Changes in Biodiversity and Functioning of Reef Fish Assemblages following Coral Bleaching and Coral Loss
Abstract
: Coral reef ecosystems are increasingly subject to severe, large-scale disturbances caused by climate change (e.g., coral bleaching) and other more direct anthropogenic impacts. Many of these disturbances cause coral loss and corresponding changes in habitat structure, which has further important effects on abundance and diversity of coral reef fishes. Declines in the abundance and diversity of coral reef fishes are of considerable concern, given the potential loss of ecosystem function. This study explored the effects of coral loss, recorded in studies conducted throughout the world, on the diversity of fishes and also on individual responses of fishes within different functional groups. Extensive (>60%) coral loss almost invariably led to declines in fish diversity. Moreover, most fishes declined in abundance following acute disturbances that caused >10% declines in local coral cover. Response diversity, which is considered critical in maintaining ecosystem function and promoting resilience, was very low for corallivores, but was much higher for herbivores, omnivores and carnivores. Sustained and ongoing climate change thus poses a significant threat to coral reef ecosystems and diversity hotspots are no less susceptible to projected changes in diversity and function.1. Introduction
Biodiversity varies greatly along latitudinal and longitudinal gradients [1,2], and may lead to important regional-scale differences in resilience and ecosystem function [3]. Biological diversity has long been argued to ensure continuity of ecosystem function during disturbances [4], and it is expected, therefore, that more diverse communities will be more resilient to natural and anthropogenic disturbances [5,6]. However, the maintenance of ecological functions depends upon functional redundancy (the extent to which sympatric species are capable of fulfilling the same ecological roles) and response diversity (the extent to which different species vary in their vulnerability and response to a given disturbance), rather than biodiversity per se [7]. Intuitively, ecosystem functions performed by single species will be much more vulnerable to disturbances compared to functions performed by multiple species. With high levels of functional redundancy, it is expected that ecosystem functioning will be insensitive to the loss of a few individual species. Alternatively, the loss of some species may be compensated by increases in the abundance or function of remaining species fulfilling the same ecological role [3]. However, species with common ecological functions will tend to have similar resource requirements and may therefore, be similarly affected by most major disturbances [7]. This suggests that functional groups with high redundancy may be highly vulnerable to disturbance, leading to loss of ecosystem function despite high levels of biodiversity [7,8].
Coastal marine ecosystems are increasingly subject to a wide range of anthropogenic and natural disturbances, leading to declines in quality, quantity and connectivity of habitats [9,10]. Significant declines in the abundance and diversity of major habitat-forming species have been recorded in seagrass habitats [11], kelp forests [12] mangroves [13] and coral reefs [10], with generally negative effects on overall abundance, diversity, and productivity of organisms within these ecosystems [6]. The vulnerability of coastal marine ecosystems to climate change is further heightened by long-term degradation caused by more direct anthropogenic disturbances, such as exploitation, sedimentation, pollution, and habitat modification [6,10]. For the most part, effects of climate change compound upon habitat degradation and losses that have occurred as a result of more localized anthropogenic disturbances [10]. The cumulative effects of direct anthropogenic disturbances may also make coastal species and ecosystems much more vulnerable to sustained and ongoing climate change. For example, Wooldridge [14] estimated that significant improvements in water quality on the inshore Great Barrier Reef (GBR) would enable local corals to withstand temperature increases of 2.0–2.5 °C.
Coral reefs are considered to be among the most vulnerable ecosystems to climate change [15], owing to the temperature sensitivities of corals, which may bleach and die when sea temperatures exceed normal local limits by as little as 1.0 °C [16]. With sustained increases in the temperature of tropical ocean waters, periodic temperature anomalies are increasingly exceeding the temperature threshold of corals, resulting in severe bleaching and extensive coral mortality. The severity and geographic extent of mass (multi-specific) bleaching events has been increasing since 1979 [17,18], and culminated in the 1998 global mass-bleaching event, which caused widespread bleaching in the Pacific, Indian and Atlantic oceans [19]. The 1998 global mass-bleaching event contributed greatly to global degradation of coral reef environments, especially in the Indian Ocean [20]. Moreover, the frequency, extent and magnitude of climate-induced coral bleaching is expected to increase over coming decades [21], potentially causing even greater coral loss and marked changes in the structure of coral assemblages [10].
Coral loss and degradation of coral reef habitats have a significant influence on the abundance and diversity of coral reef fishes [22-24]. Thus far, the fishes that appear most susceptible to acute disturbances and coral loss are highly specialized coral-dependent species, such as coral-feeding butterflyfishes [24]. Of greater concern however, are declines in the abundance of fishes that are critically important in maintaining the ecosystem function and resilience of coral reef habitats [8]. Herbivorous fishes are widely regarded as the single most important functional group of coral reef fishes [25], promoting ecosystem resilience by regulating abundance of macroalgae and thereby, ensuring availability of suitable substrate for settlement and population replenishment of habitat-forming corals [26,27]. Functional group approaches are being increasingly used to examine the way in which reef fish assemblages respond to disturbances and habitat degradation [22]. While functional groups may be defined as groups of species that perform similar ecological functions, irrespective of their taxonomic affinities [25,28]; a variety of methods have been used to classify species into functional groups. Coral reef fishes have been classified into functional groups based on trophic level, ecological role, body size, home range, habitat associations, or a combination of these factors [25,29-31].
The purpose of this review is to explore changes in the biodiversity and functioning of coral reef fish assemblages following distinct episodes of coral loss caused by acute disturbances, such as bleaching, severe tropical storms (cyclones), outbreaks of coral-feeding crown-of-thorns starfish (Acanthaster planci), or experimentally imposed disturbances. In particular, this study will aim to test whether high diversity among reef fish assemblages ensures the maintenance of key ecosystem functions during acute disturbances, and also quantify response diversity among reef fishes within distinct functional groups (e.g., among different species of coral-feeding fishes). The effects of coral loss on diversity and function of reef fish assemblages is considered to be among the most crucial knowledge gap in understanding the potential threat of climate change on corals reefs [32]. Losses in live coral and structural complexity have been shown to cause losses in fish diversity and abundance [22,23,33] but little is understood regarding a loss of functional diversity in the fish assemblage or how such a loss may affect the subsequent recovery and resilience of coral reef ecosystems. Previous studies have demonstrated marked variation in responses to coral loss amongst functional groups [22-24], but there has been limited consideration of differential responses among species within key groups [7].
2. Threats to Coral Reef Assemblages
Historically, the greatest threat to marine fishes has been from exploitation of direct fisheries [34,35]. Coastal fisheries throughout the world are generally regarded as unsustainable, if not already grossly overexploited [36]. Inshore fisheries and specifically, coral reef fisheries have collapsed in 18% of tropical island countries, and are fully exploited or overexploited in a further 17% of countries [35]. Moreover, destructive fishing practices and direct habitat alteration, combined with extrinsic contributors to habitat degradation (pollution, sedimentation, eutrophication, and climate change), have greatly increased the impact of human populations on marine fishes. Importantly, habitat degradation affects a much greater range of different fishes compared to fisheries exploitation, as changes to habitat structure not only reduce availability of resources, but may influence the outcomes of key biological interactions, such as competition and predation [37]. Fisheries exploitation typically targets larger individuals and species at higher trophic levels [38], whilst habitat alterations and destruction may affect an altogether different component of fish assemblages [8,39]. However, over-fishing and habitat-degradation tend to co-occur, leading to comprehensive declines in the abundance of fishes, especially in heavily populated regions of the world [8,39].
Habitat degradation on coral reefs is typically manifest as declines in the abundance of habitat-forming corals, and replacement of coral by macroalgae or other non-coral organisms [26]. Extensive coral loss may also result in declines in habitat and topographical complexity [23,40], which are critical for sustaining high diversity of reef fishes and other reef-associated organisms [23,24]. Major causes of coral loss include both biological disturbances (e.g., climate-induced coral bleaching, outbreaks of A. planci, and coral disease), which kill or remove coral tissue but leave the underlying coral skeletons intact [41,42] and physical disturbances (e.g., severe tropical storms or cyclones), which simultaneously reduce both live coral cover and structural complexity [43,44]. In the Caribbean, marked declines (80%) in coral cover observed over the last three decades are mainly attributed to severe tropical storms (cyclones) and outbreaks of coral disease [45]. While mass-mortalities of Diadema antillarum in the 1980s did not directly contribute to coral mortality, the subsequent growth of macroalgae hindered recovery and resilience of coral communities, leading to a general decline in coral cover with natural attrition of coral colonies. In the Pacific, average coral cover is believed to have declined by 20–50% during the last 20 years, but causes of these declines differ among regions [46]. Outbreaks of the coral-feeding crown-of-thorns starfish (A. planci) have caused extensive coral loss on Australia's Great Barrier Reef (GBR), Guam, Fiji, and Palau, whereas cyclones are considered to be the primary cause of recent coral loss in the central Pacific (e.g., Hawaii) [46]. Elsewhere, such as Indonesia and the Philippines, habitat degradation has resulted from more direct anthropogenic disturbances, such as destructive fishing practices [46]. Widespread coral bleaching has also contributed to coral mortality throughout the Indo-Pacific, especially in 1998, 1999, and 2002 [46], and the 1998 event has been identified as the main driver of coral cover change in the Indian Ocean over a 30-year period [47].
