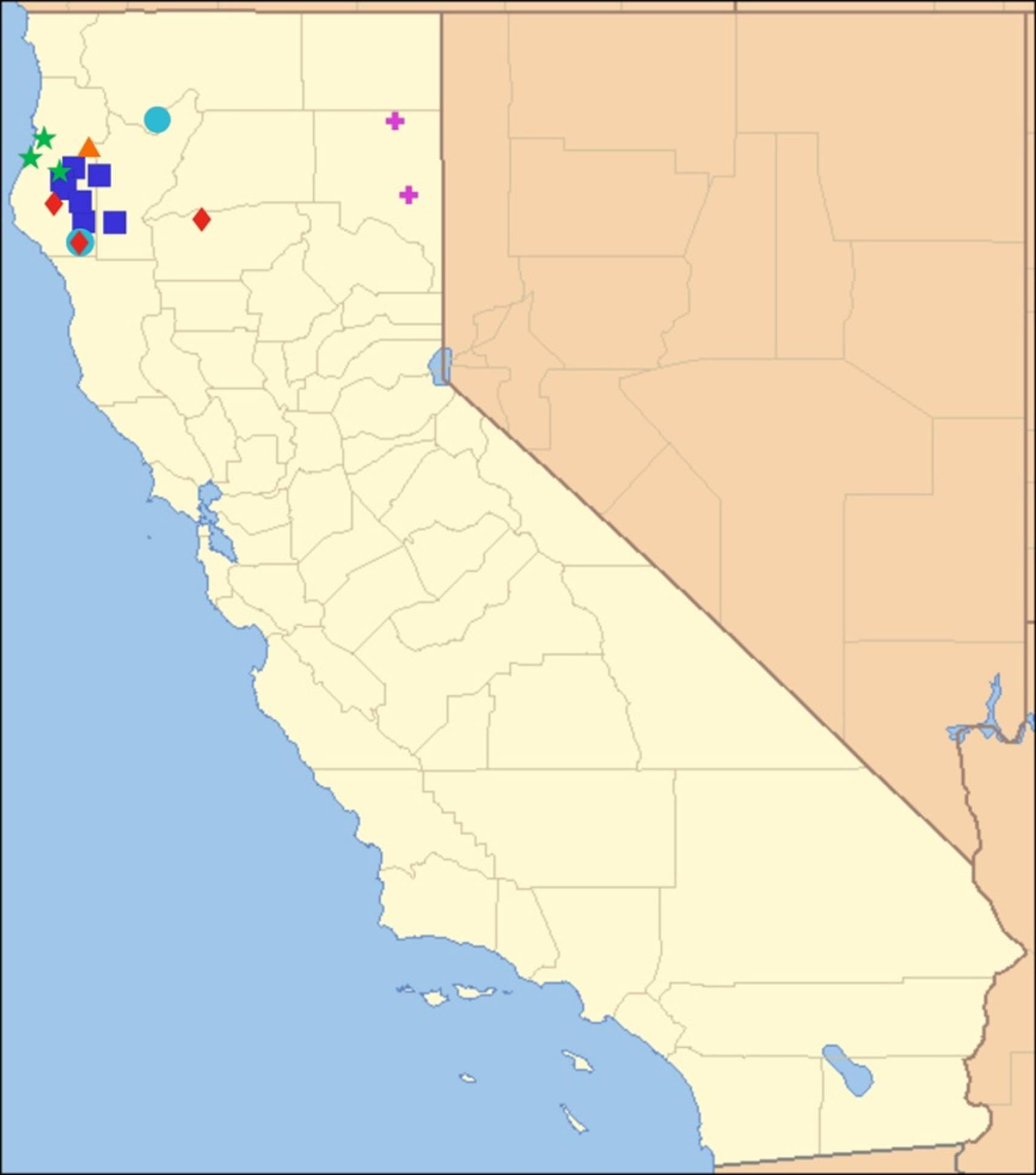
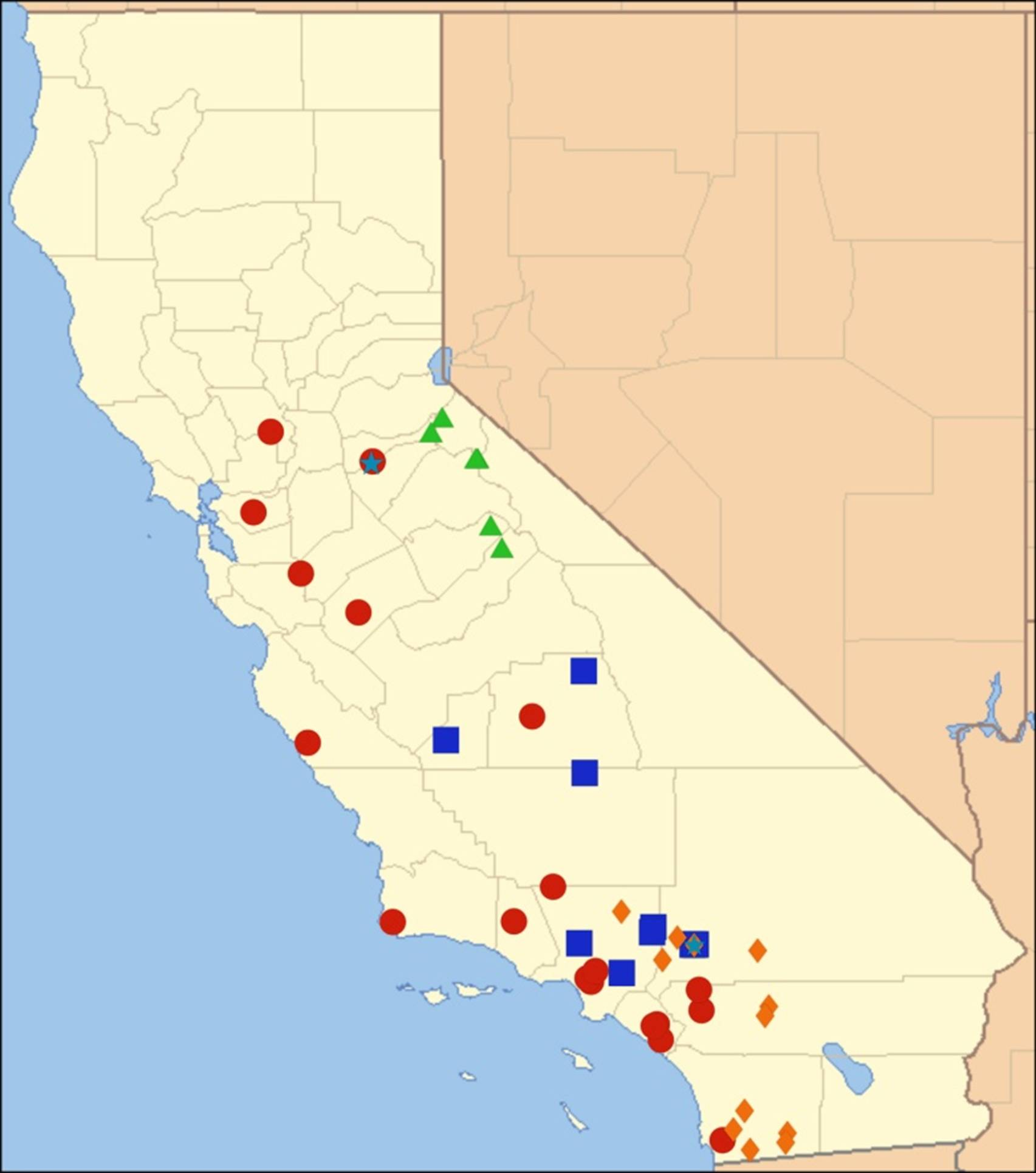
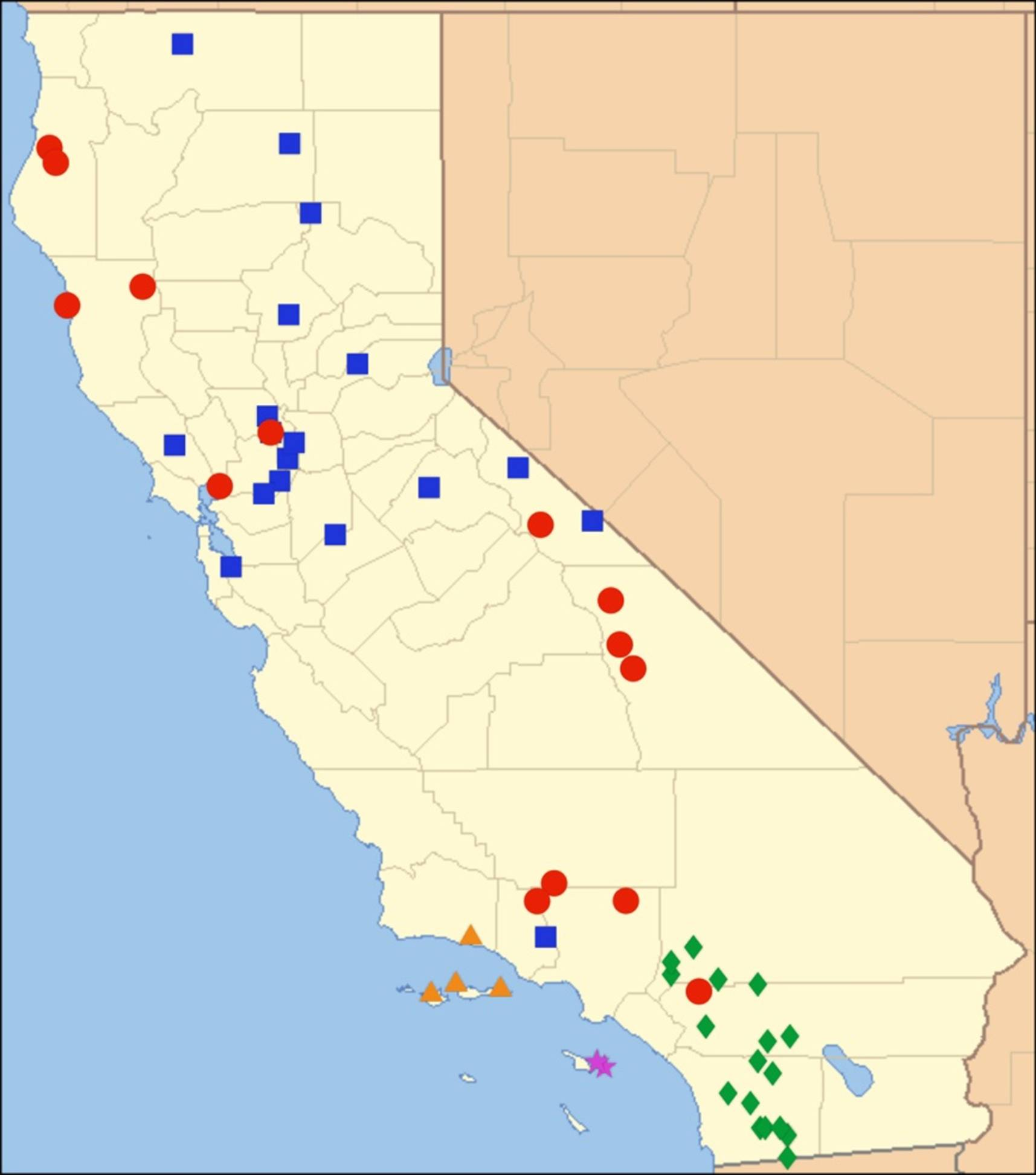
2.15. Biogeography of the Okanagodes Species
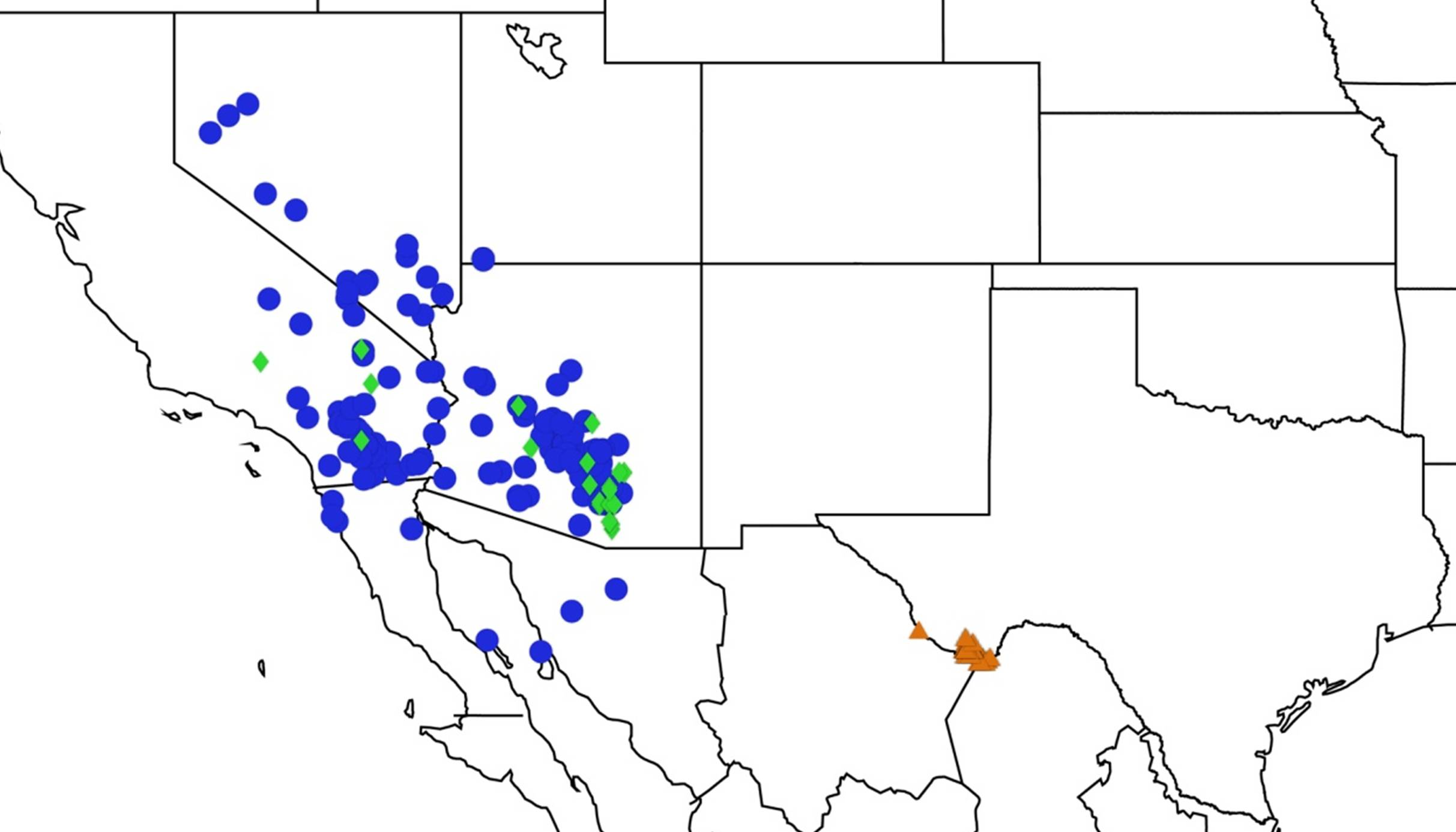
2.16. Biogeography of the Tibicinoides Species
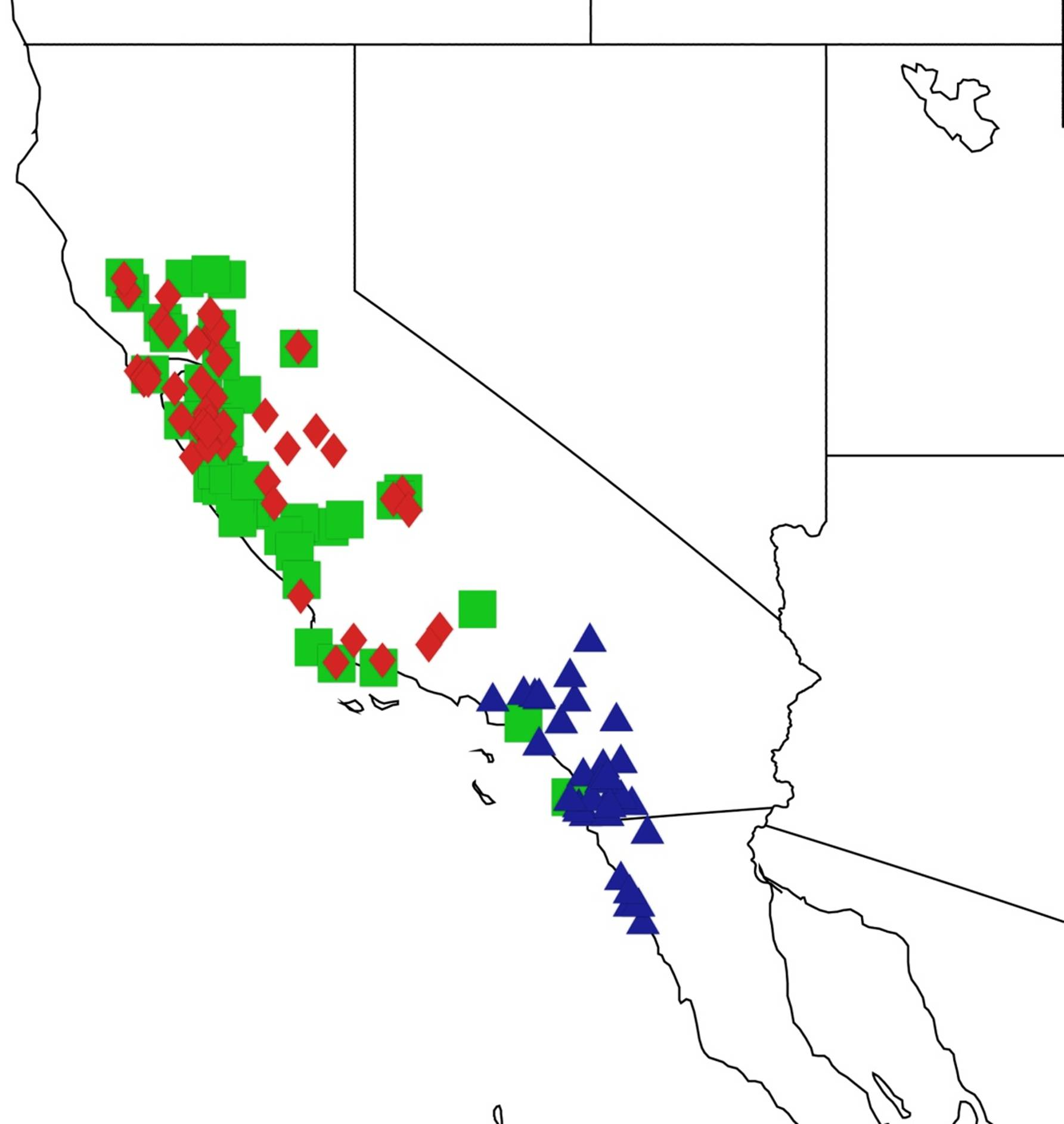
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Sanborn, A.F. A first contribution to a knowledge of the cicada fauna of El Salvador (Homoptera: Cicadoidea). Fla. Entomol. 2001, 84, 449–450. [Google Scholar] [CrossRef]
- Sanborn, A.F. Distribution of the cicadas (Homoptera: Cicadidae) of the Bahamas. Fla. Entomol. 2001, 84, 733–734. [Google Scholar] [CrossRef]
- Sanborn, A.F. Two new cicada Zammara. species from South America (Hemiptera: Cicadoidea:Cicadidae). Fla. Entomol. 2004, 87, 365–371. [Google Scholar] [CrossRef]
- Sanborn, A.F. Fidicina. variegata, a new cicada species from Costa Rica (Hemiptera: Cicadomorpha: Cicadidae). Ann. Entomol. Soc. Am. 2005, 98, 187–190. [Google Scholar] [CrossRef]
- Sanborn, A.F. New records for the cicada fauna from four Central American countries (Hemiptera: Cicadoidea: Cicadidae). Fla. Entomol. 2006, 89, 75–79. [Google Scholar] [CrossRef]
- Sanborn, A.F. New records of cicadas from Mexico (Hemiptera: Cicadoidea: Cicadidae). Southwest. Nat. 2006, 51, 255–257. [Google Scholar] [CrossRef]
- Sanborn, A.F. Additions to the cicada fauna of Venezuela with the description of a new species and checklist of the Venezuelan cicada fauna (Hemiptera: Cicadoidea: Cicadidae). Zootaxa. 2007, 1503, 21–32. [Google Scholar]
- Sanborn, A.F. New species, new records and checklist of cicadas from Mexico (Hemiptera: Cicadoidea: Cicadidae). Zootaxa. 2007, 1651, 1–42. [Google Scholar]
- Sanborn, A.F. New records of Brazilian cicadas including the description of a new species (Hemiptera: Cicadoidea, Cicadidae). Neotrop. Entomol. 2008, 37, 685–690. [Google Scholar]
- Sanborn, A.F. Checklist, new species and key to the cicadas of Cuba (Hemiptera: Cicadoidea: Cicadidae). Deut. Entomol. Z. 2009, 59, 85–92. [Google Scholar]
- Sanborn, A.F. A new species of Cicadetta. (Hemiptera: Cicadomorpha: Cicadidae) from Hispaniola. Caribb. J. Sci. 2009, 45, 1–7. [Google Scholar]
- Sanborn, A.F. Redescription and neotype designation for Okanagana. noveboracensis (Emmons, 1854) (Hemiptera: Cicadidae). P. Entomol. Soc. Wash. 2009, 111, 867–873. [Google Scholar]
- Sanborn, A.F. The cicadas of Colombia including new records and the description of a new species (Hemiptera: Cicadidae). J. Nat. Hist. 2010, 44, 1577–1607. [Google Scholar] [CrossRef]
- Sanborn, A.F. Two new species and new records of cicadas from Central America (Hemiptera: Cicadoidea: Cicadidae). Stud. Neotrop. Fauna E. 2010, 45, 67–76. [Google Scholar] [CrossRef]
- Sanborn, A.F. Checklist of the cicadas (Insecta: Hemiptera: Cicadidae) of Paraguay including new records for six species. Check List 2011, 7, 465–467. [Google Scholar]
- Sanborn, A.F. A new species of Orellana. Distant from Brazil (Hemiptera: Cicadoidea: Cicadidae). P. Entomol. Soc. Wash. 2011, 113, 377–384. [Google Scholar]
- Sanborn, A.F. Checklist of the cicadas of French Guiana including new records and the description of nine new species (Insecta, Hemiptera, Cicadoidea, Cicadida. Zoosystema. 2011, 33, 377–418. [Google Scholar] [CrossRef]
- Heath, M.S.; Sanborn, A.F. A new species of cicada of the genus Okanagana. (Hemiptera: Cicadoidea: Cicadidae) from Arizona. Ann. Entomol. Soc. Am. 2007, 100, 483–489. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Heath, M.S.; Sueur, J.; Phillips, P.K. The genus Neocicada. Kato, 1925 (Hemiptera: Cicadomorpha: Cicadidae), with descriptions of three new species. Syst. Entomol. 2005, 30, 191–207. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Moore, T.E.; Young, A.M. Two new cicada species from Costa Rica (Hemiptera: Cicadomorpha: Cicadidae) with a key to Fidicinoides. in Costa Rica. Zootaxa. 2008, 1846, 1–20. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K.; Gillis, P. The cicadas of Florida (Hemiptera: Cicadoidea: Cicadidae). Zootaxa. 2008, 1916, 1–43. [Google Scholar]
