Human-Induced Disturbance Alters Pollinator Communities in Tropical Mountain Forests
Abstract
:1. Introduction
- (1) Do pollinator richness and abundance of bee and butterfly/moth pollinator guilds decrease from the forest interior towards fire-degraded habitats?
- (2) Does the community composition of both pollinator guilds change between forest and fire-degraded habitats?
- (3) Do changes in habitat conditions cause changes in the body size of the pollinator guilds?
2. Methods
2.1. Site Description

2.2. Sampling
2.3. Statistical Analysis
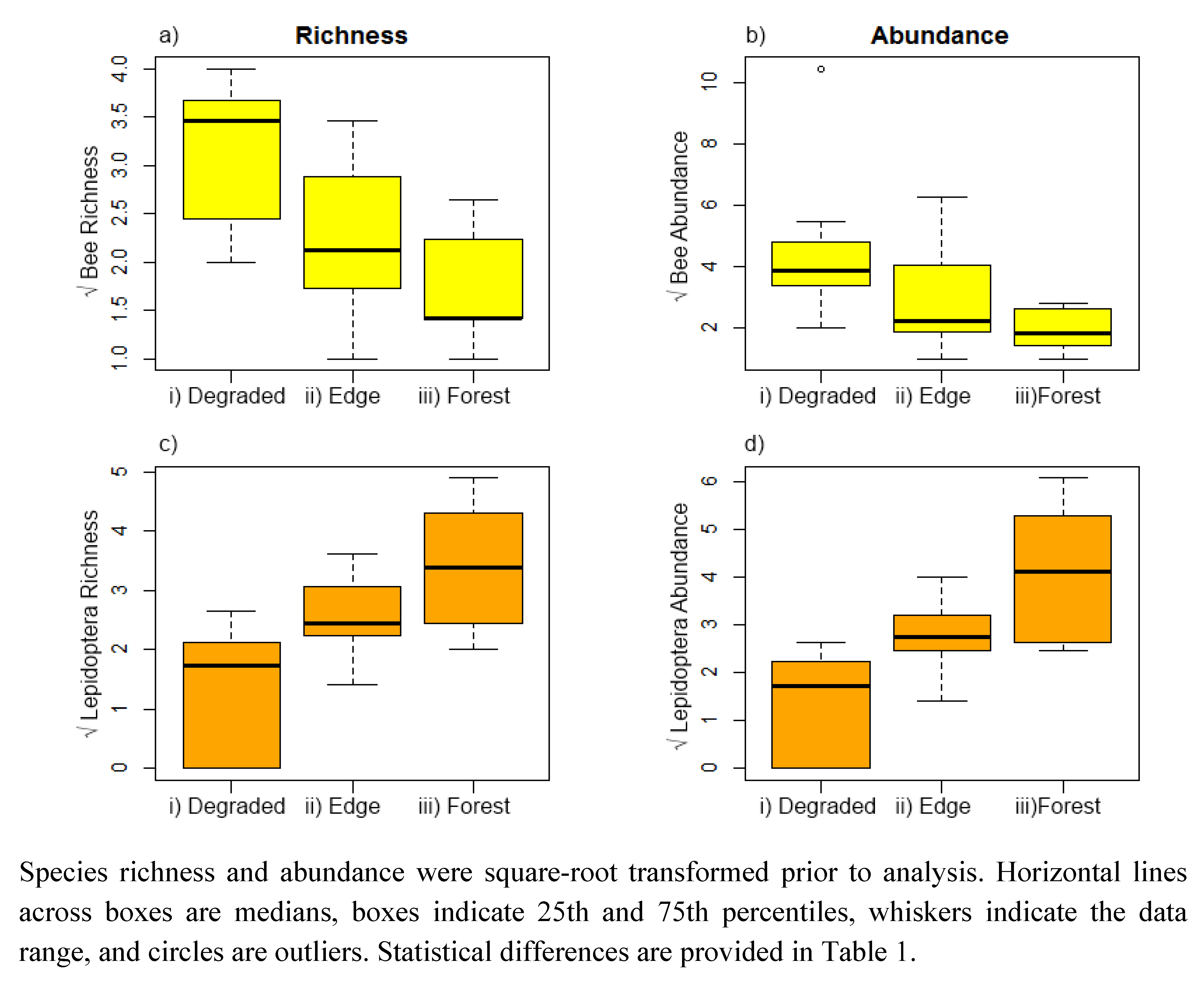
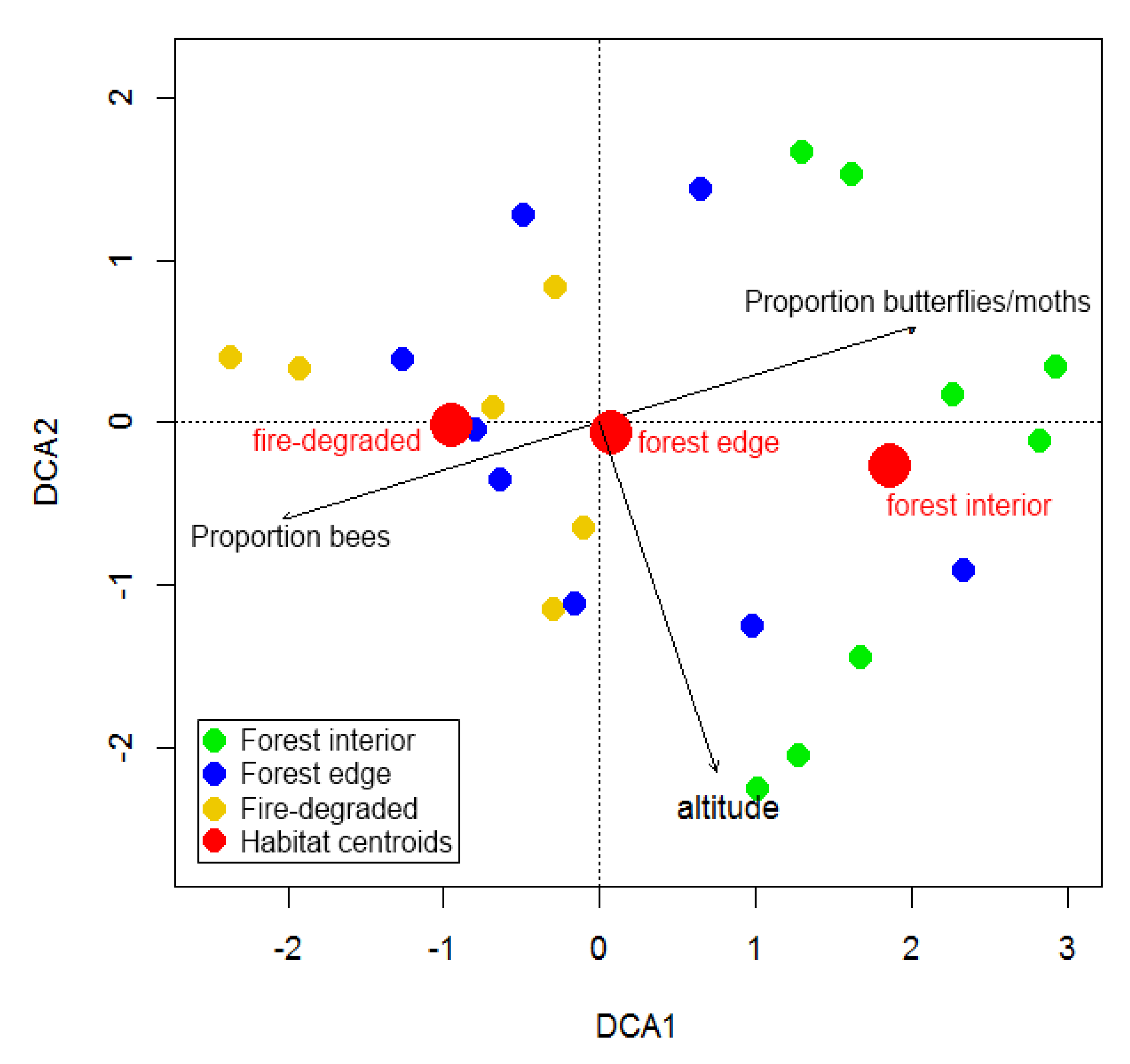
3. Results
| Bees | Estimate | Std. Error | DF | t-value | p-value | |
|---|---|---|---|---|---|---|
| Richness | Forest vs. degraded | −0.53 | 0.13 | 13 | −3.80 | 0.002 |
| Interior vs. edge | −0.25 | 0.15 | 13 | −1.70 | 0.112 | |
| Abundance | Forest vs. degraded | −1.07 | 0.37 | 13 | −2.83 | 0.014 |
| Interior vs. edge | −0.49 | 0.41 | 13 | −1.20 | 0.251 | |
| Lepidoptera | ||||||
| Richness | Forest vs. degraded | 0.88 | 0.14 | 13 | 6.05 | <0.001 |
| Interior vs. edge | 0.41 | 0.15 | 13 | 2.64 | 0.020 | |
| Abundance | Forest vs. degraded | 0.17 | 0.17 | 13 | 6.31 | <0.001 |
| Interior vs. edge | 0.64 | 0.18 | 13 | 3.51 | 0.004 |
| Bees | Lepidoptera | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (F) | Small (χ2) | Large (χ2) | Size (F) | Small (χ2) | Large (χ2) | ||||||
| Fire-degraded | 0.1 | 8.6 | 11.4 | + | 0.3 | − | 5.5 | 4.1 | − | ||
| p-Value | 0.187 | 0.720 | 0.015 | 0.003 | 0.132 | 0.006 | |||||
| Forest edge | 0.1 | 8.4 | 9.2 | 0.0 | 10.2 | 11.3 | |||||
| p-Value | 0.178 | 0.720 | 0.720 | 0.399 | 0.452 | 0.489 | |||||
| Forest interior | 0.2 | − | 11.1 | 3.4 | − | 0.2 | + | 10.6 | 17.4 | + | |
| p-Value | 0.024 | 0.720 | 0.006 | 0.016 | 0.435 | 0.006 | |||||
4. Discussion
4.1. Species Richness and Abundance
4.2. Species Turn-Over
4.3. Changes in Body Size
5. Conclusions
Acknowledgements
Supplementary Files
References
- Barthlott, W.; Lauer, W.; Placke, A. Global Distribution of Species Diversity in Vascular Plants: Towards A World Map Of Phytodiversity (Globale Verteilung der Artenvielfalt Höherer Pflanzen: Vorarbeiten zu einer Weltkarte der Phytodiversität). Erdkunde 1996, 50, 317–327. [Google Scholar]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; da Fonseca, G.A.; Olivieri, S. Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar]
- IPCC, Summary for Policymakers. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L.; Canziani, O.F.; Palutikof, J.P.; van der Linden, P.J.; Hanson, C.E. (Eds.) Cambridge University Press: Cambridge, UK, 2007.
