Abstract
Hurricanes Irma and Maria, two powerful storms that hit the U.S. Virgin Islands less than 2 weeks apart in September 2017, caused extensive damage to the natural resources on St. John. Damage was particularly severe in a unique mangrove/coral ecosystem in three bays within Virgin Islands Coral Reef National Monument, a National Park Service marine protected area. Many Red Mangrove (Rhizophora mangle) trees were uprooted and tossed into the sea, and the prop roots of others were stripped of corals, sponges and other marine life. No other mangrove area in the Caribbean is known to have so many scleractinian corals (about 30 species before the storms). Although many corals were overturned or buried in rubble, colonies of most of the species, including four that are listed as threatened under the U.S. Endangered Species Act, survived. Recovery of this ecosystem will depend on Red Mangrove propagules becoming established and producing prop roots to support rich marine life along with a canopy to provide the shade that was critical to the biodiversity that was present before the storms. Unlike in many situations where major disturbances reduce coral cover, the substrate that must be restored for full recovery to occur is a living substrate—the prop roots of the mangroves. Larvae of corals and sponges will need to recruit on to the roots. Future storms could hinder this process.
1. Introduction
Hurricanes are part of life cycles in the Caribbean. However, the combination of Hurricanes Irma and Maria in September 2017 was unprecedented in terms of destructive forces which smashed the US Virgin Islands. Irma, a Category 5 storm, with sustained winds of 185 mph and gusts over 220 mph, was followed less than two weeks later by Hurricane Maria, a Category 4 storm in St. John and a Category 5 in St. Croix and Puerto Rico (Figure 1).
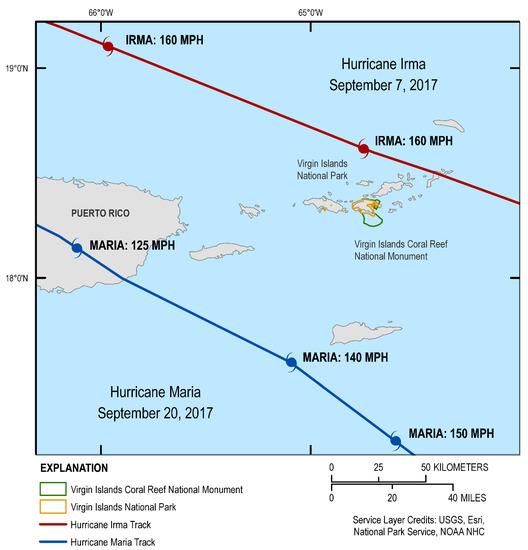
Figure 1.
Tracks of Hurricanes Irma and Maria, September 2017 as the storms moved through the U.S. Virgin Islands and Puerto Rico. (credit, Breanna Williams and Betsy Boynton, Cherokee Nation Technologies, contracted to the USGS).
2. Unprecedented Damage to Terrestrial and Marine Resources
Over the course of only a few hours on 6 September 2017, Hurricane Irma caused massive destruction in the British and U.S. Virgin Islands, particularly on St. John, the site of Virgin Islands National Park and Virgin Islands Coral Reef National Monument. As it was not possible for anyone to safely get out and see the conditions either above or below the water, it is not clear if Irma or Maria caused the most overall damage, although it is suspected that Irma was the most destructive as its eye went right over the island of St. John [1].
This unprecedented combination of severe storms over such a short time period resulted in extreme damage to almost everything on land, to boats hauled out on shore and anchored in the water, to trees including mangroves, and to reefs and other marine resources below the surface. Deeper reefs sustained less damage than shallow, nearshore ecosystems [2].
Hurricane Hole, the most visited portion of Virgin Islands Coral Reef National Monument, includes four mangrove-lined bays (Figure 2). These bays range in size from 0.06 to 0.11 km2 with watersheds of 0.11 to 0.43 km2 and maximum elevation from 60 to 146 m [3]. Before these powerful storms, research documented that the biodiversity in three of these bays (Princess Bay, Otter Creek and Water Creek) was remarkable, perhaps unique for mangroves in the Caribbean; about 30 coral species, including four that are listed as threatened under the Endangered Species Act, grew on and near the prop roots of Red Mangrove (Rhizophora mangle) trees (Figure 3) [3,4]. Approximately 80 species of fish, and 60 species of sponges have also been identified [4]. The objective of this paper is to provide an overview of the initial effects of the storms on the mangroves and shallow marine communities in this unusual coral/mangrove ecosystem using still photos and videos taken while snorkeling in waters less than 5 m around the perimeters of the bays and aerial images taken before and after the hurricanes.
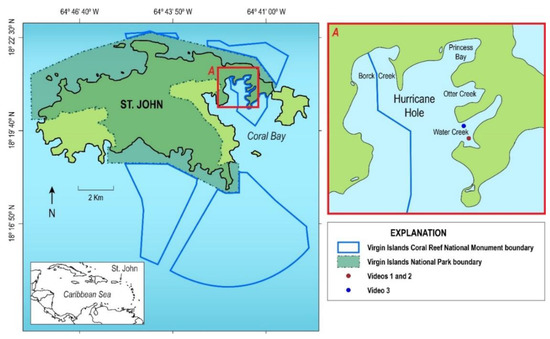
Figure 2.
Map of Hurricane Hole, within Virgin Islands Coral Reef National Monument. (credit, Betsy Boynton, Cherokee Nation Technologies contracted to the USGS, adapted from base map by Simon Pittman, NOAA). (Note that the term “Creek” does not refer to a freshwater source and is synonymous with Bay).