- Sanborn, A.F.; Heath, J.E.; Phillips, P.K.; Heath, M.S.; Noriega, F.G. Thermal adaptation and diversity in tropical ecosystems: Evidence from cicadas (Hemiptera, Cicadidae). PLoS One 2011, 6, e29368. [Google Scholar]
- Sanborn, A.F.; Heath, M.S.; Phillips, P.K.; Heath, J.E. A new species of the genus Beameri. (Hemiptera: Cicadidae) from North America. J. Nat. Hist. 2011, 45, 1589–1605. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Heath, M.S.; Phillips, P.K.; Heath, J.E. The genus Cacama. Distant, 1904 (Hemiptera: Cicadidae) with the description of three new species. Zootaxa. 2011, 2897, 35–50. [Google Scholar]
- Sanborn, A.F.; Maes, J.-M. Checklist of the cicadas (Insecta: Hemiptera: Cicadidae) of Nicaragua including new records for seventeen species. Check List 2012, 8, 437–442. [Google Scholar]
- Sanborn, A.F.; Heath, M.S. Catalogue of the Cicadas (Hemiptera: Cicadoidea: Cicadidae) of Continental North America North of Mexico. Thomas Say Publications in Entomology: Mongraphs.; Entomological Society of America: Lanham, MD, USA, 2012; p. 227. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K. Re-evaluation of the Diceroprocta. delicata species complex (Homoptera: Cicadidae). Ann. Entomol. Soc. Am. 2001, 94, 159–165. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Phillips, P.K. Neotype and allotype description of Tibicen. superbus (Hemiptera: Cicadomorpha: Cicadidae) with description of its biogeography and calling song. Ann. Entomol. Soc. Am. 2004, 97, 647–652. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Phillips, P.K. Reevaluation of the Diceroprocta. texana species complex (Hemiptera: Cicadoidea: Cicadidae). Ann. Entomol. Soc. Am. 2010, 103, 860–865. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Phillips, P.K. Elevation of a subspecies of Tibicen. (Hemiptera: Cicadoidea: Cicadidae) to a full species. Southwest. Nat. 2011, 56, 363–368. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Phillips, P.K. Ecology, acoustic behavior and morphlogy of the cicada Cornuplura. nigroalbata (Hemiptera: Cicadidae). Ann. Entomol. Soc. Am. 2012, 56, 879–883. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Heath, M.S.; Phillips, P.K.; Heath, J.E. The genus Pacarina. Distant, 1905(Hemiptera: Cicadidae) with the description of a new species. J. Nat. Hist. 2012, 46, 923–941. [Google Scholar] [CrossRef]
- Barber, H.G. Insects of Florida Hemiptera-Homoptera. B. Am. Mus. Nat. Hist. 1914, 33, 495–535. [Google Scholar]
- Davis, W.T. Mississippi cicadas, with a key to the species of the southeastern United States. J. New York Entomol. Soc. 1918, 26, 141–155. [Google Scholar]
- Lawson, P.B. The Cicadidae of Kansas. U. KS Sci. B. 1920, 12, 307–376. [Google Scholar]
- Davis, W.T. An annotated list of the cicadas of Colorado with descriptions of a new species. J. New York Entomol. Soc. 1921, 29, 43–57. [Google Scholar]
- Osborn, H. Homoptera of Florida. Fla. Entomol. 1921, 5, 1–19. [Google Scholar] [CrossRef]
- Davis, W.T. An annotated list of the cicadas of Virginia with description of a new species. J. New York Entomol. Soc. 1922, 30, 36–52. [Google Scholar]
- Davis, W.T. Family Cicadidae in Brittonʹs Guide to the Insects of Connecticut. Part IV. The Hemiptera or sucking insects of Connecticut. Conn. St. Geol. Nat. Hist. Sur. B. 1923, 34, 1–807. [Google Scholar]
- Davis, W.T. The cicadas or harvest flies of New Jersey. St. New Jersey Dept. Agr. Cir. 1926, 97, 3–26. [Google Scholar]
- Davis, W.T. Cicadas belonging to the genus Diceroprocta. with descriptions of a new species. J. New York Entomol. Soc. 1928, 36, 439–458. [Google Scholar]
- Bibby, F.F. The cicadas of Texas. MSc Thesis, Agricultural and Mechanical College of Texas, College Station, TX, USA, 1936. [Google Scholar]
- Froeschner, R.C. A synopsis of the Cicadidae of Missouri (Homoptera). J. New York Entomol. Soc. 1952, 60, 1–13. [Google Scholar]
- Simons, J.N. The cicadas of California. B. Calif. Insect Sur. 1954, 2, 153–192. [Google Scholar]
- Moore, T.E. The cicadas of Michigan (Homoptera: Cicadidae). Pap. Mich. Acad. Sci. Arts Lett. 1966, 51, 75–95. [Google Scholar]
- Alexander, R.D.; Pace, A.E.; Otte, D. The singing insects of Michigan. Great Lakes Entomol. 1972, 5, 33–69. [Google Scholar]