Climate change is an important emerging threat to coral reef assemblages, though recent effects of extreme temperature anomalies have been greatly exacerbated by chronic long-term degradation of coral reef ecosystems [10]. The long-term and cumulative effects of more direct anthropogenic disturbances (sedimentation and eutrophication) and increased fragmentation of coral reef habitats have greatly eroded reef resilience [10], thereby reducing the capacity for coral assemblages to recover in the aftermath of climatic disturbances [27]. As a result, ongoing increases in sea-surface temperatures, combined with climate-related declines in ocean pH, pose a significant threat to coral reefs [17,48], especially if habitat-forming corals are unable to adapt to changing conditions [10]. By 2050, coral reefs are expected to be subject to annual thermal anomalies equivalent to those that caused global bleaching and extensive widespread coral loss in 1998 [17], suggesting that mass bleaching will occur at intervals much less than the time required for corals (populations and communities) to recover [21]. Aside from contributing to habitat degradation, climate change may also have direct effects on the distribution, abundance and fitness of coral reef fishes [49]. Experimental studies testing direct effects of climate change on coral reef fishes have shown that 2–4 °C increases in temperature will significantly reduce aerobic scope [50], and may constrain somatic growth [49] and reproductive output [51]. Increases in CO2 concentrations to ≥1,000ppm exacerbate the effects of temperature on aerobic scope [52], but lower levels (∼700 ppm CO2) may also cause impaired ability to distinguish between olfactory cues, such as odors from different habitat types or the smell of predators [53,54]. However, recent changes in environmental conditions have not had any apparent effects on coral reef fishes, and experimental levels shown to significantly affect reef fishes are not likely to be experienced until after 2070 [49]. In contrast, climate-induced coral bleaching is already having significant effects on habitat structure, with secondary effects on reef fishes [24].
3. Effects of Coral Loss on Diversity of Reef Fishes
A significant proportion of coral reef fishes live very close to reef substrates and strongly associate with habitat structure (biological and/or physical) provided by scleractinian corals. Jones et al. [33] suggested that up to 75% of coral reef fishes rely on live corals for food, shelter or settlement. Accordingly, there is often a strong positive relationship between coral cover versus abundance [55-57] and diversity [58] of coral reef fishes. However, coral reef fishes vary in their level of reliance on corals, ranging from highly specialist fishes that are critically dependent on a single coral species for food or habitat [59,60], to fishes that only very loosely associate with live corals [39]. It is also difficult to separate the relative importance of live coral versus the physical structure provided by high cover and diversity of scleractinian corals [61]. Therefore, the effects of coral loss on reef fish assemblages may vary depending upon absolute levels of coral cover (especially after the disturbances), changes in coral composition, and corresponding changes in structural complexity [23]. While it is clear that severe and widespread coral depletion, caused by large-scale disturbances (e.g., coral bleaching), can have broad impacts across a wide range of different fishes and lead to marked reductions in local diversity [23,33], moderate declines in coral cover may actually lead to short-term increases in the local diversity of fishes [23].
The global degradation of coral reef ecosystems [62] has generated considerable concern regarding ecosystem-level consequences of sustained and ongoing coral loss, such that there are a large and increasing number of studies considering effects of coral loss on abundance and diversity of coral reef fishes. To explore variability in the effects of coral loss on local diversity of coral reef fishes, we compiled data from 26 separate studies (Table 1), which collectively considered effects of coral loss on fish assemblages at 75 different reefs throughout the world. Proportional declines in coral cover at individual reefs were related to proportional decreases or increases in the species richness of fishes recorded during the course of an acute natural, anthropogenic, or experimentally-induced reduction in total coral cover. Only studies that present data on coral cover and species richness from before and after distinct disturbances were included. Moreover, analyses were restricted to those studies (and reefs) where coral cover declined by >10%. This meta-analysis of data collected from throughout the world was intended to address two critical questions:
What proportion of reef fish species is likely to be lost for a given decline in live coral cover? Given that many reef fishes associate with coral-rich habitats, extensive coral loss is expected to have a negative effect on the diversity of coral reef fishes [58]. It is possible however, that some fishes may benefit from changes in habitat-structure and disappearance of coral-dependent fishes, such that fish diversity may be unchanged [63,64]. Predicting the responses of fish assemblages to coral loss is particularly important given sustained and ongoing climate change, which is likely to alter the structure of coral reef habitats [48].
Do geographical regions with naturally depauperate fish and coral assemblages exhibit disproportionate vulnerability to acute disturbances? If increased biological diversity enhances the stability and resilience of natural communities [1], we would expect to find that locations with comparatively low biodiversity (e.g., Caribbean) are much more susceptible to environmental perturbations compared to biodiversity hotpots (e.g., the Indo-Australian Archipelago).
3.1. Relationship between Coral Loss and Species Richness of Reef Fishes
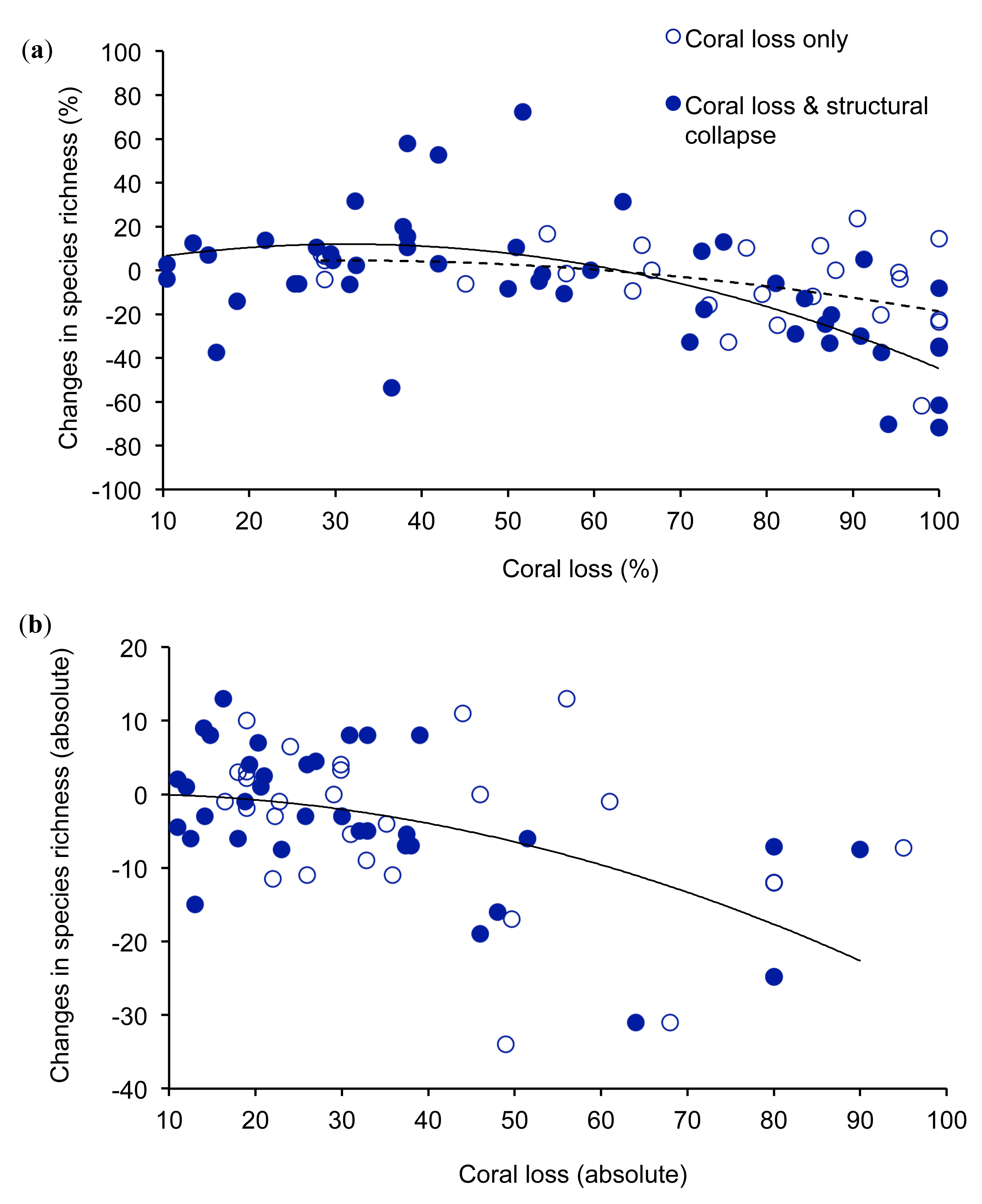
Studies on changes in the local diversity of coral reef fishes following acute disturbances show that very extensive coral loss has a marked effect on the diversity of reef fishes (Figure 1). The overall relationship between percentage coral loss and changes in fish diversity was non-linear and best represented using a polynomial (r2 = 0.21, n = 75), where the y-intercept is set to 0. Where coral loss exceeded 60%, diversity of fishes generally declined, although there were some examples of negligible loss of diversity when coral decline was >80%. In contrast, moderate levels of coral loss often had limited or positive effects on the local diversity of reef fishes (Figure 1a). This complex relationship may reflect differential responses of coral loss on coral-dependent compared to generalist fishes, which may benefit from some reduction in overall coral cover [63]. Increases in local diversity of fishes following moderate declines in coral cover may result from increases in overall habitat heterogeneity, whereby reefs with intermediate levels of coral cover are likely to support a diverse mix of fishes that live on live coral versus non-coral substrates [23]. However, extensive (>60%) coral loss may lead to a net decline in the diversity because increases in diversity of generalist species cannot compensate for the local extirpation of coral-dependent species. There were however, several examples where moderate declines in live coral cover had a disproportionate effect on the local diversity of fishes. During experimentally induced disturbances on the GBR, in which coral cover was reduced by 16–36%, the proportional decline in diversity of fishes was 1.8–2.3 times the proportional coral loss [76]. Studies that revealed marked declines in diversity of fishes following minor (<20%) coral loss are mostly conducted at very restricted spatial scales and tend to consider a very restricted set of fishes, mostly coral-dependent fishes. It is likely therefore, that these studies overestimate changes in local diversity of coral reef fishes.