- Webster, B.D.; Steeves, T.A. Morphogenesis in Pteridium aquilinum (L.) Kuhn-General morphology and growth habit. Phytomorphology 1958, 8, 30–41. [Google Scholar]
- Hartig, K.; Beck, E. The bracken fern (Pteridium arachnoideum (Kaulf.) Maxon) Dilemma in the Andes of southern Ecuador. Ecotropica 2003, 9, 3–13. [Google Scholar]
- Silva Matos, D.M.; Belinato, T.A. Interference of Pteridium arachnoideum (Kaulf.) Maxon. (Dennstaedtiaceae) on the establishment of rainforest trees. Braz. J. Biol. 2010, 70, 311–316. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Martins, S.V. High Diversity Forest Restoration in Degraded Areas: Methods and Projects In Brazil; Nova Publishers: São Paulo, Brazil, 2007. [Google Scholar]
- Bawa, K.S. Plant-pollinator interactions in tropical rain forests. Annu. Rev. Ecol. Syst. 1990, 21, 399–422. [Google Scholar]
- Schemske, D.W.; Mittelbach, G.G.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.L. Pollen limitation of plant reproduction: Pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Hagen, M.; Kraemer, M. Agricultural surroundings support flower–visitor networks in an afrotropical rain forest. Biol. Conserv. 2010, 143, 1654–1663. [Google Scholar] [CrossRef]
- Winfree, R.; Griswold, T.; Kremen, C. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 2007, 21, 213–223. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar]
- Steffan-Dewenter, I.; Münzenberg, U.; Bürger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Hamer, K.C.; Hill, J.K. Scale-dependent effects of habitat disturbance on species richness in tropical forests. Conserv. Biol. 2000, 14, 1435–1440. [Google Scholar] [CrossRef]
- Quesada, M.; Sanchez-Azofeifa, G.A.; Alvarez-Añorve, M.; Stoner, K.E.; Avila-Cabadilla, L.; Calvo-Alvarado, J.; Castillo, A.; Espírito-Santo, M.M.; Fagundes, M.; Fernandes, G.W.; et al. Succession and management of tropical dry forests in the Americas: Review and new perspectives. Forest Ecol. Manag. 2009, 258, 1014–1024. [Google Scholar] [CrossRef]
- Heithaus, E.R. Community structure of neotropical flower visiting bees and wasps: Diversity and phenology. Ecology 1979, 60, 190–202. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Breitbach, N.; Tillmann, S.; Schleuning, M.; Grünewald, C.; Laube, I.; Steffan-Dewenter, I.; Böhning-Gaese, K. Influence of habitat complexity and landscape configuration on pollination and seed-dispersal interactions of wild cherry trees. Oecologia 2012, 168, 425–437. [Google Scholar] [CrossRef]