- Drew, W.A.; Spangler, F.L.; Molnar, D. Oklahoma Cicadidae (Homoptera). P. Okla. Acad. Sci. 1974, 54, 90–97. [Google Scholar]
- Marshall, D.C.; Cooley, J.R.; Alexander, R.D.; Moore, T.E. New records of Michigan Cicadidae (Homoptera), with notes on the use of songs to monitor range changes. Great Lakes Entomol. 1996, 29, 165–169. [Google Scholar]
- Kondratieff, B.C.; Ellington, A.; Leatherman, D.A. The cicadas (Homoptera.: Cicadidae., Tibicinidae.) of Colorado; Gillette Museum of Arthropod Diversity, Dept. of Bioagricultural Sciences and Pest Management, Colorado State University: Fort Collins, CO, USA, 2002; p. 81. [Google Scholar]
- Hill, K.B.R.; Marshall, D.C. Confirmation of the cicada Tibicen. pronotalis walkeri stat. nov. (=T. walkeri, Hemiptera: Cicadidae) in Florida: Finding singing insects through their songs. Zootaxa. 2009, 2125, 63–66. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K.; Villet, M.H. Thermal responses in some Eastern Cape African cicadas (Hemiptera: Cicadidae). J. Therm. Biol. 2003, 28, 347–351. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Heath, M.S.; Heath, J.E.; Noriega, F.G.; Phillips, P.K. Convergence and parallelism among cicadas of Argentina and the southwestern United States (Hemiptera: Cicadoidea). Biol. J. Linn. Soc. 2004, 83, 281–288. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Heath, J.E.; Heath, M.S.; Phillips, P.K. Temperature responses and habitat selection by mangrove cicadas in Florida and Queensland (Hemiptera Cicadoidea). Trop. Zool. 2004, 17, 65–72. [Google Scholar]
- Puissant, S.; Defaut, B. Les synusies de cigales en France (Hemiptera, Cicadidae). Mat. Orthopt. Entomocénot. 2005, 10, 115–129. [Google Scholar]
- Puissant, S. Contribution a la Connaissance. des Cigales. de France: Geonemie. et Ecologie. des Populations (Hemiptera., Cicadidae.); Association pour la Caractérisation et lʹEtude des Entomocénoses, Bedeilhac et Aynat: Paris, France, 2006; p. 193. [Google Scholar]
- Phillips, P.K.; Sanborn, A.F. Phytogeography influences biogeography of the Cicadidae. Acta. Zool. Sinica. 2007, 53, 454–462. [Google Scholar]
- Sueur, J.; Puissant, S. Similar look but different song: A new Cicadetta. species in the montana complex (Insecta, Hemiptera, Cicadidae. Zootaxa. 2007, 1442, 55–68. [Google Scholar]
- International Commission Of Zoological Nomenclature, International Code of Zoological Nomenclature, 4th ed; International Trust for Zoological Nomenclature: London, UK, 1999; p. 306.
- Van Duzee, E.P. Check list of the Hemiptera. (excepting the Aphididae., Aleurodidae. and Coccidae.) of America, North. of Mexico; New York Entomological Society: New York, NY, USA, 1916; p. 111. [Google Scholar]
- Davis, W.T. The distribution of cicadas in the United States with descriptions of new species. J. New York Entomol. Soc. 1930, 38, 53–73. [Google Scholar]
- Davis, W.T. Six new cicadas from the Western United States. J. New York Entomol. Soc. 1935, 43, 299–310. [Google Scholar]
- Simons, J.N. New California cicadas with taxonomic notes on other species. Pan-Pac. Entomol. 1953, 29, 191–198. [Google Scholar]
- Miller, S.E. The California Channel Islands—Past, present, and future: An entomological perspective. In Entomology of the California Channel Islands. Proceedings of the First Symposium; Menke, A.S., Miller, D.R., Eds.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1985; pp. 3–27. [Google Scholar]
- Van Duzee, E.P. A preliminary list of the Hemiptera of San Diego County, California. Trans. San Diego Soc. Nat. Hist. 1914, 2, 1–57. [Google Scholar]
- Davis, W.T. Cicadas of the genus Cacama., with descriptions of several new species. J. New York Entomol. Soc. 1919, 27, 68–79. [Google Scholar]
- Beamer, L.D.; Beamer, R.H. Biological notes on some western cicadas. J. New York Entomol. Soc. 1930, 38, 291–305. [Google Scholar]
- Heath, J.E.; Hanagan, J.L.; Wilkin, P.J.; Heath, M.S. Adaptation of the thermal responses of insects. Am. Zool. 1971, 11, 147–158. [Google Scholar]
- Heath, J.E.; Wilkin, P.J.; Heath, M.S. Temperature responses of the cactus dodger, Cacama. valvata (Homoptera, Cicadidae). Physiol. Zool. 1972, 45, 238–246. [Google Scholar]
