Effectiveness of Seed Traps for Assessing Seed Rain in Periurban Grasslands
Abstract
:1. Introduction
2. Materials and Methods
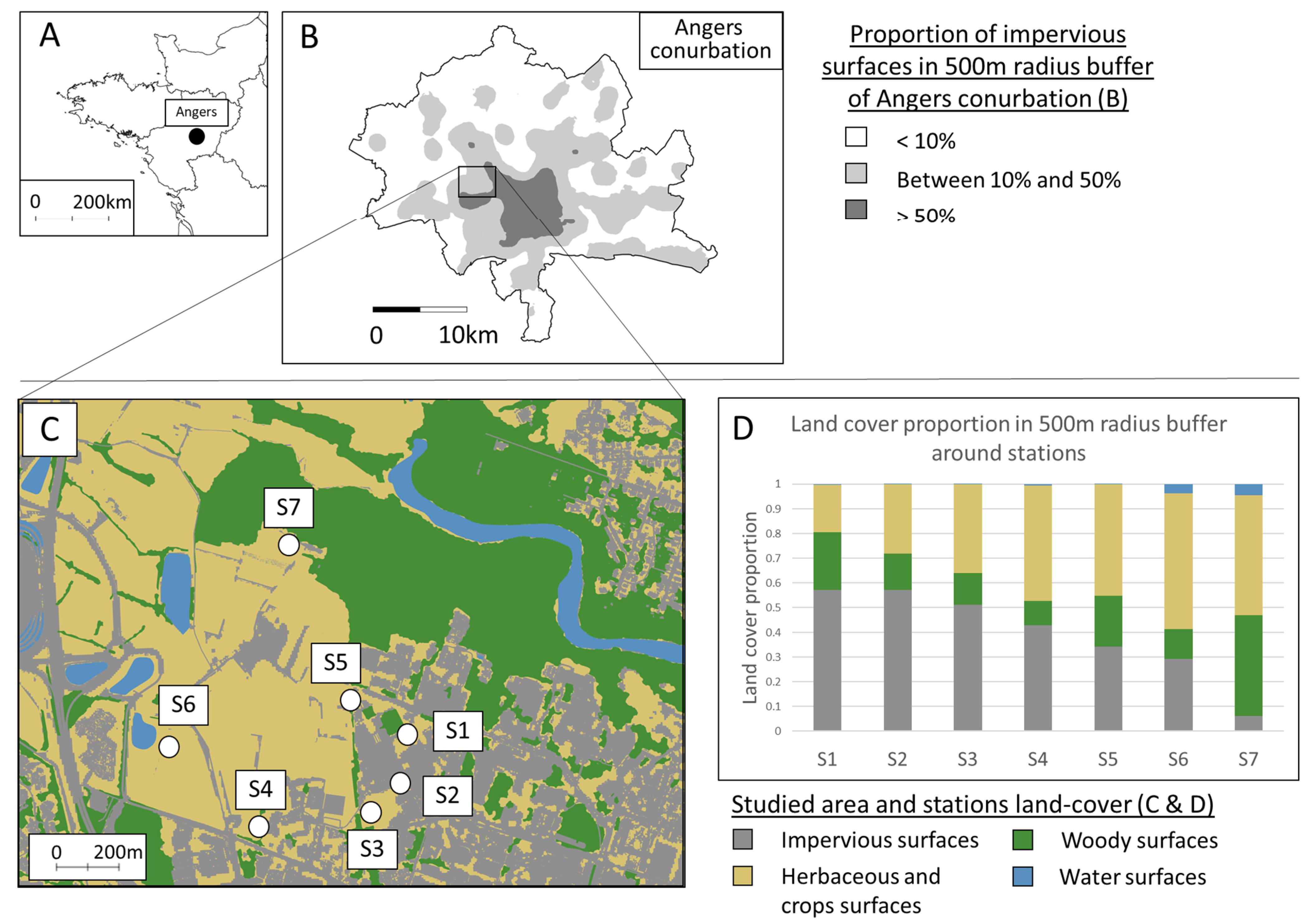
2.1. Study Area
2.2. Spatial Variation in Seed Rain
2.3. Comparative Study of Seed Trap Characteristics
2.4. Data Collection
2.5. Data Analysis
3. Results
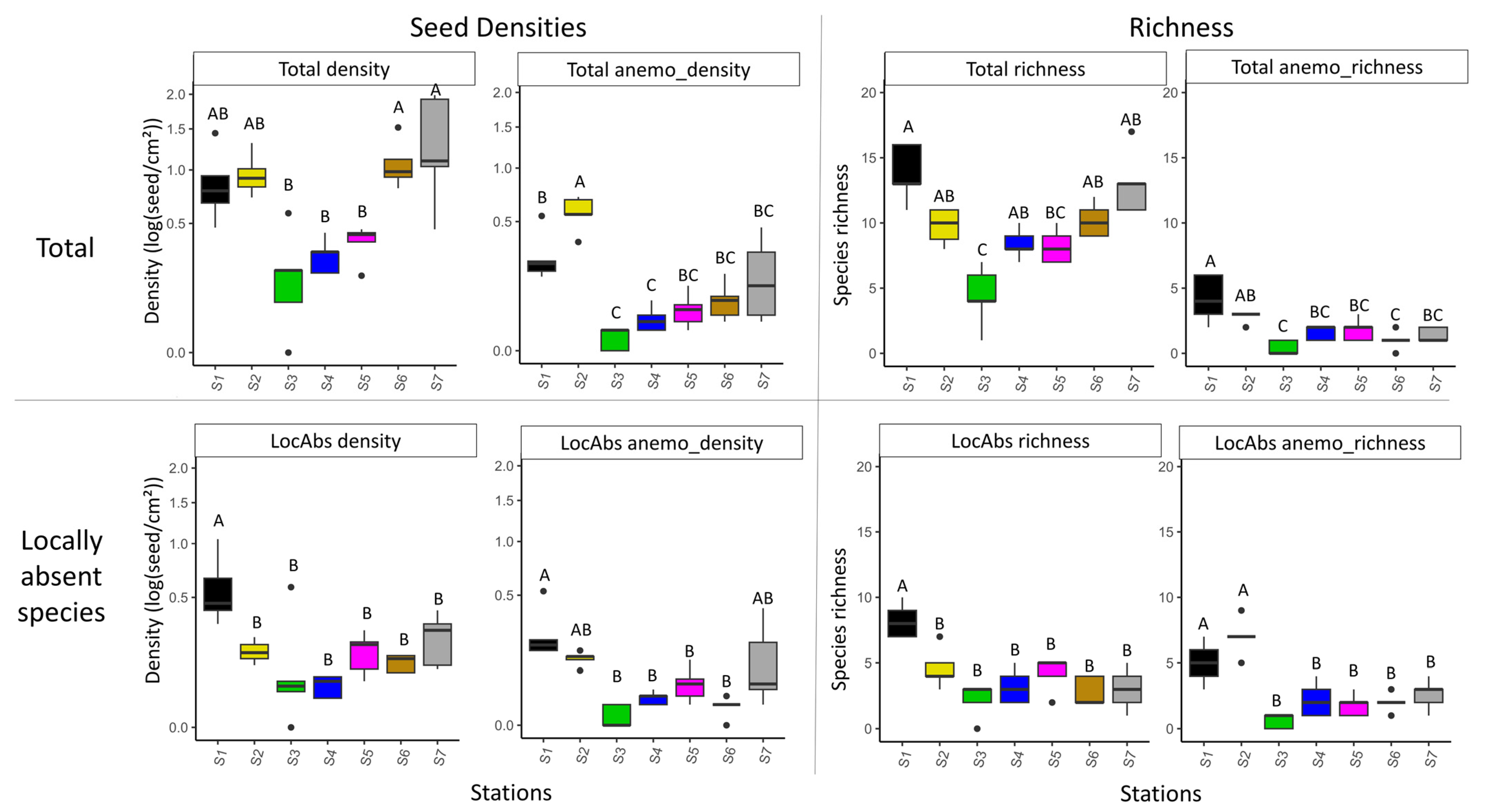
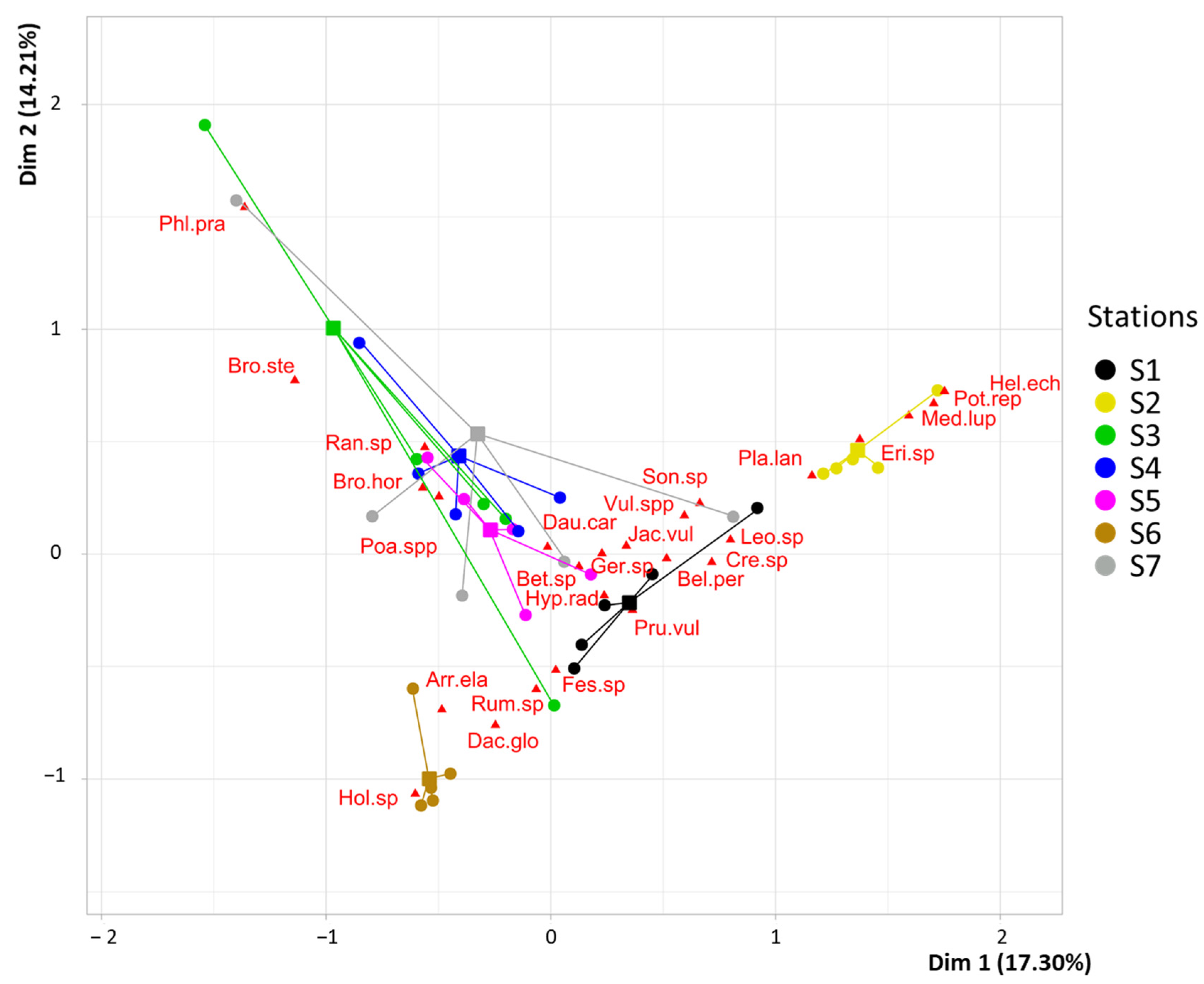
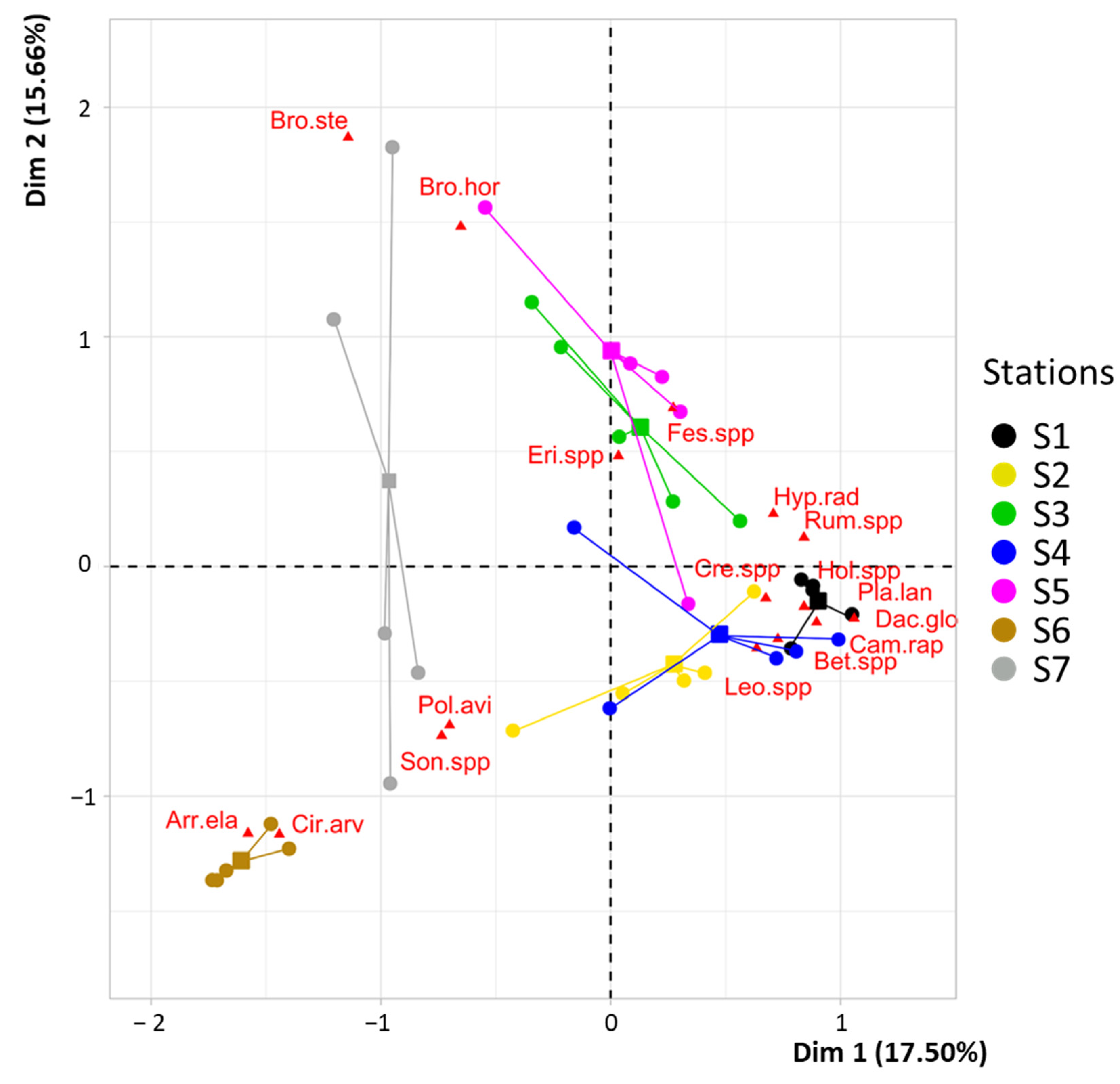
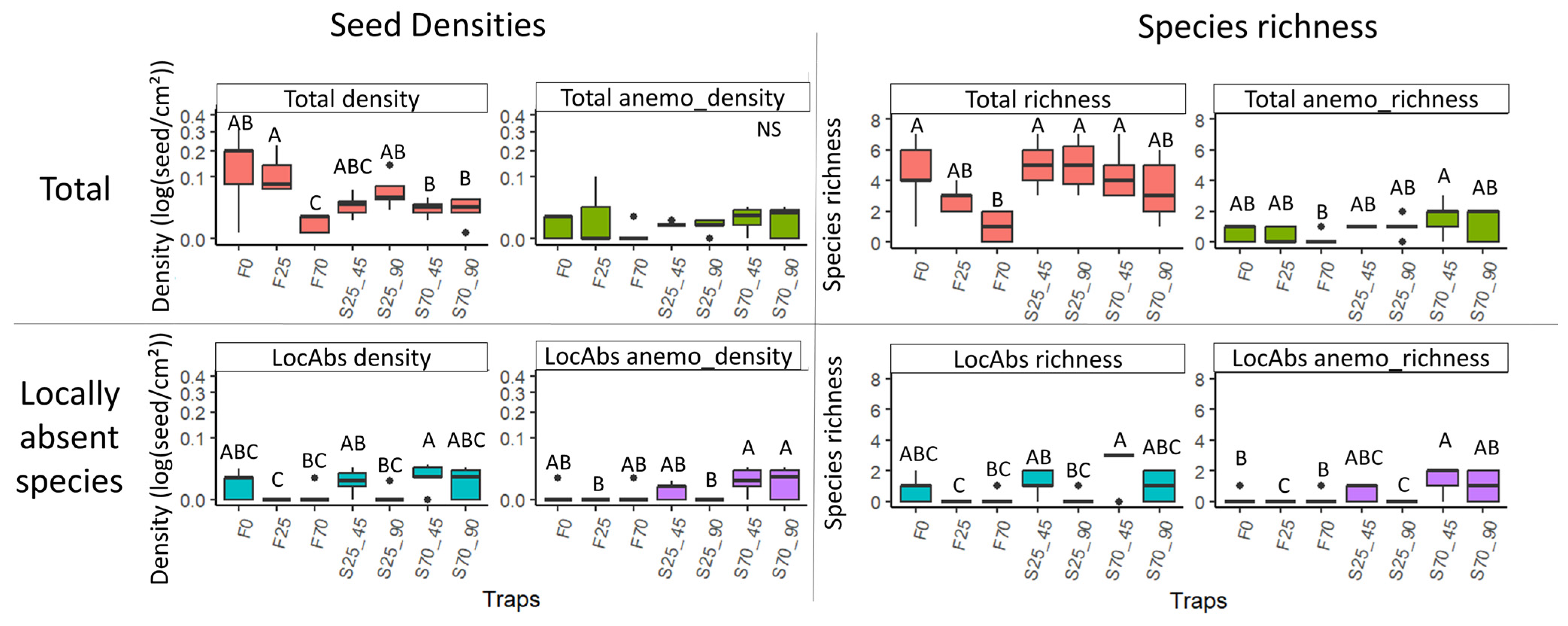
3.1. Effectiveness of Funnel Traps in Capturing Spatial Variation in Seed Rain
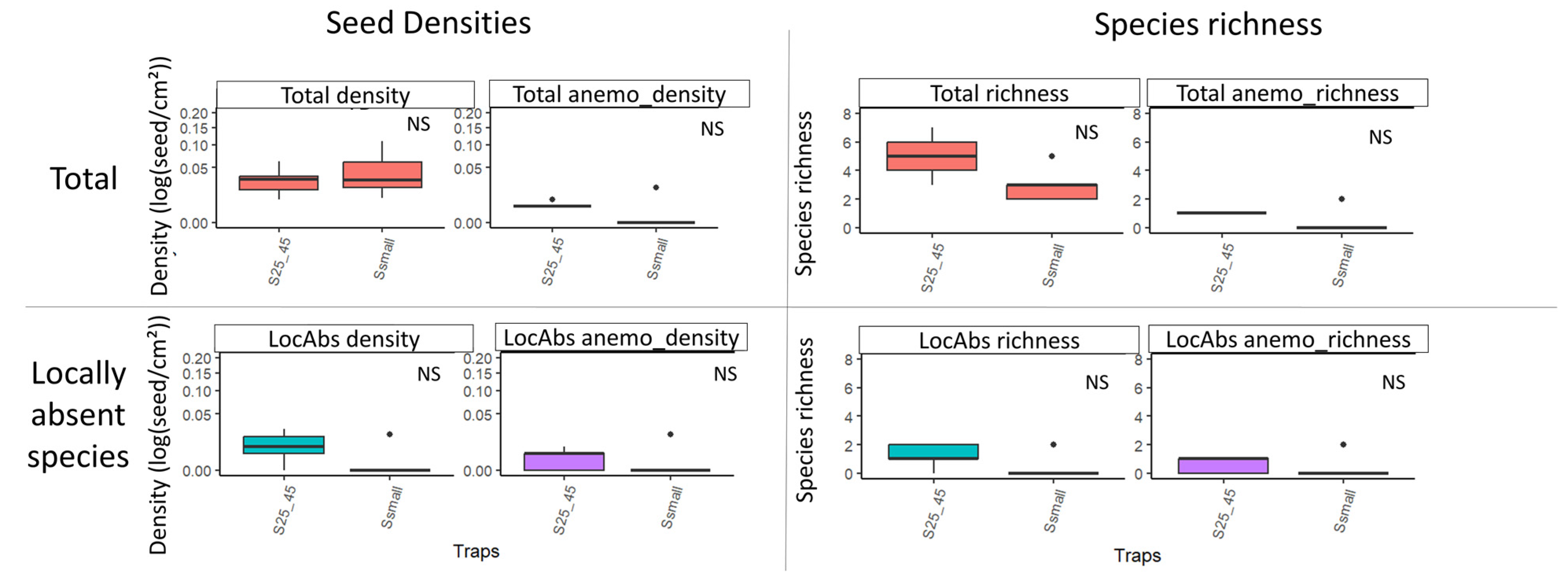
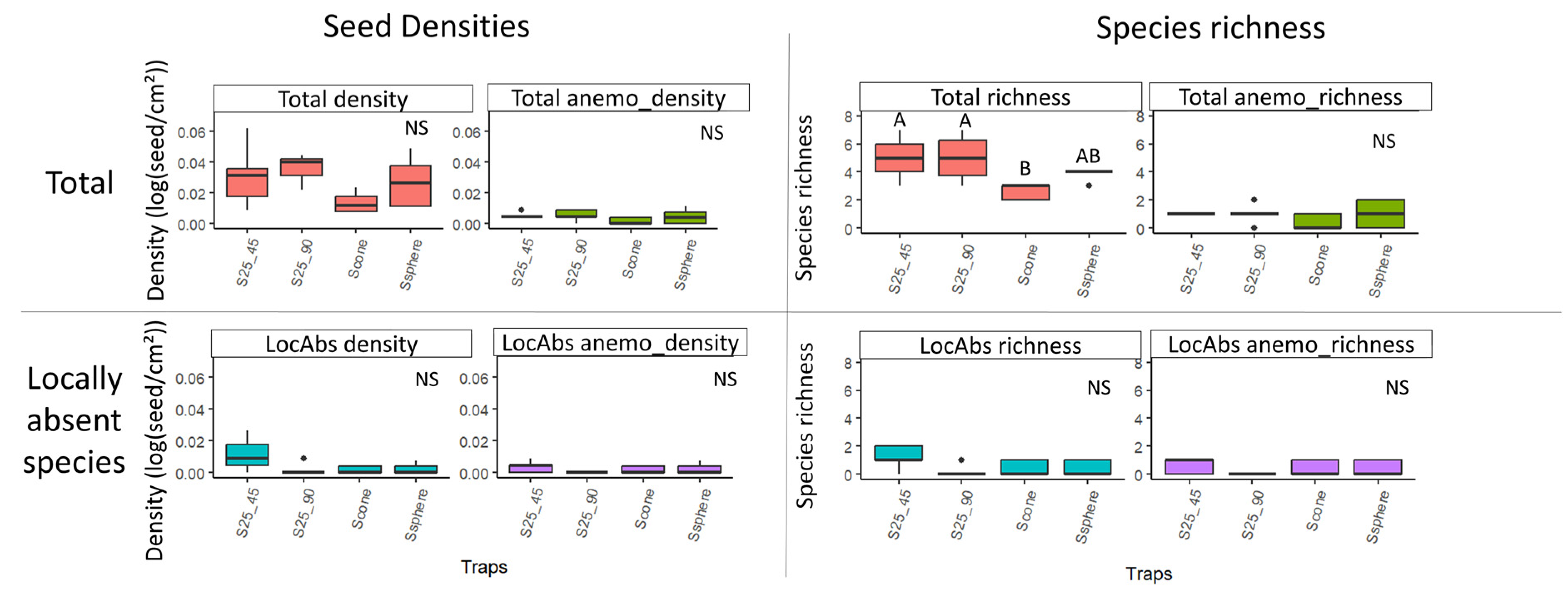
3.2. Comparison of Seed Traps
3.2.1. Trap Types, Heights, and Tilt
3.2.2. Trap Surface Area and Forms
4. Discussion
4.1. Efficiency of Seed Traps in Spatial Variation Assessment
4.2. Effectiveness of Seed Traps According to Their Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species Code | Genus | Species | Family | Dispersal Strategies (Julve) |
| Ach.mil | Achillea | millefolium | ASTERACEAE | anemochorous |
| Agr.sp | Agrostis | sp. | POACEAE | autochorous |
| Arr.ela | Arrhenatherum | elatius | POACEAE | zoochorous |
| Ave.sp | Avena | sp. | POACEAE | zoochorous |
| Bel.per | Bellis | perennis | ASTERACEAE | autochorous |
| Bet.sp | Betula | sp. | BETULACEAE | anemochorous |
| Bro.hor | Bromus | hordeaceus | POACEAE | zoochorous |
| Bro.ste | Bromus | sterilis | POACEAE | autochorous |
| Cam.rap | Campanula | rapunculus | CAMPANULACEAE | autochorous |
| Cen.sp | Centaurea | sp. | ASTERACEAE | autochorous |
