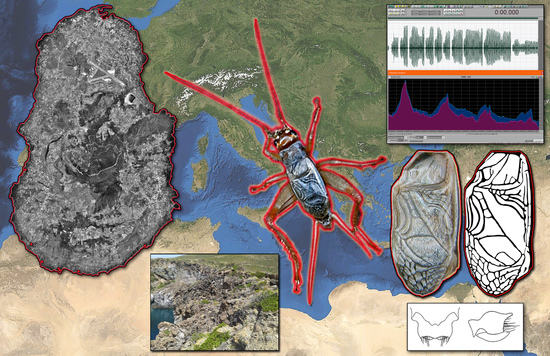
New Unexpected Species of Acheta (Orthoptera, Gryllidae) from the Italian Volcanic Island of Pantelleria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Discovery of the Cricket
2.2. Morphological Analysis
2.3. Abbreviations for Collections and Museums
2.4. Recording and Audio Analysis Equipment
2.5. Crowdsourcing of the New Species Name
- pantescus, adjective indicating the inhabitants of Pantelleria;
- marinus, indicating the peculiar eco-ethology of the species, linked to the rocks on the sea level;
- petrosus, as an alternative to the previous one;
- phantasma, as a noun in apposition, indicating the difficulty of observing this cricket, whose song was recorded only by chance.
3. Results
3.1. Acheta pantescus n. sp., English Name: Pantelleria Cricket
Species Name
- pantescus, 396 votes (56.6%);
- phantasma, 146 votes (20.8%);
- petrosus, 136 votes (19.4%);
- marinus, 22 votes (3.1%).
3.2. Song Description
3.2.1. Calling/Advertising Song
3.2.2. “Type 2” Song
3.2.3. Generalities and Bioacoustic Comparison with Other Acheta Songs
3.2.4. Material Examined
3.2.5. Diagnosis
3.2.6. Description
3.2.7. Measurements
3.2.8. Etymology
3.2.9. Affinities
3.2.10. Distribution and Conservation Assessment
3.2.11. Habitat on Pantelleria Island
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massa, B.; Fontana, P.; Buzzetti, F.M.; Kleukers, R.; Odé, B. Fauna d’Italia. Orthoptera; Calderini de Il Sole 24 Ore: Bologna, Italy, 2012; Volume XLVIII, pp. 1–563, (Includes Audio DVD-ROM). [Google Scholar]
- Fontana, P.; Buzzetti, F.M.; Cogo, A.; Odé, B. Guida al Riconoscimento e allo Studio di Cavallette, Grilli, Mantidi e Insetti Affini del Veneto; Guide Natura/1; Museo Naturalistico Archeologico di Vicenza: Vicenza, Italy, 2002; pp. 1–592, (Includes Audio CD-ROM). [Google Scholar]
- Gorochov, A.V. Grylloidea (Orthoptera) of Saudi Arabia and Adjacent Countries. Fauna Saudi Arab. 1993, 13, 79–97. [Google Scholar]
- Mertens, J.E.J.; Van Roje, M.; Merckx, J.; Dekoninck, W. The use of low cost compact cameras with focus stacking functionality in entomological digitization projects. Zookeys 2017, 712, 141–154. [Google Scholar] [CrossRef]
- Hadley, A.; Combine, Z. Available online: www.hadleyweb.pwp.blueyonder.co.uk (accessed on 15 February 2009).
- Baker, E.; Chesmore, D. Standardisation of bioacoustic terminology for insects. Biodivers. Data J. 2020, 8, e54222. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, F.M.; Barrientos-Lozano, L. Bioacoustics of some Mexican Orthoptera (Insecta: Orthoptera: Ensifera, Caelifera). Bioacoustics 2011, 20, 193–213. [Google Scholar] [CrossRef]
- Welch, T.B.; Wright Cameron, H.G.; Morrow, M.G. Real-Time Digital Signal Processing from MATLAB to C with the TMS320C6x DSPs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- National Instruments Corporation. White Paper 4278 (2018)–The Fundamentals of FFT-Based Signal Analysis and Measurement in LabVIEW and LabWindows/CVI. Available online: http://www.ni.com/white-paper/4278/en/ (accessed on 10 June 2022).