- Essig, E.O. Insects and Mites of Western North. America; Macmillan Company: New York, NY, USA, 1958; p. 1050. [Google Scholar]
- Sims, P.L. Grasslands. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 265–286. [Google Scholar]
- Barbour, M.G. California Upland Forests and Woodlands. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 131–164. [Google Scholar]
- Christensen, N.L. Vegetation of the Southeastern Coastal Plain. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 317–364. [Google Scholar]
- MacMahon, J.A. Warm Deserts. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 232–264. [Google Scholar]
- Keeley, J.E.; Keeley, S.C. Chaparral. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 165–208. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K.; Heath, J.E.; Heath, M.S. Comparative thermal adaptation in cicadas (Hemiptera: Cicadidae) inhabiting Mediterranean ecosystems. J. Therm. Biol. 2011, 36, 150–155. [Google Scholar] [CrossRef]
- West, N.E. Intermountain Deserts, Shrub Steppes, and Woodlands. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 209–230. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K. Thermal responses of the Diceroprocta. cinctifera species group (Homoptera: Cicadidae). Southwest. Nat. 1996, 41, 136–139. [Google Scholar]
- Davis, W.T. Additional records of North American cicadas with descriptions of new species. J. New York Entomol. Soc. 1932, 40, 241–265. [Google Scholar]
- Stucky, B.J. Discovery of the cicada Diceroprocta. azteca in Kansas, with notes on its ecology (Hemiptera: Cicadidae). Trans. KS Acad. Sci. 2009, 112, 123–126. [Google Scholar] [CrossRef]
- Davis, W.T. Sonoran cicadas collected by Harry H. Knight, Dr. Joseph Bequaert and others, with descriptions of a new species. J. New York Entomol. Soc. 1917, 25, 203–215. [Google Scholar]
- Griffith, G.E.; Omernik, J.M.; Rohm, C.M.; Pierson, S.M. Florida Regionalization Project; EPA/Q-95/002; Environmental Research Laboratory, US Environmental Protection Agency: Corvallis, OR, USA, 1994; p. 83. [Google Scholar]
- Van Duzee, E.P. Observations on some Hemiptera taken in Florida in the spring of 1908. B. Buffalo Soc. Nat. Sci. 1909, 9, 149–230. [Google Scholar]
- Metcalf, Z.P.; Osborn, H. Some observations on insects of the between tidal zone of the North Carolina coast. Ann. Entomol. Soc. Am. 1920, 13, 108–117. [Google Scholar]
- Metcalf, Z.P. Some ecological aspects of the tidal zone of the North Carolina coast. Ecology 1920, 1, 193–197. [Google Scholar] [CrossRef]
- Davis, W.T. New North American cicadas with notes on described species. J. New York Entomol. Soc. 1938, 46, 291–310. [Google Scholar]
- Osborn, H. Observations on the Cicadidae of Iowa. P. Iowa Acad. Sci. 1896, 3, 194–203. [Google Scholar]
- Davis, W.T. Cicada tibicen, a South American species, with records and descriptions of North American cicada. J. New York Entomol. Soc. 1925, 33, 35–51. [Google Scholar]
- Sanborn, A.F.; Heath, J.E.; Heath, M.S. Thermoregulation and evaporative cooling in the cicada Okanagodes. gracilis (Homoptera: Cicadidae). Comp. Biochem. Physiol. A 1992, 102, 751–757. [Google Scholar] [CrossRef]
- Andersen, D.C. Are cicadas (Diceroprocta. apache) both a keystone and a critical-link species in lower Colorado River riparian communities? Southwest. Nat. 1994, 39, 26–33. [Google Scholar] [CrossRef]
- Ellingson, A.R.; Andersen, D.C. Spatial correlations of Diceroprocta. apache and its host plants: Evidence for a negative impact from Tamarix. invasion. Ecol. Entomol. 2002, 27, 16–24. [Google Scholar] [CrossRef]
- Glinski, R.L.; Ohmart, R.D. Factors of reproduction and population densities in the Apache Cicada (Diceroprocta. apache). Southwest. Nat. 1984, 29, 73–79. [Google Scholar] [CrossRef]
- Davis, W.T. Records of cicadas from North America with descriptions of new species. J. New York Entomol. Soc. 1921, 29, 1–16. [Google Scholar]
- Davis, W.T. Notes on cicadas with descriptions of new species. J. New York Entomol. Soc. 1942, 50, 169–187. [Google Scholar]
- Greller, A.M. Deciduous Forests. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 287–316. [Google Scholar]