The unexplained variation in changes in local diversity of coral reef fishes for a given level of coral loss is likely due to a number of confounding influences [23], whereby effects of acute disturbances on reef fishes may be more or less severe depending on associated changes in biological and physical structure of reef habitats. Most importantly, the effects of coral loss on coral reef fishes are expected to be much more pronounced when coral loss is combined with declines in structural complexity of reef habitats [22,82], but few studies have explicitly measured changes in structural complexity associated with acute disturbances. In this study, we assumed that biological disturbances (e.g., bleaching and outbreaks of A. planci) would have limited influence on structural complexity of reef habitats in the short-term, compared to physical disturbances (e.g., cyclones) [23,24]. However, the longer-term effects of biological disturbances may be equivalent to that of physical disturbances [83] as the skeletons of dead corals are highly susceptible to biological and physical erosion [84]. Declines in topographic complexity tend to occur 4–5 years after acute biological disturbances [24], so we tested whether short-term (<4 years) effects of coral loss are less severe compared to physical disturbances or longer-term (>4 years) effects of biological disturbances, where coral loss is likely to be compounded by declines in the physical structure of reef habitats. When considering only those studies where coral loss may be accompanied by changes in structural complexity, the relationship between coral loss and biodiversity loss for fishes is much stronger (r2 = 0.36, n = 48), and at high levels of coral loss, resulting declines in diversity of fishes are more pronounced (Figure 1). Structural collapse of coral habitat is not a necessary consequence of coral loss; in some environments, such as highly exposed reef fronts, corals contribute very little to topographic complexity [64,83]. However, in the absence of direct and consistent measures of structural complexity, comparisons between biological versus physical disturbances may indicate the extent to which changes in structural collapse accounts for observed variation in the relationship between coral loss and changes in species diversity of reef fishes.
Variation in the effects of coral loss on diversity of fishes may also depend on the absolute level of coral loss [23]. When looking at absolute (rather than proportional) changes in coral cover and fish diversity, it is clear that high levels of coral loss (e.g., >40% coral loss) almost universally, lead to declines in diversity of reef fishes, especially when caused by physical disturbances (Figure 1b). In this instance, large declines in absolute coral cover are only possible when coral cover is very high prior to disturbance, such that habitats are likely to be dominated by fishes with strong-reliance on habitat-forming corals. Overall, there was a strong negative relationship between absolute coral loss and absolute changes in fish diversity (r2 = 0.41, n = 74), which did not differ greatly between biological versus physical disturbances.
3.2. Geographical Variation in Effects of Coral Loss
The Indo-Australia Archipelago (IAA) is the world's richest coral reef biodiversity hotspot, encompassing coral reefs of northern Australia, Papua New Guinea, Solomon's, Indonesia, and Philippines [85]. Species richness of coral reef fishes declines five-fold as you move from the IAA to the central Pacific, and declines even further as you move further east [85]. Reef fish diversity also declines (but much less rapidly) as you move west across the Indian Ocean. However, the lowest diversity of coral reef fishes (and corals) is found in the Caribbean [25]. Furthermore, these global diversity gradients are reflected within most major families of coral reef fishes [1]; declines in the diversity of reef fishes as you move away from the IAA are attributable to declines in the number of species within each major family, rather than loss of entire families. Consequently, some key functional groups are represented by only one (or very few) species in highly depauperate locations. These locations are thus expected to be highly susceptible to acute disturbances, whereby the loss of entire families will compromise ecosystem function and may have a range of feedbacks that ultimately result in extensive biodiversity loss [1]. Accordingly, the most pronounced changes in the structure of benthic assemblages (e.g., phase shifts from coral- to macroalgal-dominance) have occurred in depauperate and marginal reef locations, such as the Caribbean and eastern Pacific [1].
Despite regional differences in the extent of coral decline recorded, recent episodes of coral loss appear to have had a disproportionate effect on fishes in areas of high, not low, diversity (Table 2). Observed changes in the species richness of reef fishes were greatest in the central Pacific (e.g., Samoa, and French Polynesia) but these locations also experienced the greatest coral loss. When changes in species richness were considered relative to local levels of coral loss, the standardized declines in fish diversity were greatest for the IAA (Table 2). These data suggest that low diversity systems are not necessarily more susceptible to acute disturbances, though we must also consider geographical differences in disturbance history. In the Caribbean, for example, there has been a long-history of anthropogenic disturbances that have greatly altered contemporary fish communities [86], possibly favoring more generalist species that are less susceptible to environmental perturbations. Our results are however, very consistent with Mora et al [31], who conducted a comprehensive global analysis of interactions between human population density, biodiversity of fishes, and ecosystem functioning on coral reefs. This study showed strong positive relationships between biodiversity and ecosystem functioning (measured as standing biomass of coral reef fishes), but also found that more diverse locations are more (not less) susceptible to anthropogenic pressures (e.g., fishing) imposed by high human populations [31]. Mora et al. [31] argued that high diversity systems have evolved greater specialization and increased efficiency in energy transfer, such that the loss of species will have disproportionate effects on ecosystem functioning. More importantly, the relationship between biodiversity and ecosystem functioning was non-asymptotic (non-saturating) suggesting that even in high diversity systems the addition or loss of species will have potentially devastating effects.
4. Response Diversity among Coral Reef Fishes
Declines in diversity of coral reef fishes have significant connotations for ecosystem function and productivity of coral reef environments [25]. The extent to which species losses will translate to loss of key ecological functions depends on the number of species that fulfill a particular function (functional redundancy) and variation in responses to environmental perturbations among fishes within each functional group (response diversity). In this study, responses of fishes were calculated based on proportional changes in the individual abundance of each species relative to proportional loss of coral cover recorded during an acute distrubance event (e.g., 10% decreases in both coral and fish species abundance means a one-fold decrease in fish abundance while a 10% decrease in coral cover and 30% decrease in fish abundance would mean a three-fold decrease in fish abundance). To explicitly test for response diversity among reef fishes, data was compiled from a variety of studies that have looked at species-specific responses to distinct episodes of coral loss following Pratchett et al. [24]. Each of these studies quantified changes in the abundance of one or more species of coral reef fishes before and then one-seven years after distinct episodes of coral loss. The total data set involved 1360 observations (changes in the abundance of a specific species at a specific location), from 30 different studies, most of which are listed in Table 2. This study did not consider those studies that have considered differential responses among pre-defined functional groups [87], rather data on the individual responses of fish species were used, and we independently assigned species to specific functional groups. The appropriate recognition and classification of key functional groups is fundamental in testing for response diversity among coral reef fishes. To date, there has not been any widely accepted systematic approach to identify key functional groups of coral reef fishes equivalent to functional assessments completed for other major groups (e.g., macroalgae [88]). While many studies have used functional classifications of coral reef fishes, they have generally tailored the classifications to the objectives of the study. Consequently, there has been considerable variation in the choice of functional groups and the criteria used to assign species to these groups.
To provide an objective assessment of the response diversity of coral reef fishes, and to allow comparisons among broadly and narrowly defined functional groups, we employed a hierarchical classification of functional groups. Species were first assigned to one of four primary functional groups based on their trophic function (i.e., carnivores, omnivores, corallivores, and herbivores). These primary functional groups have been widely used and generally reflect their role in the flow of energy through reef food chains [25]. It should be noted, however, that detritivores were included in the herbivore functional group, based on the mechanical removal of algal or detrital material from the reef (i.e., nominally herbivorous fish [89]). This holistic approach was necessary, as several studies have shown that, from a nutritional perspective, many of the fish traditionally classified as herbivores are better defined as detritivores [89,90]. Species were subsequently assigned to secondary functional groups based on feeding mode [91], diet [60,89,92], and behavior [93]. These secondary functional groups are not exhaustive but were selected to reflect their role in ecosystem processes and/or the source of their prey (Table 3).
4.1. Variation in Responses
Species within a particular functional group may differ in their responses to disturbances (and resource depletion) owing to differential reliance on highly susceptible resources (e.g., coral), differences in their ecological versatility [24], distribution relative to disturbed sites and habitats [94], or variation in the scales at which they operate (e.g., due to differences in home ranges) [7,95]. Species in strong competition for specific resources are also expected to exhibit compensatory changes in abundance during acute disturbances, which would further contribute response diversity within functional groups [95]. However, given that most functional groups are based on dietary composition and feeding behavior, it is also possible that entire functional groups will respond similarly during disturbances [7], especially highly specialist groups, such as corallivores [8]. If so, high redundancy and high biodiversity will contribute little to ecological stability and ecosystem resilience [25]. Previous studies have demonstrated contrasting responses to disturbance between functional groups (e.g., herbivores versus corallivores) [24,39], but how variable is the response of species within a functional group and how does the within-group variation compare to differences among groups?
To compare variation in responses (changes in the abundance of fishes relative to local coral loss) within versus among functional groups, we used a three-level nested ANOVA. We also calculated variance components to assess the relative contributions of (i) primary functional groups (four-levels), (ii) secondary functional groups (19-levels), and (iii) species (403 species) in accounting for differential responses of fishes to coral loss. If there is very high response diversity among fishes within functional groups, we would expect to find a disproportionate amount of variance explained by species. If however, responses of fishes are strongly influenced by their specific ecological functions (e.g., herbivores versus carnivores) and there is relatively limited response diversity within groups, then most of the variation is likely to be explained by either primary of secondary functional groups. Variance components in responses of fishes from 30 different studies and 403 species revealed very limited response diversity within functional groups (Figure 2). Despite significant variation in responses of individual species (nested within primary and secondary functional groups), it was the distinction between primary functional groups (corallivores, herbivores, omnivores and carnivores) that accounted for the greatest proportion of variation (62.4%) in responses of fishes. Differences among secondary primary groups (which provides greatly increased resolution in separating functionally-important groups of fishes) accounted for much greater variation compared to individual species, but differences in mean responses between these groupings were not significant (Figure 2). This analysis shows that fishes with fundamentally different trophic functions vary in their responses to coral loss (which is already known [24]), but also that there is relatively low response diversity within most functional groups. Perhaps more important, however, is the relative levels of response diversity apparent within each functional group (Figure 3, Table 4).