- Blackman, R.B.; Tukey, J.W. The measurement of power spectra from the point of view of communications engineering. Bell Syst. Tech. J. 1958, 37, 185–282. [Google Scholar] [CrossRef]
- Harris, F.J. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE 1978, 66, 51–83. [Google Scholar] [CrossRef]
- Nuttall, A.H. Some windows with very good sidelobe behavior. IEEE Trans. Acoust. Speech Signal Process. 1981, 29, 84–91. [Google Scholar] [CrossRef]
- Montealegre-Zapata, F.; Morris, G.K. Songs and Systematics of Some Tettigoniidae from Colombia and Ecuador I. Pseudophyllinae (Orthoptera). J. Orthoptera Res. 1999, 8, 162–236. [Google Scholar] [CrossRef]
- Elsner, N.; Popov, A.V. Neuroethology of acoustic communication. Adv. Insect Physiol. 1978, 13, 229–355. [Google Scholar] [CrossRef]
- Brizio, C.; Buzzetti, F.M.; Pavan, G. Beyond the audible: Wide band (0–125 kHz) field investigation on Italian Orthoptera (Insecta) songs. Biodivers. J. 2020, 11, 443–496. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File Online, Version 2.0/4.0; 2022. Available online: http://Orthoptera.SpeciesFile.org (accessed on 15 June 2022).
- Capra, F. Risultati Zoologici della Missione Inviata dalla R. Società Geografica Italiana per l’Esplorazione dell’Oasi de Giarabub (1926–1927); Ortotteri e Dermatteri. Ann. Mus. Civ. Stor. Nat. Giacomo Doria 1929, 53, 122–159. [Google Scholar]
- Uvarov, B.P. On the native country of the common house-cricket (Gryllus domesticus L.). Entomol. Mon. Mag. 1921, 57, 138–140. [Google Scholar]
- Uvarov, B.P.; Popov, G.B. The saltatorial Orthoptera of Socotra. Zool. J. Linn. Soc. 1957, 43, 359–389. [Google Scholar] [CrossRef]
- Costa, O.G. Ortotteri. In Fauna del Regno di Napoli; Azzolino e Compagno: Napoli, Italy, 1856; pp. 46–47. [Google Scholar]
- Massa, B.; Buzzetti, F.M.; Fontana, P. Order Orthoptera. Arthropod Fauna UAE 2010, 3, 70–112. [Google Scholar]
- Gorochov, A.; Llorente, V. Estudio taxónomico preliminar de los Grylloidea de España (Insecta, Orthoptera). Graellsia 2001, 57, 95–139. [Google Scholar] [CrossRef]
- Gianguzzi, L., II. Paesaggio Vegetale dell’Isola di Pantelleria. Contributo alla Conoscenza della Flora, della Vegetazione e delle Serie di Vegetazione; Collana Sicilia Foreste, Azienda Foreste Demaniali della Regione Siciliana: Palermo, Italy, 2000; pp. 1–192. [Google Scholar]
- Massa, B. (Ed.) Arthropoda di Lampedusa, Linosa e Pantelleria. Il Nat. Sicil. 1995, 19, 3–910. [Google Scholar] [CrossRef]
- Chopard, L. Orthoptéroides de l’Afrique du Nord. Faune de l’Empire Français; Librairie Larose: Paris, France, 1943; Volume I, pp. 1–450. [Google Scholar]
- Massa, B. Annotated check-list of Orthoptera of Libya. J. Orthoptera Res. 2009, 18, 75–93. [Google Scholar] [CrossRef]
- Sahnoun, A.M.; Doumandji, S.E.; Desutter-Grandcolas, L. A check-list of Ensifera from Algeria (Insecta: Orthoptera). Zootaxa 2010, 2432, 1–44. [Google Scholar] [CrossRef]
- Orthoptères Acridomorpha de l’Afrique du Nord-Ouest. Available online: http://acrinwafrica.mnhn.fr/SiteAcri/accueil.html (accessed on 20 June 2022).