| Cir.arv | Circium | arvense | ASTERACEAE | anemochorous |
| Con.arv | Convolvulus | arvensis | CONVOLVULACEAE | autochorous |
| Con.sp | Convlovulus | sp. | CONVOLVULACEAE | autochorous |
| Cre.sp | Crepis | sp. | ASTERACEAE | anemochorous |
| Cyn.cri | Cynosurus | cristatus | POACEAE | zoochorous |
| Cyn.dac | Cynodon | dactylon | POACEAE | zoochorous |
| Dac.glo | Dactylis | glomerata | POACEAE | zoochorous |
| Dau.car | Daucus | carota | APIACEAE | zoochorous |
| Ech.vul | Echium | vulgare | BORAGINACEAE | autochorous |
| Eri.sp | Erigeron | sp. | ASTERACEAE | anemochorous |
| Fes.sp | Festuca | sp. | POACEAE | zoochorous |
| Gal.sp | Gallium | sp. | RUBIACEAE | autochorous |
| Ger.sp | Geranium | sp. | GERANIACEAE | autochorous |
| Hed.hel | Hedera | helix | ARALIACEAE | zoochorous |
| Hel.ech | Helminthotheca | echioides | ASTERACEAE | anemochorous |
| Hol.sp | Holcus | sp. | POACEAE | zoochorous |
| Hyp.per | Hypericum | perforatum | HYPERICACEAE | anemochorous |
| Hyp.rad | Hypochaeris | radicata | ASTERACEAE | anemochorous |
| Jac.vul | Jacobaea | vulgaris | ASTERACEAE | anemochorous |
| Lap.com | Lapsana | communis | ASTERACEAE | autochorous |
| Leo.sp | Leotodon | sp. | ASTERACEAE | anemochorous |
| Lep.cam | Lepidium | campestre | BRASSICACEAE | zoochorous |
| Leu.vul | Leucanthemum | vulgar | ASTERACEAE | autochorous |
| Lol.mul | Lolium | multiflorum | POACEAE | autochorous |
| Lol.spp | Lolium | sp. | POACEAE | autochorous |
| Mal.sp | Malva | sp. | MALVACEAE | anemochorous |
| Med.lup | Medicago | lupulina | FABACEAE | autochorous |