To compare within-group variation across different functional groups we constructed frequency distributions of species responses (Figure 3). These plots revealed a wide range of responses for planktivores, carnivores and herbivores, but relatively limited response diversity among corallivorous fishes (Figure 3). Importantly, modal responses were much more pronounced for corallivores, compared to all other groups, whereby most species studied exhibited a > one-fold decline in abundance following local coral loss. The strong modality in the distribution of responses for corallivorous fishes is reflected in the low value for kurtosis (Table 3). Kurtosis is a commonly used descriptor of size-frequency distributions, which accounts for both the concentration and spread of data [96]; high levels of kurtosis generally reflect “fatter tails” or an over-representation of extreme values. As such, we used kurtosis as a proxy for response diversity, and compared values across primary and secondary functional groups, wherever there was sufficient data (>30 observations) to construct meaningful frequency distributions of individual responses (Table 3). Kurtosis was very low (4.3–5.3) across all groups of corallivores, but was generally much higher (>30.0) for herbivores, omnivores and carnivores. However, this does not in itself guarantee the persistence of key ecological functions during natural or anthropogenic disturbances. Despite marked differences in response diversity, it was apparent that more species declined in abundance than increased in abundance across all four groups (Figure 3), such that the median response was always negative.
Overall, there were strong negative effects of coral loss on the individual abundance of coral reef fishes. In 60% (815 out of 1360) of all observations, local abundance of fishes declined following declines in live coral cover. Moreover, declines in the abundance of fishes were often (in 499 out of 1360 cases) disproportionately (up to five-fold) greater than proportionate loss of live coral cover. For example, Syms and Jones [76] documented the local extirpation of Chaetodon trifascialis following a 14% decline in live coral cover. As such, the decline in abundance of C. trifascialis was >five-fold greater than declines in the abundance of live coral. Disproportionate declines in the abundance of fishes are most likely attributable to selective effects of coral loss. In the former example, marked declines in the abundance C. trifascialis probably relate to extensive loss of preferred coral prey even though overall coral loss was very moderate. Chaetodon trifascialis is among the most highly specialized of coral reef fishes and feeds almost exclusively on only one type of coral, tabulate Acropora [60]. Accordingly, spatial and temporal changes in the abundance of this species are strongly related to changes in the abundance of tabulate Acropora, rather than overall changes in live coral cover [97]. Similarly, for other fishes (e.g., among diurnal planktivores that often rely on coral for shelter) the extent to which coral loss affects local population size, probably relates to the changes in the local availability of very specific coral-types [98], or even the relative size and complexity of different coral hosts. However, the high proportion of fishes that decline in abundance following coral depletion supports the notion that many different fishes benefit from extensive cover (and probably diversity) of corals [33], even though relatively few species may be explicitly reliant on coral for food or shelter [24]. However, widespread declines in abundance of fishes were sometimes offset by very pronounced increases in the abundance for the small number of species that seemingly benefit from local coral loss. In extreme examples, the abundance of fishes (often herbivorous species) increased 10-fold following coral depletion, such that there was an overall increase in the mean abundance of herbivores, omnivores and carnivores (Table 4).
Most studies ascertain effects of coral loss on reef fishes by quantifying changes in their distribution and abundance [99], which implicitly assumes that such disturbances will lead to widespread mortality and/or migration. It is possible, however that fishes that do not exhibit immediate and pronounced changes in abundance are nonetheless negatively affected by severe coral loss, whereby coral loss leads to declines in growth or condition [100,101]. Coral feeding fishes are generally considered to be among the first and worst affected fishes following extensive coral loss. On the GBR however, local densities of all coral feeding butterflyfishes were unchanged for >2 years after 90% loss of live coral, caused by severe bleaching in 2001–02 [77]. These butterflyfishes did eventually (after five years) succumb to local coral depletion, and several species went locally extinct, but short-term effects of coral loss on these species were only apparent by quantifying changes in the physiological condition of individual fishes [101]. It is also possible, that some species may fluctuate in abundance independent of local changes in habitat structure. For example, rapid and pronounced increases in the abundance of herbivorous fishes following localized coral loss may be caused by aggregation of adult fishes at highly disturbed locations [23] or pronounced pulses of recruitment, but it is unclear to what extent these are attributable to habitat changes. Many fishes, especially short-lived species, exhibit random fluctuation in local abundance due to marked inter-annual variation in recruitment [102]. There are also likely to be apparent changes in the abundance of some species due to vagaries associated with random sampling, whereby there may be as much variation in abundance of species between successive surveys conducted minutes versus years apart [103]. As a consequence, our meta-analyses of data from different studies are likely to have significant overestimated response diversity within functional groups. Moreover, the concept of functional redundancy implicitly assumes that all species contribute equally in fulfilling necessary functions, whereas common or large species often exert a disproportionate role in ecosystem stability and function [104]. We must also consider the relative importance of different functional groups, relative to their apparent level of response diversity. For example, it is apparent that corallivorous fishes (especially, obligate coral feeding species) have limited response diversity (Figure 3), but are coral-feeding fishes critically important to ecosystem function? The remainder of this study focuses on corallivorous fishes, which are the worst affected groups of fishes, and herbivorous fishes, which are potentially the most important functional group.
4.2. Corallivorous Fishes
Corallivorous fishes have the most apparent and direct reliance on live corals [105,106] and are often among the worst affected fishes following distinct episodes of coral loss [24]. Accordingly, the response diversity among corallivorous fishes was much lower than for all other functional groups (Figure 3). It is also possible that the strong consistency in responses of corallivorous fishes is attributable to the extensive research that has been undertaken to clarify and confirm the trophic function and dietary composition of nominal corallivores [106], whereas some other functional groups (e.g., herbivores and carnivores) are less well defined. However, the extent to which corallivorous fishes feed on corals (vs. other non-coral prey) is highly variable [60], such that most studies distinguish between those fishes that feed exclusively on live corals (obligate corallivores) versus those fishes that only partly or occasionally consume corals (facultative corallivores) [60]. There is also a third group of coral-feeding butterflyfishes, which specialize on soft (alcyonarian) corals as opposed to hard (scleractinian) corals [60]. Corallivores within each of these secondary functional groups tend to decline in response to coral loss, however the magnitude of this response is highest, and the response diversity (kurtosis) is lowest for obligate corallivores (Table 3). These differences were not large (particularly when compared to differences among primary groups), but are consistent with expected differences in their responses to coral loss, whereby obligate coral feeders are much more reliant on live corals and likely to be much more affected by coral loss compared to facultative coral feeders. Detailed research on Chaetodon butterflyfishes [72,77], shows that facultative corallivores are relatively unaffected by moderate decline in abundance of corals, presumably because they can compensate by increasing intake of non-coral prey. However, corals may still represent an important and necessary component of their diet, such that even facultative corallivores are adversely affected by extensive coral loss [77].
Coral feeding represents a relatively unique and potentially important trophic link between scleractinian corals and higher consumers [106]. Corallivorous fishes make this carbon immediately available to higher consumers, and greatly increase transfer efficiency of energy from corals to fishes. While many early researchers suggested that there were few fishes capable of feeding on corals [105], recent reviews of corallivory reveal that there are at least 128 species of reef fishes from 11 families that feed on coral [106,107]. Despite their diversity, the functional-importance of corallivorous fishes is equivocal. One interesting observation, which suggest that corallivorous fishes are not overly important, is that obligate corallivores are one of the only functional groups of fishes (e.g., among all secondary functional groups listed in Table 3) that are not represented across all coral reef regions. Although recently degraded, coral reef assemblages in Caribbean have been isolated from Indo-Pacific reefs for >three million years and persisted despite the lack of obligate coral feeding fishes [107,108]. This lack of obligate coral-feeding fishes may be compensated by the local diversity of obligate coral-feeding invertebrates [109], whereby there are a very large number of invertebrate species known to feed (both obligately and facultatively) on live coral tissues. However, overall effects of corallivores on habitat-forming corals and entire reef ecosystems are generally considered to be damaging [107], reducing growth, fitness, or ultimately abundance of live corals.
4.3. Nominal Herbivores
Concern regarding the loss of key functional groups on coral reefs mostly centers on herbivorous fishes, and particularly grazing species [25,94]. Herbivorous fishes (and invertebrates) are believed to play a critical role in controlling macroalgae, which might otherwise monopolize reef substrates [25] and prevent population replenishment by corals and other habitat-forming species [110]. This is especially important given the increasing diversity, frequency and severity of disturbances that threaten corals, because continual grazing of reef algae is fundamental to the recovery and resilience of coral assemblages [26]. Extensive coral mortality provides space on the reef that is rapidly colonized by turf algae [111]. On reefs where herbivorous fishes and/or sea urchins are abundant, algal assemblages may remain as cropped turf forms following coral bleaching and coral loss. However, where grazing pressure is low, or if coral mortality is extensive, herbivores may be unable to counter increased algal abundance and macroalgal blooms can develop [27,111,112], bringing about a phase shift from coral- to macroalgal-dominated reef habitats.
Although our results show that there is a degree of response diversity among herbivorous fish species as a whole (Figure 2), there was moderate response diversity within each of the herbivorous functional groups, with low response diversity among the territorial farmers (Table 4). This distinction is important as inferences from the primary ‘herbivore’ functional group imply that all herbivorous species perform a similar role. There is, however, considerable variation in the feeding behavior of these fishes [113], and this variation is directly related to the impact on reef processes [25]. Whilst excavating, scraping, and cropping taxa generally consume algal turfs, they perform different and complimentary roles in helping reefs to resist shifts to alternate states and reassemble following disturbances. Croppers remove the upper portions of the algae (and associated epiphytic material), and in doing so prevent the growth and development of macroalgal species; scraping taxa remove pieces of the substratum together with the algae, clearing space for the settlement of other organisms; and excavating taxa remove large amounts of carbonate material when feeding and play an important role in external bioerosion. Furthermore, the removal of adult macroalgae appears to be restricted to a limited suite of species (i.e., macroalgal browsers [30,89,114]) and represents a separate but critical process in the reversal of phase-shifts [115]. Differential responses of herbivorous fishes to coral loss probably reflects their differential reliance on coral structures; territorial farmers showed the most consistent responses, whereby all species were negatively affected by severe coral loss. This finding may relate to their smaller size and increased site fidelity, making them more prone to habitat modification from disturbances compared to other larger more mobile herbivorous species. It is also apparent that many territorial farming damselfishes favor sites with high coral cover in which to establish territories [116].