- Davis, W.T. New cicadas from North America. J. New York Entomol. Soc. 1934, 42, 37–63. [Google Scholar]
- Davis, W.T. New cicadas from North America with notes. J. New York Entomol. Soc. 1941, 49, 85–99. [Google Scholar]
- Sanborn, A.F.; Maté, S. Thermoregulation and the effect of body temperature on call temporal parameters in the cicada Diceroprocta. olympusa (Homoptera: Cicadidae). Comp. Biochem. Physiol. A 2000, 125, 141–148. [Google Scholar] [CrossRef]
- Beamer, R.H. Studies on the biology of Kansas Cicadidae. U KS Sci. B. 1928, 18, 155–263. [Google Scholar]
- Boulard, M. Arguments pour la suppression du nom de genre Tibicen. et de ses dérivés dans la nomenclature de la superfamille des Cicadoidea. B. Zool. Nom. 1984, 41, 163–184. [Google Scholar]
- Boulard, M. Taxonomie et nomenclature superieures des Cicadoidea. Histoire, problems et solutions. EPHE, Trav. Lab. Biol. Evol. Ins. Hemipt. 1988, 1, 1–89. [Google Scholar]
- Boulard, M. Nomenclature et taxonomie supérieures des Cicadoidea ou vraies cigales. Histoire, problèmes et solutions (Rhynchota Homoptera Cicadomorpha). EPHE, Trav. Lab. Biol. Evol. Ins. Hemipt. 1998, 10, 79–129. [Google Scholar]
- Boulard, M. Higher taxonomy and nomenclature of the Cicadoidea or true cicadas: History, problems and solutions (Rhynchota Auchenorhyncha Cicadomorpha). EPHE, Trav. Lab. Biol. Evol. Ins. Hemipt. 2001, 14, 1–48. [Google Scholar]
- Boulard, M. Tibicen. Latreille, 1825: <<Fatal error>> . Nouv. Rev. d’Entomol. (N.S.) 2003, 25, 371–372. [Google Scholar]
- Melville, R.V.; Sims, R.W. Tibicina. Amyot, 1847 and Lyristes. Horvath, 1926 (Insecta, Hemiptera, Homoptera): Proposed conservation by the suppression of Tibicen. Berthold, 1827. Z.N. (S.). B. Zool. Nom. 1984, 41, 163–184. [Google Scholar]
- Boulard, M. Comment on the proposed conservation of Tibicina. Amyot 1847 and Lyristes. Horváth 1926. Z.N. (S.) 239. B. Zool. Nom. 1985, 42, 212–214. [Google Scholar]
- Hamilton, K.G.A. Comment on the proposed conservation of Tibicina. Amyot 1847 and Lyristes. Horváth 1926. Z.N. (S.) 239. B. Zool. Nom. 1985, 42, 211. [Google Scholar]
- Lauterer, P. Comment on the proposed conservation of Tibicina. Amyot 1847 and Lyristes. Horváth 1926. Z.N. (S.) 239. B. Zool. Nom. 1985, 42, 214. [Google Scholar]
- Latreille, P.A. Famille. Naturelles. du Regue. Animal, Exposées. Succinctement. et Dans. un Ordre. Analytique., Avec L'indication de Leurs. Genres; (In French). J.B. Ballière: Paris, France, 1825; pp. 1–570. [Google Scholar]
- Sanborn, A.F. Unpublished work. Preliminary stages of development.
- Sanborn, A.F. The identity of Cicada tibicen Linné [=Tibicen. chloromerus (Walker, 1850)] (Hemiptera: Cicadoidea: Cicadidae). Entomol. News 2008, 119, 227–231. [Google Scholar] [CrossRef]
- Elliott, L.; Hershberger, W. 2006. The Songs of Insects; Houghton Mifflin Company: Boston, MA, USA, 2006; p. 228. [Google Scholar]
- Beamer, R.H. On the oviposition of some Kansas cicadas. Ann. Entomol. Soc. Am. 1925, 18, 479–482. [Google Scholar]
- Stucky, B. First record of the cicada Tibicen. pruinosus in Colorado, with a key to the Colorado species of Tibicen. (Hemiptera: Cicadidae). West. N. Am. Naturalist 2012, 72, 100–104. [Google Scholar] [CrossRef]
- Davis, W.T. Notes on North American cicadas with descriptions of new species. J. New York Entomol. Soc. 1923, 31, 1–15. [Google Scholar]
- Elzinga, R.J. Observations on Sandalus. niger Knoch (Coleoptera: Sandalidae) with a description of the Triungulin larva. J. Kansas Entomol. Soc. 1977, 50, 324–328. [Google Scholar]
- Hoffman, C.H. Annotated list of elm insects in the United States. USDA Misc. Pub. 1942, 466, 1–20. [Google Scholar]
- Jacobs, M.E. Observations on the two forms of the periodical cicada Magicicada. septendecim (L.). P. Indiana Acad. Sci. 1953, 63, 177–179. [Google Scholar]
- Shelford, V.E. Animal communities in temperate America as illustrated in the Chicago region. A study in animal ecology. Geogr. Soc. Chicago B. 1913, 5, 1–362. [Google Scholar]
- Emmons, E. Agriculture of New York, comprising an account of the classification, composition and distribution of the soils, rocks, and of the climate and agricultural productions of the State; together with descriptions of the more common and injurious species of insects.; C. VanBenthuysen and Company: Albany, New York, USA, 1854; Volume 5, pp. 1-viii–47, 1-viii, 1–272, plates A-C, 1–47. [Google Scholar]