| Myo.sp | Myosotis | sp. | BORAGINACEAE | zoochorous |
| Ort.dio | Urtica | dioica | URTICACEAE | zoochorous |
| Phl.pra | Phleum | pratense | POACEAE | zoochorous |
| Pla.cor | Plantago | coronopus | PLANTAGINACEAE | autochorous |
| Pla.lan | Plantago | lanceolata | PLANTAGINACEAE | autochorous |
| Poa.spp | Poa | sp. | POACEAE | autochorous |
| Pol.avi | Polygonum | aviculare | POLYGONACEAE | autochorous |
| Pol.sp | Polygonum | sp. | POLYGONACEAE | autochorous |
| Pot.rep | Potentilla | reptans | ROSACEAE | autochorous |
| Pru.vul | Prunella | vulgaris | LAMIACEAE | autochorous |
| Que.sp | Quercus | sp. | FAGACEAE | zoochorous |
| Ran.sp | Ranunculus | sp. | RANUNCULACEAE | zoochorous |
| Rum.sp | Rumex | sp. | POLYGONACEAE | anemochorous |
| Sen.vul | Senecio | vulgare | ASTERACEAE | anemochorous |
| Son.sp | Sonchus | sp. | ASTERACEAE | anemochorous |
| Tar.sp | Taraxacum | sp. | ASTERACEAE | anemochorous |
| Tri.pra | Trifolium | pratense | FABACEAE | zoochorous |
| Ver.arv | Veronica | arvensis | PLANTAGINACEAE | autochorous |
| Vic.hir | Vicia | hirsuta | FABACEAE | autochorous |
| Vul.spp | Vulpia | sp. | POACEAE | zoochorous |
References
- De Blois, S.; Domon, G.; Bouchard, A. Landscape Issues in Plant Ecology. Ecography 2002, 25, 244–256. [Google Scholar] [CrossRef]
- Schupp, E.W.; Fuentes, M. Spatial Patterns of Seed Dispersal and the Unification of Plant Population Ecology. Écoscience 1995, 2, 267–275. [Google Scholar] [CrossRef]