Clearly, consideration needs to be given to the role of the species in ecosystem processes rather than broad trophic groups (e.g., herbivores) when assessing response diversity and functional redundancy. Recent studies have shown that even the functional redundancy of herbivores appears to have been overestimated, with single species performing a disproportionate role in ecosystem processes [30,117]. In such instances, the loss of a single species may compromise the entire process. For example, overfishing of the excavating parrotfish, Bolbometopon muricatum, has resulted in a near total loss of external bioerosion on several Indo-Pacific reefs [117]. This loss of function has occurred despite the presence of several other excavating species (four-eight species) on these reefs. It has also been suggested that functional redundancy and response diversity may be influenced by the scales at which different species operate, with species operating at different scales providing mutual reinforcement for function maintenance [94]. Among reef fishes, however, there are limited differences in the scales at which species operate, and nearly all species operate at scales below the spatial extent of most major disturbances (e.g., cyclones, bleaching and outbreaks of A. planci). Recent studies have demonstrated that herbivorous coral reef fish are relatively site attached, at least for those species examined to date, and have a more restricted home range than previously assumed (<10 ha; [118,119]).
5. Loss of Species versus Loss of Functions
The global (comprehensive) extinction of a species represents the most extreme (and irreversible) consequence of environmental perturbations and habitat degradation, and there is an entire field of conservation research dedicated to establishing which species are most at risk of global extinction. Several authors have argued that rates of global extinction are burgeoning and we are entering the Earth's sixth mass extinction event [120-122]. There is however, very sparse evidence for global extinctions among coral reef fishes [123]. Local extinction, the complete loss of a species from part of its range, is far more common. The local extinction of reef fishes following coral mortality events has been documented from several Indo-Pacific locations, including PNG [59], the Great Barrier Reef [77] and the Seychelles [22]. Furthermore, a recent predictive framework to assess extinction risk of coral reef fish to coral loss and fishing, estimated that approximately one third of species are vulnerable to local extinction [8]. To date, the species that have gone locally extinct tend to be highly coral dependent species [77,100]. The relevance of these losses to ecosystem function is equivocal, as there is limited evidence of their role within the wider ecosystem. However, changes in species abundances and biomass can have profound impacts on ecosystem functioning long before the last individual disappears [120].
Perhaps the best way to conceptualize the impacts of large declines in species abundance is through ecological extinction. Ecological extinction occurs when a species is unable to maintain key functions because the species has become too rare or too widely dispersed. This concept is particularly useful because it is often the most abundant species within a community or functional group that perform the greatest roles in terms of ecosystem function [124]. Identifying large fluctuations in the abundance of species, and the loss of key functions, may therefore be a far more useful exercise in assessing ecosystem impacts than identifying how many rare species have been lost.
Following large-scale disturbances on coral reefs, there are well documented declines in the abundance of corallivorous fishes [77,125,126], and planktivores [22,23], as well as longer-term reductions in the abundance of herbivorous fishes [24]. Of these changes, it is the decline in abundance of herbivorous fishes that is considered to have the greatest influence on ecosystem function [25,110,127]. A critical goal, therefore, is to assess the relationship between abundance and function of herbivorous fishes with a view to predicting what level of disturbance (changes in coral cover and relative declines in the abundance of fishes) is likely to compromise ecosystem function and state. There is often a direct negative relationship between macroalgal cover and biomass of herbivorous fishes [128], but the extent to which these fishes can graze reef substrates with sufficient frequency to keep areas of algal turfs from developing in macroalgae, depends on the proportion of reef substrates occupied by algae versus corals and other sessile benthos. It is possible therefore, that pronounced declines in live coral cover may lead to a proliferation of macroalgae independent of any changes in abundance of herbivorous fishes [129]. Research in to the functional importance of reef species must also consider invertebrates (e.g., urchins), which may complement ecological functions of coral reef fishes. Importantly, herbivorous sea urchins may provide the redundancy and response diversity necessary to maintain herbivory following overfishing of herbivorous fishes [110]. However, the reliance of an ecosystem function on a limited or distinct set of species is likely to increase susceptibility to future disturbances [12,110].
6. Conclusions
Marine fishes are an important component of shallow coastal environments, not only in sustaining global fisheries, but also in maintaining ecosystem function. On coral reefs, Bellwood et al. [25] showed that certain fishes fulfill ecological functions that are fundamental in maintaining ecosystem state. Declines in the abundance of functionally important fishes, may for example, result in a phase shift to a less desirable ecosystem state [26,86]. The loss of an entire functional group, especially those comprising multiple species, may appear unlikely except during extreme (severe or prolonged) disturbances [110]. However, functional redundancy among coral reef fishes may have been significantly overstated [92]; even in high diversity systems, some functions may be performed by just one or few species [30]. It is also important to separate functional redundancy from response diversity [7]. If entire groups of fishes respond similarly to disturbance (e.g., if all species are extirpated following climate-induced coral bleaching), then ecological functions will stop irrespective of how many species fulfill that role [8]. Thus, functional redundancy in the absence of response diversity will give a false sense of security [25]. Even within high diversity locations and functional groups, all species may be important and individually contribute to increased efficiency in biogeochemical and trophic functions [3,31,130].
This study has shown that variation in responses of coral reef fishes to acute episodes of coral loss is greater among, rather than within, key functional groups. There was however, marked variation in apparent levels of response diversity among key functional groups. Relatively high response diversity among herbivorous fishes, and their often positive response to coral loss, is of course heartening, as it is this group that are arguably the most important in terms of resilience and reef recovery [25]. However, high levels of response diversity among herbivorous fishes were at least partly attributable to the differential functions of herbivorous fishes (e.g., among secondary functional groups), and some functional groups (e.g., territorial damselfishes) have relatively limited response diversity. As such, regions with high biodiversity and presumed increases in functional redundancy will not necessarily have any increased resilience compared to low diversity regions. Current levels of habitat degradation for coral reef ecosystems are actually much higher within high biodiversity regions, such as the Indo-Pacific archipelago [131], such that sustained and ongoing disturbances may have a disproportionate impact on the global biodiversity of coral reef organisms.
Despite its importance in coral reef resilience [7], this is the first study that has attempted to explicitly measure response diversity within and among functional groups of coral reef fishes. This is a critical step in moving beyond theoretical discussions regarding possible consequences of biodiversity loss, but there is still considerable work required to better understand of the role of response diversity in coral reef resilience. Most importantly, variation in the responses of individual species during acute disturbance events should be related not only to wholesale loss of coral cover, but also changes in the relative abundance of different benthic organisms, including different coral species, soft corals and specific algal types [77]. There is also a need to explicitly measure changes in structural complexity of coral reef habitats to tease apart the relative role of corals loss versus structural collapse, which can be readily measured based on visual assessments of landscape complexity [132]. Finally, previous authors that have measured responses of fishes to acute disturbances (Table 1) should consider re-visiting study sites to assess recovery in habitat and fish assemblages. Resilience is the capacity of populations, communities, or ecosystems to withstand disturbances, based on either resistance or recovery [133]. A critical component of response diversity, which is yet to be considered, is therefore, the differential capacity of species within distinct functional groups to recover (and restore ecosystem function) in the aftermath of acute disturbances. There are a few studies that have considered differential recovery of fishes to major disturbances [44,134], but still too few to conduct a meaningful meta-analysis and establish general patterns of differential resilience among coral reef fishes. These data are however critical in understanding the responses of reef fishes and coral reef ecosystems to sustained and ongoing increases in climate change and other anthropogenic pressures.