- Bishop, J.A.; Armbruster, W.S. Thermoregulatory abilities of Alaskan bees: Effects of size, phylogeny and ecology. Funct. Ecol. 1999, 13, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Pereboom, J.J.M.; Biesmeijer, J.C. Thermal constraints for stingless bee foragers: The importance of body size and coloration. Oecologia 2003, 137, 42–50. [Google Scholar]
- Schulze, C.H.; Linsenmair, K.E.; Fiedler, K. Understorey versus canopy: Patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. Plant. Ecol. 2001, 153, 133–152. [Google Scholar] [CrossRef]
- Hill, J.K.; Hamer, K.C.; Tangah, J.; Dawood, M. Ecology of tropical butterflies in rainforest gaps. Oecologia 2001, 128, 294–302. [Google Scholar]
- Handel, S.N. The role of plant-animal mutualisms in the design and restoration of natural communities. In Restoration Ecology and Sustainable Development; Urbanska, K.M., Webb, N.R., Edwards, P.J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 111–132. [Google Scholar]
- Dixon, K.W. Pollination and restoration. Science 2009, 325, 571–573. [Google Scholar] [CrossRef]
- Molina Carpio, J. Régimen de precipitación en la cuenca de Huarinilla-Cotapata, La Paz-Bolivia. Ecol. Boliv. 2005, 40, 43–55. [Google Scholar]
- Toler, T.R.; Evans, E.W.; Tepedino, V.J. Pan-trapping for bees (Hymenoptera: Apiformes) in Utah’s west desert: The importance of color diversity. Pan-Pac. Entomol. 2005, 81, 103–113. [Google Scholar]
- Roulston, T.H.; Smith, S.A.; Brewster, A.L. A Comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J. Kansas Entomol. Soc. 2007, 80, 179–181. [Google Scholar] [CrossRef]
- Schleuning, M.; Farwig, N.; Peters, M.K.; Bergsdorf, T.; Bleher, B.; Brandl, R.; Dalitz, H.; Fischer, G.; Freund, W.; Gikungu, M.W. Forest fragmentation and selective logging have inconsistent effects on multiple animal-mediated ecosystem processes in a tropical forest. PloS One 2011, 6, e27785. [Google Scholar]
- Campbell, J.W.; Hanula, J.L. Efficiency of malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Cons. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community ecology package, 2011. In R package version 2.0-1.
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. The R development core team (2011) nlme: Linear and nonlinear mixed effects models, 2011. In R package version 3.1-106.
- Dray, S.; Dufour, A.B. The Ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar]
- Legendre, P.; Galzin, R.; Harmelin-Vivien, M.L. Relating behaviour to habitat: Solutions to the fourth-corner problem. Ecology 1997, 78, 547–562. [Google Scholar]