- Lortie, C.J.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Michalet, R.; Pugnaire, F.I.; Callaway, R.M. Rethinking Plant Community Theory. Oikos 2004, 107, 433–438. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Nathan, R.; Perry, G.; Richardson, D.M. The Importance of Long-Distance Dispersal in Biodiversity Conservation. Divers. Distrib. 2005, 11, 173–181. [Google Scholar] [CrossRef]
- Auffret, A.G.; Rico, Y.; Bullock, J.M.; Hooftman, D.A.P.; Pakeman, R.J.; Soons, M.B.; Suárez-Esteban, A.; Traveset, A.; Wagner, H.H.; Cousins, S.A.O. Plant Functional Connectivity—Integrating Landscape Structure and Effective Dispersal. J. Ecol. 2017, 105, 1648–1656. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J.M. Seed Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed Dispersal Effectiveness Revisited: A Conceptual Review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef]
- Uroy, L.; Ernoult, A.; Mony, C. Effect of Landscape Connectivity on Plant Communities: A Review of Response Patterns. Landsc. Ecol. 2019, 34, 203–225. [Google Scholar] [CrossRef]
- Janzen, D.H. Seed Predation by Animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Urbanska, K.M.; Erdt, S.; Fattorini, M. Seed Rain in Natural Grassland and Adjacent Ski Run in the Swiss Alps: A Preliminary Report. Restor. Ecol. 1998, 6, 159–165. [Google Scholar] [CrossRef]
- Zeiter, M.; Schärrer, S.; Zweifel, R.; Newbery, D.M.; Stampfli, A. Timing of Extreme Drought Modifies Reproductive Output in Semi-Natural Grassland. J. Veg. Sci. 2016, 27, 238–248. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; McCarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Duncan, R.P.; Norton, B.A.; Thompson, K.; et al. A Conceptual Framework for Predicting the Effects of Urban Environments on Floras. J. Ecol. 2009, 97, 4–9. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Cadenasso, M. How Many Principles of Urban Ecology Are There? Landsc. Ecol. 2017, 32, 699–705. [Google Scholar] [CrossRef]
- Kowarik, I.; Lippe, M. von der Plant Population Success across Urban Ecosystems: A Framework to Inform Biodiversity Conservation in Cities. J. Appl. Ecol. 2018, 55, 2354–2361. [Google Scholar] [CrossRef]
- Schott, G.W. A Seed Trap for Monitoring the Seed Rain in Terrestrial Communities. Can. J. Bot. 1995, 73, 794–796. [Google Scholar] [CrossRef]
- Page, M.J.; Newlands, L.; Eales, J. Effectiveness of Three Seed-Trap Designs. Aust. J. Bot. 2002, 50, 587–594. [Google Scholar] [CrossRef]
- Arruda, A.; Buisson, E.; Poschlod, P.; Silveira, F.A.O. How Have We Studied Seed Rain in Grasslands and What Do We Need to Improve for Better Restoration? Restor. Ecol. 2018, 26, S84–S91. [Google Scholar] [CrossRef]
- Arruda, A.J.; Silveira, F.A.O.; Buisson, E. A Simple Standardized Protocol to Evaluate the Reliability of Seed Rain Estimates. Seed Sci. Res. 2020, 30, 304–309. [Google Scholar] [CrossRef]
- Bakker, J.P.; Poschlod, P.; Strykstra, R.J.; Bekker, R.M.; Thompson, K. Seed Banks and Seed Dispersal: Important Topics in Restoration Ecology. Acta Bot. Neerl. 1996, 45, 461–490. [Google Scholar] [CrossRef]
- Chabrerie, O.; Alard, D. Comparison of Three Seed Trap Types in a Chalk Grassland: Toward a Standardised Protocol. Plant Ecol. 2005, 176, 101–112. [Google Scholar] [CrossRef]