| Disturbance | Location | Coral cover | Time (years) | Source | |
|---|---|---|---|---|---|
| Before | After | ||||
| Bleaching and coral disease | Arabian Gulf | 90 | 22–26 | 3 | Riegl [65] |
| Kenya | 26 | 23 | 5 | McClanahan [66] | |
| Tanzania | 33 | 0–3 | 6 | Garpe et al. [42] | |
| Seychelles | 23–50 | 0–17 | <1 | S. Jennings, unpub. data | |
| Seychelles | 29–64 | 0–10 | 1 | Spalding & Jarvis [67] | |
| Chagos | 25–35 | 7–33 | 8 | Graham et al. [20] | |
| Chagos | 39–69 | 8–47 | 3 | Sheppard et al. [40] | |
| Japan | 95 | 0 | 2 | Sano [68] | |
| Japan | 39 | 3 | 1 | Shibuno et al. [69] | |
| GBR, Australia | 39–63 | 9–45 | <1 | Thompson & Malcolm [70] | |
| Crown-of-thorns | Japan | 80 | 0 | 2 | Sano et al. [41] |
| Samoa | 70 | 57 | 9 | Buckley [71] | |
| French Polynesia | 36 | 20 | 1 | Bouchon-Navaro et al. [72] | |
| Cyclone | GBR, Australia | 13–57 | 9–51 | 1 | Cheal et al. [43] |
| GBR, Australia | 85 | 5 | 4 | Halford et al. [44] | |
| Tuvalu | 4–93 | 1–54 | 3 | G.P. Jones, unpub. data | |
| Cozumel | 13–49 | 1–29 | 1 | Fenner et al. [73] | |
| Martinique | 37 | 23–26 | <1 | Rousseau et al. [74] | |
| Experimental | GBR, Australia | 66 | 29 | 1 | Lewis [75] |
| GBR, Australia | 63–68 | 40–57 | 1 | Syms & Jones [76] | |
| GBR, Australia | 33 | 3 | 4 | Pratchett et al. [77] | |
| French Polynesia | 48–50 | 0–1 | 1 | Holbrook et al. [78] | |
| Japan | 90 | 0 | 1 | Sano [79] | |
| Multiple | Papua New Guinea | 30–53 | 7–8 | 3 | Jones et al. [33] |
| GBR, Australia | 23–45 | 2–20 | 2 | Cheal et al. [80] | |
| French Polynesia | 51 | 25 | 1 | Adjeroud et al. [81] | |
| Data | N | Coral loss | Change in species richness | Standardized response |
|---|---|---|---|---|
| Indo-Australia Archipeligo | 20 | 60.0% (6.3) | −11.0% (5.1) | −20.8% (6.6) |
| Western and central Indian | 26 | 61.1% (6.1) | −1.9% (3.0) | 1.8% (3.1) |
| Central Pacific | 21 | 70.1% (6.4) | −17.5% (7.6) | −13.7% (5.8) |
| Caribbean | 9 | 49.1% (6.8) | 14.2% (10.0) | 36.54% (5.5) |
| Global | 76 | 62.1% (3.3) | −6.7% (3.1) | −4.3% (5.5) |
| Primary functional group | Secondary functional group | Examples |
|---|---|---|
| Corallivore | Obligate corallivore | Chaetodon trifascialis, Labrichthys unilineatus |
| Soft coral feeder | Chaetodon melannotus, C. ulientensis | |
| Facultative corallivore | Chaetodon citrinellus, C. kleinii | |
| Herbivore (including detritivore) | Roving detritivore | Ctenochaetus striatus, Siganus lineatus |
| Site-attached detritivore | Atrosalarias fuscus, Asterropteryx semipunctatus | |
| Excavator | Chlorurus microrhinos, Chlorurus sordidus | |
| Scraper | Scarus ghobban, S. rivulatus | |
| Turf algal cropper | Acanthurus nigrofuscus, Siganus doliatus | |
| Macroalgal browser | Naso lituratus, N. unicornis | |
| Territorial farmer | Pomacentrus adelus, Stegastes apicalis | |
| Omnivore (including planktivore) | Diurnal planktivore | Caesio cuning, Cirrhilabrus punctatus |
| Nocturnal planktivore | Apogon cyanosoma,Myripristis jacobus | |
| Omnivorous planktivore | Abudefduf sexfasciatus, Dascyllus aruanus | |
| Benthic omnivore | Pomacanthus sexstriatus, Neoglyphidodon melas | |
| Carnivore | Piscivore | Plectropomus maculatus, Cephalopholis urodeta |
| Generalist carnivore (fish and invertebrates) | Lutjanus carponotatus, Plectorhinchus flavomaculatus | |
| Macro-invertivore (>5 mm) | Balistapus undulatus, Choerodon anchorago | |
| Micro-invertivore (<5 mm) | Halichoeres melanurus, Stethojulis bandanensis | |
| Ectoparasite feeder | Labroides dimidiatus | |
| Functional group | n | Kurtosis | Mean | Median |
|---|---|---|---|---|
| Corallivore | 220 | 5.2 | −0.8 | −1.0 |
| Obligate corallivore | 127 | 4.3 | −1.0 | −1.0 |
| Soft coral feeder | 10 | - | −0.7 | −1.0 |
| Facultative corallivore | 83 | 5.3 | −0.6 | −0.8 |
| Herbivore | 342 | 45.7 | 0.6 | −0.3 |
| Roving detritivore | 32 | - | 0.0 | −0.8 |
| Site-attached detritivore | 10 | - | −0.3 | −0.2 |
| Excavator | 27 | - | 2.9 | 0.0 |
| Scraper | 78 | 34.7 | 1.0 | −0.4 |
| Turf algal cropper | 100 | 59.3 | 0.4 | −0.4 |
| Macroalgal browser | 6 | - | 1.9 | 1.2 |
| Territorial farmer | 89 | 12.0 | 0.0 | −0.2 |
| Omnivore | 348 | 79.4 | 0.7 | −0.5 |
| Diurnal planktivore | 170 | 71.2 | 1.1 | −0.6 |
| Nocturnal planktivore | 8 | - | 0.5 | 0.0 |
| Omnivorous planktivore | 121 | 26.7 | −0.1 | −0.7 |
| Benthic omnivore | 49 | 45.2 | 1.0 | −0.2 |
| Carnivore | 450 | 219.4 | 0.3 | −0.4 |
| Piscivore | 93 | 87.6 | 0.7 | −0.7 |
| Generalist carnivore | 105 | 47.2 | 0.5 | −0.4 |
| Macro-invertivore | 147 | 3.7 | −0.1 | −0.3 |
| Micro-invertivore | 84 | 46.3 | 0.3 | −0.3 |
| Ectoparasite feeder | 20 | - | 0.5 | −0.1 |
Acknowledgments
All authors are grateful for significant and ongoing support from their partners and families, enabling them to indulge important and interesting scientific pursuits. We are also grateful to colleagues and collaborators who provided data used in this study, especially unpublished data sets. Finally, thank you to the anonymous reviewers, whose insightful comments have greatly improved this manuscript.
References
- Bellwood, D.R.; Hughes, T.P. Regional-scale assembly rule and biodiversity of coral reefs. Science 2001, 292, 1532–1534. [Google Scholar]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar]
- McCann, K.S. The diversity-stability debate. Nature 2000, 405, 228–233. [Google Scholar]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Ann. Rev. Ecol. Syst. 2004, 35, 557–581. [Google Scholar]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.J.B.; Lotze, H.; Micheli, F.; Palumbi, S.R.; et al. Impacts of biodiversity loss on ocean ecosystem services. Science 2006, 314, 787–790. [Google Scholar]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, M.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar]
- Graham, N.A.J.; Chabanet, P.; Evans, R.D.; Jennings, S.; Letourneur, Y.; MacNeil, M.A.; McClanahan, T.R.; Ohman, M.C.; Polunin, N.V.C.; Wilson, S.K. Extinction vulnerability of coral reef fishes. Ecol. Lett. 2011, 14, 341–348. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.J.B.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar]
- Short, F.; Neckles, H. The effects of global climate changes on seagrasses. Aquat. Bot. 1999, 63, 3–4. [Google Scholar]
- Steneck, R.; Graham, M.; Bourque, B.; Corbett, D.; Erlandson, J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar]
- Alongi, D.M. Present state and future of the world's mangrove forests. Biol. Conservat. 2002, 29, 331–349. [Google Scholar]
- Wooldridge, S.; Done, T. Improved water quality can ameliorate effects of climate change on corals. Ecol. Appl. 2009, 19, 1492–1499. [Google Scholar]
- Walther, G.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.; Fromentin, J.; Hoegh-Guldberg, O.; Bairlein, F. Ecological response to recent climate change. Nature 2002, 416, 389–395. [Google Scholar]
- Jokiel, P.; Coles, S. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162. [Google Scholar]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwat. Res. 1999, 50, 839–866. [Google Scholar]
- Oliver, J.K.; Berkelmans, R.; Eakin, C.M. Coral Bleaching in Space and Time. In Coral Bleaching: Pattenrs, Processes, Causes and Consequences; van Oppen, M. J. H., Lough, J.M., Eds.; Springer-Verlag: Heidelberg, Germany, 2009; pp. 21–39. [Google Scholar]
- Goreau, T.; Mcclanahan, T.; Hayes, R.; Strong, A. Conservation of coral reefs after the 1998 global bleaching event. Conserv. Biol. 2000, 14, 5–15. [Google Scholar]
- Graham, N.A.J.; McClanahan, T.R.; MacNeil, M.A.; Wilson, S.K.; Polunin, N.V.C.; Jennings, S.; Chabanet, P.; Clark, S.; Spalding, M.D.; Letourneur, Y.; et al. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS One 2008, 3, e3039. [Google Scholar]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 2005, 11, 2251–2265. [Google Scholar]
- Graham, N.A.J.; Wilson, S.K.; Jennings, S.; Polunin, N.V.C.; Bijoux, J.P. Dynamic fragility of oceanic coral reef ecosystems. Proc. Natl. Acad. Sci. USA 2006, 103, 8425–8429. [Google Scholar]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Change Bio. 2006, 12, 2220–2234. [Google Scholar]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.J.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.C.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes—Ecological and economic consequences. Oceanogr. Mar. Biol. Ann. Rev. 2008, 46, 251–296. [Google Scholar]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar]
- Hughes, T.P.; Graham, N.A.J.; Jackson, J.B.C.; Mumby, P.J.; Steneck, R.S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 2010, 25, 633–642. [Google Scholar]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B.L. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 2007, 17, 360–365. [Google Scholar]
- Steneck, R.; Dethier, M.N. A functional group approach to the structure of algal-dominated communities. Oikos 1994, 69, 476–498. [Google Scholar]
- Hoey, A.S.; Bellwood, D.R. Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 2008, 27, 37–47. [Google Scholar]
- Hoey, A.S.; Bellwood, D.R. Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems 2009, 12, 1316–1328. [Google Scholar]
- Mora, C.; Aburto-Oropeza, O.; Ayala Bocos, A.; Ayotte, P.M.; Banks, S.; Bauman, A.G.; Beger, M.; Bessudo, S.; Booth, D.J.; Brokovich, E.; et al. Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 2011, 9, 1–30. [Google Scholar]