- Dray, S.; Legendre, P. Testing the species traits-environment relationships: The fourth-corner problem revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- Otero, J.T.; Sandino, J.C. Capture rates of male Euglossine bees across a human intervention gradient, Chocó region, Colombia. Biotropica 2006, 35, 520–529. [Google Scholar]
- Liow, L.H.; Sodhi, N.S.; Elmqvist, T. Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia. J. Appl. Ecol. 2001, 38, 180–192. [Google Scholar] [CrossRef]
- Tonhasca, A., Jr.; Blackmer, J.L.; Albuquerque, G.S. Abundance and diversity of Euglossine bees in the fragmented landscape of the Brazilian Atlantic forest. Biotropica 2002, 34, 416–422. [Google Scholar]
- Zayed, A.; Whitfield, C.W. A genome-wide signature of positive selection in ancient and recent invasive expansions of the honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2008, 105, 3421–3426. [Google Scholar] [CrossRef]
- Brosi, B.J.; Daily, G.C.; Ehrlich, P.R. Bee community shifts with landscape context in a tropical countryside. Ecol. Appl. 2007, 17, 418–430. [Google Scholar] [CrossRef]
- Brosi, B.J.; Daily, G.C.; Shih, T.M.; Oviedo, F.; Durán, G. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 2008, 45, 773–783. [Google Scholar]
- Wilms, W.; Wiechers, B. Floral resource partitioning between native Melipona bees and the introduced Africanized honey bee in the Brazilian Atlantic rain forest. Apidologie 1997, 28, 339–355. [Google Scholar] [CrossRef]
- Stefanescu, C.; Herrando, S.; Páramo, F. Butterfly species richness in the north-west Mediterranean basin: The role of natural and human-induced factors. J. Biogeogr. 2004, 31, 905–915. [Google Scholar] [CrossRef]
- Hill, J.K.; Hamer, K.C.; Lace, L.A.; Banham, W.M.T. Effects of selective logging on tropical forest butterflies on Buru, Indonesia. J. Appl. Ecol. 1995, 32, 754–760. [Google Scholar] [CrossRef]
- Kitahara, M.; Fujii, K. Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: An analysis based on the concept of generalist vs. specialist strategies. Res. Popul. Ecol. 1994, 36, 187–199. [Google Scholar] [CrossRef]
- Hogsden, K.L.; Hutchinson, T.C. Butterfly assemblages along a human disturbance gradient in Ontario, Canada. Can. J. Zoolog. 2004, 82, 739–748. [Google Scholar]
- Potts, S.G.; Dafni, A.; Ne’eman, G. Pollination of a core flowering shrub species in Mediterranean phrygana: variation in pollinator diversity, abundance and effectiveness in response to fire. Oikos 2003, 92, 71–80. [Google Scholar]