- Cassar, L.F.; Ebejer, M.J.; Massa, B. Annotated checklist of Orthoptera of the Maltese Islands. Zootaxa 2020, 4885, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.O. Quaternary deposits in the Maltese Islands: A microcosm of environmental change in the Mediterranean lands. GeoJournal 1997, 41, 101–109. [Google Scholar] [CrossRef]
- Hunt, C.O.; Schembri, P.J. Quaternary environments and biogeography of the Maltese Islands. In Facets of Maltese Prehistory. The Prehistoric Society of Malta; Mifsud, A., Savona Ventura, C., Eds.; The Prehistoric Society of Malta: Malta, 1999; Volume VII, pp. 1–243. [Google Scholar]
- Prampolini, M.; Foglini, F.; Biolchi, S.; Devoto, S.; Angelini, S.; Soldati, M. Geomorphological mapping of terrestrial and marine areas, northern Malta and Comino (central Mediterranean Sea). J. Maps 2017, 13, 457–469. [Google Scholar] [CrossRef]
- Civetta, L.; Cornette, Y.; Gillot, P.Y.; Orsi, G. The eruptive history of Pantelleria (Sicily channel) in the last 50 ka. Bull. Volcanol. 1988, 50, 47–57. [Google Scholar] [CrossRef]
- Romano, P.; White, J.C.; Ciulla, A.; Di Carlo, I.; D’Oriano, C.; Landi, P.; Rotolo, S.G. Volatiles and trace element contents in melt inclusions from the zoned Green Tuff ignimbrite (Pantelleria, Sicily): Petrological inferences. Ann. Geophys. 2019, 61, 1–18. [Google Scholar] [CrossRef]
- Chopard, L. Gryllides. Fam. Gryllidae; Subfam. Gryllinae (Trib. Grymnogryllini, Gryllini, Gryllomorphini, Nemobiini). In Orthopterorum Catalogus; Beier, M., Ed.; Uitgeverij Dr. W. Junk: Den Haag, The Netherlands, 1967; Volume 10, pp. 1–213. [Google Scholar]
- Lodolo, E.; Ben-Avraham, Z. A submerged monolith in the Sicilian Channel (central Mediterranean Sea): Evidence for Mesolithic human activity. J. Archaeol. Sci. Rep. 2015, 3, 398–407. [Google Scholar] [CrossRef]
- Thiel, M.; Gutow, L. The Ecology of the Rafting in the Marine Environment. II. The Rafting Organisms and Community. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 43, pp. 279–418. [Google Scholar] [CrossRef]
- Rapisarda, M. L’età dell’ossidiana di Pantelleria. Atti Accad. Peloritana Pericolanti. Cl. Sci. Fis. Mat. Nat. 2007, 13, 1–21. [Google Scholar] [CrossRef]
- Kenyeres, Z.; Rácz, I.A.; Varga, Z. Endemism hot spots, core areas and disjunctions in European Orthoptera. Acta Zool. Crac. 2009, 52, 189–211. [Google Scholar] [CrossRef]
- Otte, D.; Pérez-Gelabert, D.E. Caribbean Crickets. In Orthopterists’ Society; Wayne State University: Detroit, MI, USA, 2009; pp. 1–792. [Google Scholar]
- Hembry, D.H.; Bennett, G.; Bess, E.; Cooper, I.; Jordan, S.; Liebherr, J.; Magnacca, K.N.; Percy, D.M.; Polhemus, D.A.; Rubinoff, D.; et al. Insect Radiations on Islands: Biogeographic Pattern and Evolutionary Process in Hawaiian Insects. Q. Rev. Biol. 2021, 96, 247–296. [Google Scholar] [CrossRef]