- Wilson, S.K.; Adjeroud, M.; Bellwood, D.R.; Berumen, M.L.; Booth, D.; Bozec, Y.M.; Chabanet, P.; Cheal, A.; Cinner, J.; Depczynski, M.; et al. Crucial knowledge gaps in current understanding of climate change impacts on coral reef fishes. J. Exp. Biol. 2010, 213, 894–900. [Google Scholar]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar]
- Cheung, W.W.L.; Pitcher, T.J.; Pauly, D. A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol.Conservat. 2005, 124, 97–111. [Google Scholar]
- Newton, K.; Côté, I.M.; Pilling, G.M.; Jennings, S.; Dulvy, N.K. Current and future sustainability of island coral reef fisheries. Curr. Biol. 2007, 17, 655–658. [Google Scholar]
- Pauly, D.; Christensen, V.; Guenette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar]
- Coker, D.J.; Pratchett, M.S.; Munday, P.L. Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 2009, 20, 1204–1210. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.J.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V.C.; Rushton, S.P. Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob. Change Biol. 2008, 14, 2796–2809. [Google Scholar]
- Sheppard, C.; Spalding, S.; Bradshaw, C.; Wilson, S. Erosion vs. recovery of coral reefs after 1998 El Niño: Chagos reefs, Indian Ocean. AMBIO 2002, 31, 40–48. [Google Scholar]
- Sano, M.; Shimizu, M.; Nose, Y. Long-term effects of destruction of hermatypic corals by Acanthaster planci infestation on reef fish communities at Iriomote Island, Japan. Mar. Ecol. Prog. Ser. 1987, 37, 191–199. [Google Scholar]
- Garpe, K.C.; Yahya, S.A.S.; Lindahl, U.; Ohman, M.C. Long-term effects of the 1998 coral bleaching event of reef fish assemblages. Marine Ecology Progress Series 2006, 315, 237–247. [Google Scholar]
- Cheal, A.; Coleman, G.; Delan, S.; Miller, I.; Osborne, K.; Sweatman, H.P.A. Responses of coral and fish assemblages to a severe but short-lived tropical cyclone on the Great Barrier Reef, Australia. Coral Reefs 2002, 21, 131–142. [Google Scholar]
- Halford, A.; Cheal, A.J.; Ryan, D.; Williams, D.M. Resilience to large-scale disturbance in coral and fish assemblages on the Great Barrier Reef. Ecology 2004, 85, 1892–1905. [Google Scholar]
- Gardner, T.A.; Côté, I.M.; Gill, J.A.; Grant, A.; Watkinson, A.R. Long-term region-wide declines in Caribbean corals. Science 2003, 301, 958–960. [Google Scholar]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the indo-pacific: Timing, extent, and subregional comparisons. PloS ONE 2007, 2, e711. [Google Scholar]
- Ateweberhan, M.; McClanahan, T.; Graham, N.A.J.; Sheppard, C.R.C. Episodic heterogeneous decline and recovery of coral cover in the Indian Ocean. Coral Reefs 2011, 30, 739–752. [Google Scholar]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar]
- Nilsson, G.E.; Östlund-Nilsson, S.; Munday, P.L. Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp. Biochem. Phys. 2010, 156, 389–393. [Google Scholar]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Pankhurst, N.W.; Pankhurst, P.M. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol.Prog. Ser. 2010, 401, 233–243. [Google Scholar]
- Munday, P.L.; Donelson, J.M.; Dixson, D.L.; Endo, G.G.K. Effects of ocean acidification on the early life history of a tropical marine fish. Proc.Roy. Soc. London. B Biol. Sci. 2009, 276, 3275–3283. [Google Scholar]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Doving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc.Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar]
- Dixson, D.L.; Munday, P.L.; Jones, G.P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 2010, 13, 68–75. [Google Scholar]
- Carpenter, K.E.; Miclat, R.I.; Albaladejo, V.D.; Corpuz, V.T. The influence of substrtae structure on the local abundance and diversity of Philippine reef fishes. Proceedings of the 4th International Coral Reef Symposium, Marine Science Center, University of the Philippines, Quezon, Philippines; Gomez, E.D., Birkeland, C.E., Buddemeier, R.W., Johannes, R.E., Marsh, J.A., Jr., Tsuda, R.T., Eds.; ReefBase: Penang, Malaysia; 2, pp. 497–502.
- Findley, J.S.; Findley, M.T. Global, regional, and local patterns in species richness and abundance of butterflyfishes. Ecol. Monogr. 2001, 71, 69–91. [Google Scholar]
- Jennings, S.; Boulle, D.P.; Polunin, N.V.C. Habitat correlates of the distribution and biomass of Seychelles' reef fishes. Environ.l Biol. Fishes 1996, 46, 15–25. [Google Scholar]
- Bell, J.D.; Galzin, R. Influence of live coral cover on coral-reef fish communities. Mar. Ecol. Prog. Ser. 1984, 15, 265–274. [Google Scholar]
- Munday, P. Habitat loss, resource specialisation, and extinction on coral reefs. Glob. Change Biol. 2004, 10, 1642–1647. [Google Scholar]
- Pratchett, M.S. Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Mar. Biol. 2005, 148, 373–382. [Google Scholar]
- Graham, N.A.J.; Wilson, S.K.; Pratchett, M.S.; Polunin, N.V.C.; Spalding, M.D. Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodivers. Conservat. 2009, 18, 3325–3336. [Google Scholar]
- Wilkinson, C. Global and local threats to coral reef functioning and existence: Review and predictions. Mar. Freshwat. Res. 1999, 50, 867–878. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Change Biol. 2006, 12, 1587–1594. [Google Scholar]
- Wilson, S.K.; Dolman, A.M.; Cheal, A.; Emslie, M.J.; Pratchett, M.S.; Sweatman, H.P.A. Maintenance of fish diversity on disturbed coral reefs. Coral Reefs 2009, 28, 3–14. [Google Scholar]
- Riegl, B. Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Mar. Biol. 2002, 140, 29–40. [Google Scholar]
- McClanahan, T. Interaction between fisheries management and a coral belaching distrubance on coral reef fish in Kenya. Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–-2 July, 2004; ReefBase: Penang, Malaysia, 2006; 1, pp. 688–695. [Google Scholar]
- Spalding, M.D.; Jarvis, G.E. The impact of the 1998 coral mortality on reef fish communities in the Seychelles. Mar. Pollut. Bull. 2002, 44, 309–321. [Google Scholar]
- Sano, M. Short-term effects of a mass coral bleaching event on a reef fish assemblage at Iriomote Island, Japan. Fish. Sci. 2004, 70, 41–46. [Google Scholar]
- Shibuno, T.; Hashimoto, K.; Abe, O.; Takada, Y. Short-term changesin the structureof a fish community following coral bleaching at Ishigaki Island, Japan. Galaxea 1999, 1, 51–28. [Google Scholar]
- Thompson, A.; Malcolm, H. Benthic and Fish Monitoring on Fringing Reefs in the Brook, Palm and Rattlesnake Island Groups: Status Post 1998 Coral Bleahcing Event.; Queensland Parks and Wildlife: Townsville, Australia, 1999. [Google Scholar]
- Buckley, T. The Impact of Acanthaster Planci Coral Kills on the Samoan Reef Fish Community; Pacific Island Gray Literature Project: Seattle, WA, USA, 1986. [Google Scholar]
- Bouchon-Navaro, Y.; Bouchon, C.; Harmelin-Vivien, M.L. Impact of coral degradation on a chaetodontid fish assemblage (Moorea, French Polynesia). Proceedings of The Fifth International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985; Harmelin-Vivien, M., Salvat, B., Eds.; ReefBase: Penang, Malaysia, 1985; 5, pp. 427–432. [Google Scholar]
- Fenner, D.P. Effects of hurrican Gilbert on coral reefs, fishes and spoonges at Cozumel, Mexico. Bull. Mar. Sci. 1991, 48, 719–730. [Google Scholar]
- Rosseau, Y.; Galzin, R.; Marechal, J. Impact of hurricane Dean on coral reef benthic and fish structure of Martinique, French West Indies. Cybium 2010, 34, 243–256. [Google Scholar]
- Lewis, A.R. Effects of experimental coral distrubance on the structure of fish communities on large patch reefs. Mar. Ecol. Prog. Ser. 1997, 161, 37–50. [Google Scholar]
- Syms, C.; Jones, G.P. Disturbance, habitat structure, and the dynamics of a coral-reef fish community. Ecology 2000, 81, 2714–2729. [Google Scholar]
- Pratchett, M.S.; Wilson, S.K.; Baird, A.H. Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J. Fish Biol. 2006, 69, 1269–1280. [Google Scholar]
- Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Resistance and resilience of a coral reef fish community to changes in coral cover. Mar. Ecol. Prog. Ser. 2008, 2008, 263–271. [Google Scholar]
- Sano, M.; Shimizu, M.; Nose, Y. Changes in structure of coral-reef fish communities by destruction of hermatypic corals: Observational and experimental views. Pac. Sci. 1984, 38, 51–79. [Google Scholar]
- Cheal, A.; Wilson, S.K.; Emslie, M.J.; Dolman, A.M.; Sweatman, H.P.A. Responses of reef fish communities to coral declines on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2008, 372, 211–223. [Google Scholar]
- Adjeroud, M.; Augustine, D.; Galzin, R.; Salvat, B. Natural disturbances and interannual variability of coral reef communities on the outer slope of Tiahura (Moorea, French Polynesia): 1991 to 1997. Mar. Ecol. Prog. Ser. 2002, 237, 121–131. [Google Scholar]
- Lindahl, U.; Öhman, M.C.; Schelten, C.K. The 1997/1998 mass mortality of corals: Effects on fish communites on a Tanzanian coral reef. Mar. Pollut. Bull. 2001, 42, 127–131. [Google Scholar]
- Pratchett, M.S.; Wilson, S.K.; Graham, N.A.J.; Munday, P.L.; Jones, G.P.; Polunin, N.V.C. Coral bleaching and consequence for motile reef organisms: Past, present and uncertain future effects. In Coral Bleaching: Patterns, Processes, Causes and Consequences; van Oppen, M. J. H., Lough, J., Eds.; Springer-Verlag: Heidelberg, Germany, 2009; pp. 139–158. [Google Scholar]