- Freund, L.; Eichberg, C.; Retta, I.; Schwabe, A. Seed Addition via Epizoochorous Dispersal in Restoration: An Experimental Approach Mimicking the Colonization of Bare Soil Patches. Appl. Veg. Sci. 2014, 17, 74–85. [Google Scholar] [CrossRef]
- Freund, L.; Carrillo, J.; Storm, C.; Schwabe, A. Restoration of a Newly Created Inland-Dune Complex as a Model in Practice: Impact of Substrate, Minimized Inoculation and Grazing. Tuexenia 2015, 35, 221–248. [Google Scholar] [CrossRef]
- Diacon-Bolli, J.C.; Edwards, P.J.; Bugmann, H.; Scheidegger, C.; Wagner, H.H. Quantification of Plant Dispersal Ability within and beyond a Calcareous Grassland. J. Veg. Sci. 2013, 24, 1010–1019. [Google Scholar] [CrossRef]
- Kollmann, J.; Goetze, D. Notes on Seed Traps in Terrestrial Plant Communities. Flora 1998, 193, 31–40. [Google Scholar] [CrossRef]
- Chaudron, C.; Chauvel, B.; Isselin-Nondedeu, F. Effects of Late Mowing on Plant Species Richness and Seed Rain in Road Verges and Adjacent Arable Fields. Agric. Ecosyst. Environ. 2016, 232, 218–226. [Google Scholar] [CrossRef]
- Martinkova, Z.; Honek, A. The Establishment of Taraxacum Officinale Plants in Grassland. Weed Res. 2014, 54, 501–510. [Google Scholar] [CrossRef]
- Lucero, J.E.; Allen, P.S.; McMillan, B.R. Increased Primary Production from an Exotic Invader Does Not Subsidize Native Rodents. PLoS ONE 2015, 10, e0131564. [Google Scholar] [CrossRef]
- Auffret, A.G.; Cousins, S.A.O. Past and Present Management Influences the Seed Bank and Seed Rain in a Rural Landscape Mosaic. J. Appl. Ecol. 2011, 48, 1278–1285. [Google Scholar] [CrossRef]