- Adams, C.C. An ecological study of prairie and grass invertebrates. B. Ill. Nat. Hist. Sur. 1915, 11, 33–280. [Google Scholar]
- Toolson, E.C. Comparative thermal physiological ecology of syntopic populations of Cacama. valvata and Tibicen. bifidus (Homoptera: Cicadidae): Modeling fitness consequences of temperature variation. Am. Zool. 1998, 38, 568–582. [Google Scholar]
- Hastings, J.M.; Toolson, E.C. Thermoregulation and activity patterns of 2 syntopic cicadas, Tibicen. chiricahua and T. duryi (Homoptera, Cicadidae), in central New Mexico. Oecologia. 1991, 85, 513–520. [Google Scholar] [CrossRef]
- Tinkham, E.R. Biological and faunistic notes on the Cicadidae of the Big Bend Region of Trans-Pecos, Texas. J. New York Entomol. Soc. 1941, 49, 165–183. [Google Scholar]
- Davis, W.T. New cicadas with notes on North American and West Indian species. J. New York Entomol. Soc. 1935, 43, 173–199. [Google Scholar]
- Davis, W.T. New cicadas from California and Arizona with notes on several other species. J. New York Entomol. Soc. 1926, 34, 177–197. [Google Scholar]
- Daniel, H.J.; Knight, C.; Charles, T.M.; Larkins, A.L. Predicting species by call in three species of North Carolina cicadas. J. Elisha Mitchell Sci. Soc. 1993, 109, 67–76. [Google Scholar]
- Sanborn, A.F. Thermoregulation and endothermy in the large western cicada Tibicen. cultriformis (Hemiptera: Cicadidae). J. Therm. Biol. 2004, 29, 97–101. [Google Scholar] [CrossRef]
- Bromley, S.W. Cicadas in Texas. Psyche 1933, 40, 130. [Google Scholar] [CrossRef]
- Davis, W.T. The remarkable distribution of an American cicada; a new genus, and other cicada notes. J. New York Entomol. Soc. 1944, 52, 213–222. [Google Scholar]
- Küchler, A.W. Manual to Accompany the Map Potential Natural Vegetation of the Continuous United States. In American Geographical Society Special Publication Number 36; American Geographical Society: New York, NY, USA, 1964; p. 156. [Google Scholar]
- Santos, R.S.; Martinelli, N.M.; Maccagnan, D.H.B.; Sanborn, A.F.; Ribeiro, R. Description of a new cicada species associated with the coffee plant and an identification key to the species of Fidicinoides. (Hemiptera: Cicadidae) from Brazil. Zootaxa. 2010, 2602, 48–56. [Google Scholar]
- De Santis, C.L.; Medrano, M.C.; Sanborn, A.F.; Bolcatto, P.G. Cicádidos (Insecta: Hemiptera: Cicadidae) del Museo Provincial de Ciencias Naturales “Florentino Ameghino” Santa Fe, Argentina. Mus. Prov. Cien. Nat. “Florentino. Ameghino.” Ser. Cat. 2007, 19, 1–19. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K. Scaling of sound pressure level and body size in cicadas (Homoptera: Cicadidae; Tibicinidae). Ann. Entomol. Soc. Am. 1995, 88, 479–484. [Google Scholar]
- Distant, W.L. Cicadidae and Fulgoridae. In Biologia Centrali Americana; Contributions to the Knowledge of the Fauna and Flora of Mexico and Central America; R.H. Porter: London, UK, 1905; pp. 140–146. [Google Scholar]
- Sanborn, A.F.; Heath, M.S.; Heath, J.E.; Noriega, F.G. Diurnal activity, temperature responses and endothermy in three South American cicadas (Homoptera: Cicadidae: Dorisiana. bonaerensis, Quesada gigas, and Fidicina. mannifera. J. Therm. Biol. 1995, 20, 451–460. [Google Scholar] [CrossRef]
- Martinelli, N.M.; Zucchi, R.O. Primeiros registros de plantas hospedeiras de Fidicina. mannifera, Quesada gigas e Dorisiana. drewseni (Hemiptera-Cicadidae). Rev. Agric. 1997, 72, 271–281. [Google Scholar]
- Lee, Y.J.; Hill, K.B.R. Systematic revision of the genus Psithyristria. Stål (Hemiptera: Cicadidae) with seven new species and a molecular phylogeny of the genus and higher taxa. Syst. Entomol. 2010, 35, 277–305. [Google Scholar] [CrossRef]
- Moulds, M.S. An appraisal of the higher classification of cicadas (Hemiptera: Cicadoidea) with special reference to the Australian fauna. Rec. Aust. Mus. 2005, 57, 375–446. [Google Scholar] [CrossRef]
- Marlatt, C.L. A new nomenclature for the broods of the periodical cicada. B. USDA Div. Entomol. (n.s.) 1989, 18, 52–58. [Google Scholar]