- Hutchings, P.A.; Bioerosion. Encyclopedia of Modern Coral Reefs-Structure, Form and Processes; Hopley, D., Ed.; Springer-Verlag: Hiedelberg, Germany, 2011; pp. 139–156. [Google Scholar]
- Connolly, S.R.; Bellwood, D.R.; Hughes, T.P. Indo-pacific biodiversity of coral reefs: Deviations from a mid-domain model. Ecology 2003, 84, 2178–2190. [Google Scholar]
- Pandolfi, J.M.; Bradbury, R.H.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; MsArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; Warner, R.R.; Jackson, J.J.B. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar]
- Cheal, A.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H.P.A. Coral-macroalgal phase shifts or reef resilience: Link with diversity and functional roles of herbivorous fishe on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar]
- Steneck, R.S.; Dethier, M.N. A functional group approach to the structure of algal-dominated communities. Oikos 1994, 69, 476–498. [Google Scholar]
- Choat, J.H.; Clemenets, K.D.; Robbins, W.D. The trophic status of herbivorous fishes on coral reefs 1: Dietary analyses. Mar. Biol. 2002, 140, 613–623. [Google Scholar]
- Wilson, S.K.; Bellwood, D.R.; Choat, J.H.; Furnas, M. Detritus in coral reef ecosystems and its use by coral reef fishes. Oceanogr. Mar. Biol. Ann. Rev. 2003, 41, 279–309. [Google Scholar]
- Bellwood, D.R.; Choat, J.H. A functional analysis of grazing in parrotfishes (family Scaridae): The ecological implications. Environ. Biol.Fishes 1990, 28, 189–214. [Google Scholar]
- Bellwood, D.R.; Wainwright, P.C.; Fulton, C.J.; Hoey, A.S. Functional versatility supports coral reef biodiversity. Proc.Roy. Soc. B Biol. Sci. 2006, 273, 101–107. [Google Scholar]
- Ceccarelli, D.; Jones, G.P.; McCook, L. Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr.Mar. Biol. Ann. Rev. 2001, 39, 355–389. [Google Scholar]
- Nyström, M. Redundancy and response diversity of functional groups: Implications for the resilience of coral reefs. AMBIO 2006, 35, 30–35. [Google Scholar]
- Nyström, M.; Graham, N.A.J.; Lokrantz, J.; Norström, A.V. Capturing the cornerstones of coral reef resilience: Linking theory to practice. Coral Reefs 2008, 27, 795–809. [Google Scholar]
- Adjeroud, M.; Pratchett, M.S.; Kospartov, M.C.; Lejeusne, C.; Penin, L. Small-scale variability in the size striucture of scleractinain corals around Moorea, French Polynesia: Patterns across depths and locations. Hydrobiologia 2007, 589, 117–126. [Google Scholar]
- Pratchett, M.S.; Berumen, M.L. Interspecific variation in distributions and diets of coral reef butterflyfishes (Teleostei: Chaetodontidae). J. Fish Biol. 2008, 73, 1730–1747. [Google Scholar]
- Wilson, S.K.; Burgess, S.C.; Cheal, A.J.; Emslie, M.J.; Fisher, R.; Miller, I.; Polunin, N.V.C.; Sweatman, H.P.A. Habitat utilization by coral reef fish: Implications for specialists versus generalists in a changing environment. J.Anim. Ecol. 2008, 77, 220–228. [Google Scholar]
- Williams, D.M. Temporal variation in the structure of reef slope fish communinites (central Great Barrier Reef): Short-term effects of Acanthaster planci infestation. Mar. Ecol. Prog. Ser. 1986, 28, 157–164. [Google Scholar]
- Kokita, T.; Nakazono, A. Rapid response of an obligately corallivorous filefish Oxymonocanthus lngirostris (Monocanthidae) to a mass coral bleaching event. Coral Reefs 2001, 20, 155–158. [Google Scholar]
- Pratchett, M.; Wilson, S.; Berumen, M.; McCormick, M. Sub-lethal effects of coral bleaching on an obligate coral feeding butterflyfish. Coral Reefs 2004, 23, 352–356. [Google Scholar]
- Doherty, P.J.; Fowler, P. An empirical test of recruitment limitation in a coral reef fish. Science 1994, 263, 935–939. [Google Scholar]
- McClanahan, T.R.; Ateweberhan, M.; Graham, N.A.J.; Wilson, S.K.; Sebastian, C.R.; Guillaume, M.M.M.; Bruggemann, J.H. Western Indian Ocean coral communities: Bleaching responses and susceptibility to extinction. Mar. Ecol. Prog. Ser. 2007, 337, 1–13. [Google Scholar]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar]
- Randall, J.E. The effects of fishes on coral reefs. Proceedings of the 2nd International Coral Reef Symposium, M.V. Marco Polo, Great Barrier Reef, Australia; ReefBase: Penang, Malaysia; 1, pp. 159–166.
- Cole, A.J.; Pratchett, M.S.; Jones, G.P. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish. 2008, 9, 286–307. [Google Scholar]
- Rotjan, R.D.; Lewis, S.M. Impact of coral predators on tropical coral reefs. Mar. Ecol. Prog. Ser. 2008, 367, 73–91. [Google Scholar]
- Bellwood, D.R.; Klanten, S.; Cowman, P.F.; Pratchett, M.S.; Konow, N.; van Herwerden, L. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J.Evol. Biol. 2010, 23, 335–349. [Google Scholar]
- Stella, J.; Pratchett, M.S.; Hutchings, P.A.; Jones, G.P. Coral-associated invertebrates—Diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol.Ann. Rev. 2011, 49, 43–116. [Google Scholar]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar]
- Diaz-Pulido, G.; McCook, L. The fate of bleached corals: Patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 2002, 232, 115–128. [Google Scholar]
- Ostrander, G.K.; Armstrong, K.M.; Knobbe, E.T.; Gerace, D.; Scully, E.P. Rapid transition in the structure of a coral reef community: The effects of coral bleaching and physical disturbance. Proc. Natl. Acad. Sci. USA 2000, 97, 5297–5302. [Google Scholar]
- Fox, R.J.; Sunderland, T.L.; Hoey, A.S.; Bellwood, D.R. Estimating ecosystem function: Contrasting roles of closely related herbivorous rabbitfishes (Siganidae) on coral reefs. Mar. Ecol. Prog. Ser. 2009, 385, 261–269. [Google Scholar]
- Cvitanovic, C.; Bellwood, D.R. Local variation in herbivore feeding activity on an inshore reef of the Great Barrier Reef. Coral Reefs 2009, 28, 127–133. [Google Scholar]
- Hoey, A.S.; Bellwood, D.R. Suppression of herbivory by macroalgal density: A critical feedback on coral reefs? Ecol. Lett. 2011, 14, 267–273. [Google Scholar]
- Ceccarelli, D. Modification of benthic communities by territorial damselfish: A multi-species comparison. Coral Reefs 2007, 26, 853–866. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar]
- Meyer, C.P.; Holland, K.N. Movement patterns, home range size and habitat utilization of the bluespine unicornfish, Naso unicornis (Acanthuridae) in a Hawaiian marine reserve. Environ. Biol. Fishes 2005, 73, 201–210. [Google Scholar]
- Fox, R.J.; Bellwood, D.R. Unconstrained by the clock? Plasticity of diel activity rhythm in a tropical reef fish. Siganus lineatusi. Funct. Ecol. 2011. [Google Scholar] [CrossRef]
- Chappin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavore, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar]
- Brook, B.W.; Sodhi, N.S.; Bradshaw, C.J.A. Synergies among extinction drivers under global change. Trends Ecol. Evol. 2008, 23, 453–460. [Google Scholar]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. 2003, 4, 25–64. [Google Scholar]
- Gaston, K.J.; Fuller, R.A. Commonness, population depletion and conservation biology. Trends Ecol. Evol. 2007, 23, 14–19. [Google Scholar]
- Emslie, M.J.; Pratchett, M.S.; Cheal, A.J. Effects of different distrubance types on butterflyfish communities of Australia's Great Barrier Reef. Coral Reefs 2011, 30, 461–471. [Google Scholar]
- Graham, N.A.J. Ecological versatility and the decline of coral feeding fishes following climate driven coral mortality. Mar. Biol. 2007, 153, 119–127. [Google Scholar]
- Mumby, P.J.; Dahlgren, C.P.; Harborne, A.R.; Kappel, C.V.; Micheli, F.; Brumbaugh, D.R.; Holmes, K.E.; Mendes, J.M.; Broad, K.; Sanchirico, J.N.; et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science 2007, 311, 98–101. [Google Scholar]
- Williams, I.D.; Polunin, N.V.C. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 2001, 19, 358–366. [Google Scholar]
- Mumby, P.J.; Steneck, R.S. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 2008, 23, 555–563. [Google Scholar]
- Naeem, S. Ecosystem consequences of biodiversity loss: the evolution of a paradigm. Ecology 2002, 83, 1537–1552. [Google Scholar]
- Wilkinson, C. Status of coral reefs of the world: 2004; Australian Institute of Marine Science: Townsville, Australia, 2004. [Google Scholar]
- Wilson, S.K.; Graham, N.A.J.; Polunin, N.V.C. Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 2007, 151, 1069–1076. [Google Scholar]
- Holling, C.S. Resilience and stability of ecological systems. Ann. Rev. Ecol.Syst. 1973, 4, 1–23. [Google Scholar]
- Emslie, M.J.; Cheal, A.J.; Sweatman, H.P.A.; Delan, S. Recovery from disturbance of coral and reef fish communities on the Great Barrier Reef, Australia. Coral Reefs 2008, 371, 177–190. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in Biodiversity and Functioning of Reef Fish Assemblages following Coral Bleaching and Coral Loss. Diversity 2011, 3, 424-452. https://doi.org/10.3390/d3030424
Pratchett MS, Hoey AS, Wilson SK, Messmer V, Graham NAJ. Changes in Biodiversity and Functioning of Reef Fish Assemblages following Coral Bleaching and Coral Loss. Diversity. 2011; 3(3):424-452. https://doi.org/10.3390/d3030424
Chicago/Turabian StylePratchett, Morgan S., Andrew S. Hoey, Shaun K. Wilson, Vanessa Messmer, and Nicholas A.J. Graham. 2011. "Changes in Biodiversity and Functioning of Reef Fish Assemblages following Coral Bleaching and Coral Loss" Diversity 3, no. 3: 424-452. https://doi.org/10.3390/d3030424