| Map Ref. No. | Location | Dates of Visit | Audio Recording | Specimen Collected |
|---|---|---|---|---|
| 1 | Cimitero Scauri | 5–6 July 2022 | ☑ | ☐ |
| 2 | Salto la Vecchia | 5–6 July 2022 | ☑ | ☐ |
| 3 | Balata dei Turchi | 27 April 2022–14–15 May 2022 | ☑ | ☐ |
| 4 | Punta Limarsi lighthouse | 27 April 2022–14–15 May 2022–5–6 July 2022 | ☑ | ☑ |
| 5 | Punta Limarsi | 27 April 2022–14–15 May 2022–5–6 July 2022 | ☑ | ☐ |
| 6 | Cala Rotonda—Martingana | 14–15 May 2022 | ☑ | ☑ |
| 7 | Punta del Formaggio | 5–6 July 2022 | ☑ | ☐ |
| DEVICE | Record Date | Sampling Frequency | Locality | Duration | Song Type | Interval Analysed for Frequency/Sound Pressure |
|---|---|---|---|---|---|---|
| Song Meter Micro | 27 April 2022 | 22.05 kHz | Punta Limarsi | 30′00″.000 | Calling | 2 s |
| Song Meter Micro | 27 April 2022 | 22.05 kHz | Punta Limarsi | 1′52″.676 | Calling | 2 s |
| Song Meter Micro | 27 April 2022 | 22.05 kHz | Punta Limarsi | 2′53″.493 | Calling | 2 s |
| Samsung SM-A750SN | 15 May 2022 | 32.00 kHz | Martingana | 1′30″.958 | Type 2 | 2 s |
| Edirol R09 | 6 July 2022 | 44.1 kHz | Martingana | 1′46″.701 | Calling | 10 s |
| Date | Collection Time | Hourly Average Air Temperature (°C) | Hourly Average Relative Humidity (%) | Instantaneous Wind Speed at 2 m (m/s) |
|---|---|---|---|---|
| 27 April 2022 | 21:00 | 18 | 37 | 1 |
| 14 May 2022 | 21:00 | 16.1 | 57 | 0.9 |
| 15 May 2022 | 21:00 | 23.1 | 25 | 1 |
| 6 July 2022 | 21:00 | 25.5 | 81 | 3.4 |
| Element | Frequency (Hz) | Theoretical Value | Δ | Pressure (dBfs) | |
|---|---|---|---|---|---|
| Fundamental | Harmonic | ||||
| Primary | I | 5154 | -- | -- | −23.51 |
| II | 10,270 | 10,308 | +0.37% | −45.37 | |
| III | 15,440 | 15,462 | +0.14% | −65.44 | |
| IV | 20,540 | 20,616 | +0.37% | −70.39 | |
| Secondary | I | 5028 | -- | -- | −28.46 |
| II | 9894 | 10,056 | +1.61% | −51.8 | |
| III | 14,960 | 15,084 | +0.83% | −64.02 | |
| IV | 19,500 | 20,118 | +3.14% | −76.92 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massa, B.; Cusimano, C.A.; Fontana, P.; Brizio, C. New Unexpected Species of Acheta (Orthoptera, Gryllidae) from the Italian Volcanic Island of Pantelleria. Diversity 2022, 14, 802. https://doi.org/10.3390/d14100802
Massa B, Cusimano CA, Fontana P, Brizio C. New Unexpected Species of Acheta (Orthoptera, Gryllidae) from the Italian Volcanic Island of Pantelleria. Diversity. 2022; 14(10):802. https://doi.org/10.3390/d14100802
Chicago/Turabian StyleMassa, Bruno, Camillo Antonino Cusimano, Paolo Fontana, and Cesare Brizio. 2022. "New Unexpected Species of Acheta (Orthoptera, Gryllidae) from the Italian Volcanic Island of Pantelleria" Diversity 14, no. 10: 802. https://doi.org/10.3390/d14100802