- Alexander, R.D.; Moore, T.E. The evolutionary relationships of 17-year and 13-year cicadas, and three new species (Homoptera, Cicadidae, Magicicada.). Misc. Pub. Mus. Zool. U. Mich. 1962, 2121, 1–59. [Google Scholar]
- Simon, C. Evolutionary relationships among broods of 13-year and 17-year periodical cicadas. Entomol. News 1981, 92, 169–170. [Google Scholar]
- Simon, C. Evolution of 13-year and 17-year periodical cicadas (Homoptera: Cicadidae: Magicicada.). B. Entomol. Soc. Am. 1988, 34, 163–176. [Google Scholar]
- Cooley, J.R.; Marshall, D.C.; Simon, C. The historical contraction of periodical cicada Brood VII (Hemiptera: Cicadoidea: Cicadidae). J. New York Entomol. Soc. 2004, 112, 198–204. [Google Scholar] [CrossRef]
- Cooley, J.R.; Kritsky, G.; Edwards, M.J.; Zyla, J.D.; Marshall, D.C.; Hill, K.B.R.; Krauss, R.; Simon, C. The distribution of periodical cicada Brood X in 2004. Am. Entomol. 2009, 55, 106–112. [Google Scholar]
- Cooley, J.R.; Kritsky, G.; Edwards, M.J.; Zyla, J.D.; Marshall, D.C.; Hill, K.B.R.; Bunker, G.; Neckermann, M.; Troutman, R.; Yoshimura, J.; et al. Periodical cicadas (Magicicada. spp.): A GIS-based map of Broods XIV in 2008 and “XV” in 2009. Am. Entomol. 2011, 57, 144–150. [Google Scholar]
- Edwards, M.J.; Faivre, A.E.; Crist, R.C., III; Sitvarin, M.I.; Zyla, J. Distribution of the 2004 emergence of seventeen-year periodical cicadas (Hemiptera: Cicadidae: Magicicada. spp., Brood X) in Pennsylvania, U.S.A. Entomol. News 2005, 116, 273–282. [Google Scholar]
- Zyla, J.D. First report of the 13-year periodical cicada, Magicicada. tredecim (Walsh and Riley) (Hemiptera: Cicadidae) in Maryland. P. Entomol. Soc. Wash. 2004, 106, 485–487. [Google Scholar]
- Zyla, J.D. Reports of four year accelerated occurrences of the 2004 emergence of periodical cicadas, Magicicada. spp. (Hemiptera: Cicadidae) Brood X in Maryland, Virginia, and the District of Columbia. P. Entomol. Soc. Wash. 2004, 106, 488–490. [Google Scholar]
- Dybas, H.S.; Lloyd, M. The habitats of 17-year periodical cicadas (Homoptera: Cicadidae: Magicicada. spp.). Ecol. Monogr. 1974, 44, 279–324. [Google Scholar] [CrossRef]
- Lloyd, M.; White, J. On the oviposition habits of 13-year versus 17-year periodical cicadas of the same species. J. New York Entomol. Soc. 1976, 84, 148–155. [Google Scholar]
- White, J. Resource partitioning by ovipositing cicadas. Am. Nat. 1980, 115, 1–28. [Google Scholar]
- Miller, F.D. Effects and control of periodical cicada Magicicada.septendecim and Magicicada. cassini oviposition injury on urban forest trees. J. Arboricult. 1997, 23, 225–232. [Google Scholar]
- Miller, F.; Crowley, W. Effects of periodical cicada oviposition injury on woody plants. J. Arboricult. 1998, 24, 248–253. [Google Scholar]
- Mattingly, W.B.; Flory, S.L. Plant architecture affects periodical cicada oviposition behavior on native and non-native hosts. Oikos. 2010, 120, 1083–1091. [Google Scholar] [CrossRef]
- Moulds, M.S. The status of Cicadetta. and Melampsalta. (Homoptera: Cicadidae) in Australia with the description of two new species. Gen. Appl. Entomol. 1988, 20, 39–48. [Google Scholar]
- Moulds, M.S. A review of the genera of Australian cicada (Hemiptera: Cicadoidea). Zootaxa. 2012, 3287, 1–262. [Google Scholar]
- Hendrickson, G.O. Studies on the insect fauna of Iowa prairies. Iowa St. Coll. J. Sci. 1930, 4, 149–179. [Google Scholar]
- Davis, W.T. Two ways of song communication among our North American cicadas. J. New York Entomol. Soc. 1943, 51, 185–190. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K. Analysis of the acoustic signals produced by the cicada Platypedia. putnami variety lutea (Homoptera: Tibicinidae). Ann. Entomol. Soc. Am. 1999, 92, 451–455. [Google Scholar]
- Peet, R.K. Forests of the Rocky Mountains. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 63–102. [Google Scholar]
- Franklin, J.F. Pacific Northwest Forests. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; pp. 103–130. [Google Scholar]
- Putnam, J.D. Remarks on the habits of several western Cicadidae. P. Davenpost. Acad. Nat. Sci. 1881, 3, 67–68. [Google Scholar]