- Tylianakis, J.M.; Klein, A.-M.; Tscharntke, T. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 2005, 86, 3296–3302. [Google Scholar]
- Bawa, K.S.; Bullock, S.H.; Perry, D.R.; Coville, R.E.; Grayum, M.H. Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. Am. J. Bot. 1985, 72, 346–356. [Google Scholar] [CrossRef]
- Powell, A.H.; Powell, G.V. Population dynamics of male Euglossine bees in Amazonian forest fragments. Biotropica 1987, 19, 176–179. [Google Scholar] [CrossRef]
- Janzen, D.H. Euglossine Bees as long-distance pollinators of tropical plants. Science 1971, 171, 203. [Google Scholar]
- Brehm, G.; Homeier, J.; Fiedler, K. Beta diversity of Geometrid moths (Lepidoptera: Geometridae) in an Andean montane rainforest. Divers. Distrib. 2003, 9, 351–366. [Google Scholar] [CrossRef]
- Kambach, S. Differences in diversity and composition of pollinator guilds between montane forest and arrested succession in Bolivia.
- Gathmann, A.; Greiler, H.-J.; Tscharntke, T. Trap-nesting bees and wasps colonizing set-aside fields: Succession and body size, Management by cutting and sowing. Oecologia 1994, 98, 8–14. [Google Scholar]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia 2007, 153, 589–596. [Google Scholar]
- Bommarco, R.; Biesmeijer, J.C.; Meyer, B.; Potts, S.G.; Pöyry, J.; Roberts, S.P.M.; Steffan-Dewenter, I.; Öckinger, E. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. P. Roy. Soc. B-Biol. Sci. 2010, 277, 2075–2082. [Google Scholar]
- Thomas, C.D.; Hill, J.K.; Lewis, O.T. Evolutionary consequences of habitat fragmentation in a localized butterfly. J. Anim. Ecol. 1998, 67, 485–497. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kambach, S.; Guerra, F.; Beck, S.G.; Hensen, I.; Schleuning, M. Human-Induced Disturbance Alters Pollinator Communities in Tropical Mountain Forests. Diversity 2013, 5, 1-14. https://doi.org/10.3390/d5010001
Kambach S, Guerra F, Beck SG, Hensen I, Schleuning M. Human-Induced Disturbance Alters Pollinator Communities in Tropical Mountain Forests. Diversity. 2013; 5(1):1-14. https://doi.org/10.3390/d5010001
Chicago/Turabian StyleKambach, Stephan, Fernando Guerra, Stephan G. Beck, Isabell Hensen, and Matthias Schleuning. 2013. "Human-Induced Disturbance Alters Pollinator Communities in Tropical Mountain Forests" Diversity 5, no. 1: 1-14. https://doi.org/10.3390/d5010001
APA StyleKambach, S., Guerra, F., Beck, S. G., Hensen, I., & Schleuning, M. (2013). Human-Induced Disturbance Alters Pollinator Communities in Tropical Mountain Forests. Diversity, 5(1), 1-14. https://doi.org/10.3390/d5010001