- Kirmer, A.; Mahn, E.-G. Spontaneous and Initiated Succession on Unvegetated Slopes in the Abandoned Lignite-Mining Area of Goitsche, Germany. Appl. Veg. Sci. 2001, 4, 19–27. [Google Scholar] [CrossRef]
- Larios, L.; Aicher, R.J.; Suding, K.N. Effect of Propagule Pressure on Recovery of a California Grassland after an Extreme Disturbance. J. Veg. Sci. 2013, 24, 1043–1052. [Google Scholar] [CrossRef]
- Tison, J.-M.; de Foucault, B. Flora Gallica: Flore de France; Biotope Editions: Mèze, France, 2014; ISBN 978-2-36662-012-2. [Google Scholar]
- Julve Baseflor. Index Botanique, Écologique et Chorologique de La Flore de France, Version 2015. Available online: http://philippe.julve.pagesperso-orange.fr/baseflor.xlsx (accessed on 24 July 2023).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I.; Stolle, J.; Klotz, S. Changes in the Functional Composition of a Central European Urban Flora over Three Centuries. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 235–244. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Koh, L.P.; Peh, K.S.-H.; Tan, H.T.W.; Chazdon, R.L.; Corlett, R.T.; Lee, T.M.; Colwell, R.K.; Brook, B.W.; Sekercioglu, C.H.; et al. Correlates of Extinction Proneness in Tropical Angiosperms. Divers. Distrib. 2008, 14, 1–10. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Morgan, J.W.; Mcdonnell, M.J.; Mccarthy, M.A. Plant Traits and Local Extinctions in Natural Grasslands along an Urban-Rural Gradient: Plant Extinctions along an Urban-Rural Gradient. J. Ecol. 2005, 93, 1203–1213. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, Plant Traits and the Composition of Urban Floras. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Jackel, A.-K.; Poschlod, P. Diaspore Production and the Influence of the Size of Diaspore Traps on the Quantitive Result of Seasonal Diaspore Rain in Two Calcareous Grassland Sites. Berichte Inst. Landsch.-Pflanzenökol. Univ. Hohenh. 1994, 3, 123–132. [Google Scholar]
| Type of Trap | Height (cm) | Tilt | Area (cm2) | ID | |
|---|---|---|---|---|---|
| Funnel | 70 | - | 79 | F70 | |
| 25 | - | 79 | F25 | ||
| 0 | - | 79 | F0 | ||
| Sticky plate | 70 | 45° | 225 | S70_45 | |
| 90° | 225 | S70_90 | |||
| 25 | 45° | 225 | S25_45 | ||
| 90° | 225 | S25_90 | |||
| Other shapes of sticky traps | Small plate (10 × 10 cm) | 25 | 45° | 100 | Ssmall |
| Cone | 25 | - | 257 | Scone | |
| Hemisphere | 25 | - | 266 | Ssphere | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gros, C.; Bulot, A.; Braud, F.; Aviron, S.; Daniel, H. Effectiveness of Seed Traps for Assessing Seed Rain in Periurban Grasslands. Diversity 2023, 15, 1015. https://doi.org/10.3390/d15091015
Gros C, Bulot A, Braud F, Aviron S, Daniel H. Effectiveness of Seed Traps for Assessing Seed Rain in Periurban Grasslands. Diversity. 2023; 15(9):1015. https://doi.org/10.3390/d15091015
Chicago/Turabian StyleGros, Clément, Adeline Bulot, Ferréol Braud, Stéphanie Aviron, and Hervé Daniel. 2023. "Effectiveness of Seed Traps for Assessing Seed Rain in Periurban Grasslands" Diversity 15, no. 9: 1015. https://doi.org/10.3390/d15091015