- Sanborn, A.F.; Noriega, F.G.; Phillips, P.K. Thermoregulation in the cicada Platypedia. putnami var. lutea with a test of a crepitation hypothesis. J. Therm. Biol. 2002, 27, 365–369. [Google Scholar]
- Little, E.L., Jr. Common insects on Pinyon (Pinus. edulis). J. New York Entomol. Soc. 1943, 51, 239–252. [Google Scholar]
- Fautin, R.W. Biotic communities of the northern desert shrub biome in Western Utah. Ecol. Monogr. 1947, 16, 253–310. [Google Scholar]
- Davis, W.T. North American cicadas belonging to the genera Platypedia. and Melampsalta. J. New York Entomol. Soc. 1920, 28, 95–135. [Google Scholar]
- Davis, W.T. New cicadas from the Western United States, with notes on several other species. J. New York Entomol. Soc. 1927, 35, 373–389. [Google Scholar]
- Davis, W.T. Two new cicadas from Lower California, Mexico. J. New York Entomol. Soc. 1917, 25, 6–10. [Google Scholar]
- Watts, R.J.; Williams, K. Host use patterns of adult chaparral cicadas (Homoptera: Cicadidae) in San Diego County, Califormia. B. Ecol. Soc. Am. (Suppl.) 1992, 73, 380–381. [Google Scholar]
- McDonnell, S.E.; Moskowitz, D.P. First breeding record of the cicada Okanagana. rimosa Say (Say’s Cicada) in New Jersey. Northeast. Nat. 2012, 19, 140–142. [Google Scholar] [CrossRef]
- Johnson, C.W. Okanagana. rimosa (Say) in Nova Scotia. Psyche 1921, 28, 15. [Google Scholar] [CrossRef]
- Cooley, J.R. Long-range acoustical signals, phonotaxis, and risk in the sexual pair-forming behaviors of Okanagana. canadensis and O. rimosa (Hemiptera: Cicadidae. Ann. Entomol. Soc. Am. 2001, 94, 755–760. [Google Scholar] [CrossRef]
- Sanborn, A.F.; Webb, M.D. Recognition of the types of Okanagana. occidentalis (Walker, 1866) (Hemiptera: Cicadoidea: Tibicinidae): Lectotype designation, type locality and species identity. Fla. Entomol. 2001, 84, 451–453. [Google Scholar] [CrossRef]
- Davis, W.T. Cicadas of the genera Okanagana., Tibicinoides. and Okanagodes., with descriptions of several new species. J. New York Entomol. 1919, 27, 179–223. [Google Scholar]
- Davis, W.T. A remarkable cicada from Mexico and other North American species. J. New York Entomol. Soc. 1936, 45, 101–123. [Google Scholar]
- Osborn, H. Descriptions of two new cicadas from Louisiana. Ohio Nat. 1896, 6, 497–499. [Google Scholar]
- Sanborn, A.F.; Breitbarth, J.H.; Heath, J.E.; Heath, M.S. Temperature responses and habitat sharing in two sympatric species of Okanagana. (Homoptera: Cicadoidea). West. N. Am. Naturalist 2002, 62, 437–450. [Google Scholar]
- Davis, W.T. New species of cicadas from California and Utah. J. New York Entomol. Soc. 1915, 23, 11–21. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K. No acoustic benefit to subterranean calling in the cicada Okanagana. pallidula Davis (Homoptera: Tibicinidae). Great Basin Nat. 1995, 55, 374–376. [Google Scholar]
- Bliven, B.P. Concerning cicadas: Notes and descriptions of new species. Occident. Entomol. 1964, 1, 90–102. [Google Scholar]
- Van Duzee, E.P. A preliminary review of the west coast Cicadidae. J. New York Entomol. Soc. 1915, 23, 21–44. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; p. 203. [Google Scholar]
- Sanborn, A.F.; Phillips, P.K.; Sites, R.W. Biodiversity, biogeography, and bibliography of the cicadas of Thailand (Hemiptera: Cicadoidea: Cicadidae. Zootaxa. 2007, 1413, 1–46. [Google Scholar]
- USGS Geographical Names Information System. Available online: http://geonames.usgs.gov/ (accessed on 1 January 2013).
- Heavens-Above GmbH. Available online: http://www.heavens-above.com/ (accessed on 1 January 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sanborn, A.F.; Phillips, P.K. Biogeography of the Cicadas (Hemiptera: Cicadidae) of North America, North of Mexico. Diversity 2013, 5, 166-239. https://doi.org/10.3390/d5020166
Sanborn AF, Phillips PK. Biogeography of the Cicadas (Hemiptera: Cicadidae) of North America, North of Mexico. Diversity. 2013; 5(2):166-239. https://doi.org/10.3390/d5020166
Chicago/Turabian StyleSanborn, Allen F., and Polly K. Phillips. 2013. "Biogeography of the Cicadas (Hemiptera: Cicadidae) of North America, North of Mexico" Diversity 5, no. 2: 166-239. https://doi.org/10.3390/d5020166