Figure 3.
Several species of scleractinian corals grew on and near the Red Mangrove prop roots before Hurricanes Irma and Maria (photo, C. Rogers).
The first sight of Hurricane Hole following Hurricane Irma revealed a shocking scene of desolation, with the few houses on shore in ruins, boats in the head of each of the four bays tangled and jumbled together, and trees snapped and uprooted on shore. In the past, Hurricane Hole has served as a safe haven for boats during hurricanes, but around 100 boats were transported up onto the shore or sank or capsized during these storms, causing further damage. Some of the damaged boats were able to be repaired, but many were a total loss. As in other storms in the Atlantic and Caribbean [5], the mangroves were especially hard hit (Figure 4). Large mangrove trees and some cactus have been uprooted and now lie on the bottom of the bays.

Figure 4.
Images of a portion of Water Creek before Hurricane Irma (top) and one week after Irma (bottom) showing severe damage to mangroves fringing the shore, and boats and debris up in the mangroves. 2012 High Resolution Orthoimagery courtesy of the U.S. Geological Survey. 2017 imagery citation: Office for Coastal Management, 2019: 2017 NOAA NGS Emergency Response Imagery: Hurricane Irma, https://inport.nmfs.noaa.gov/inport/item/52284.
Hurricane Irma brought higher wind speeds but Maria brought larger waves because of the larger fetch, with heights of 2 to 3 m in Coral Bay, near the entrances to Otter and Water Creeks based on the model described in Reference [1] (Figure 5).
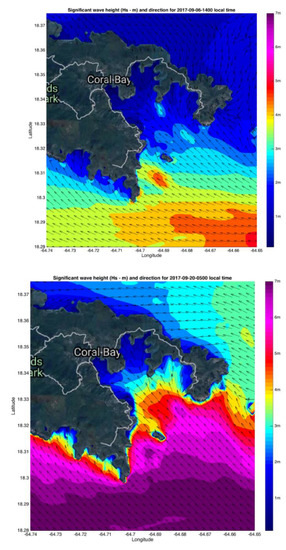
Figure 5.
Modelled wave heights for Coral Bay including Hurricane Hole during Hurricane Irma (top) and Hurricane Maria (bottom). From Reference [1]. This model does not have the ideal resolution to accurately simulate waves in very shallow water in Hurricane Hole but provides a good estimate of the forces to which these bays were subjected.
Before the storms, the south shore of Water Creek (Figure 2) had especially large corals of several different species (link to video #1 https://youtu.be/uEEVv_WQxqs) and very diverse prop root communities with colorful sponges, tunicates, anemones, and seaweeds. Numerous fish, including grunts, snappers, and the especially conspicuous angelfish, swam among the roots. For many reasons, including safety concerns and disruption of researchers’ lives, it was not possible to examine the area immediately after the hurricanes.
However, when first observed in February 2018, 5 months after the storms, there were few signs of life—the video of the area looks like a black and white film (link to video #2 https://youtu.be/EbgUlnfhOLw). Destruction of prop roots and marine communities approached 90 to 100% in some areas. A few damaged corals were present, but many if not most of the large corals that used to grow in shallow water under the Red Mangrove trees were gone, probably pulverized under piles of rock rubble that had been moved around by the waves. Many rocks have been moved up into shallower water, closer to shore (Figure 6). These rocks could hinder the settlement of mangrove seedlings and other organisms. Few sponges are present. Fewer prop roots are visible, and several have been scoured or stripped bare. Even the outer bark is gone on many.

Figure 6.
Before the storms, several species of corals and sponges grew on and among the prop roots of Red Mangrove trees. Storm waves scoured the roots and transported rocks into shallow nearshore waters (photos, C. Rogers). (a) Before; (b) After.
Similar severe damage to prop root communities was also observed in Otter Creek (Figure 7), while Princess Creek had less damage to corals and other organisms on the roots and nearby substrate, possibly because it received some protection from points of land that define its shoreline and was less exposed to the direct action of the storm waves. In general, the number and abundance of fish species seems much lower, although quantitative data are not available.

Figure 7.
Some coral colonies (including this large Orbicella faveolata) remained in place, sustaining partial damage, while others, such as the Colpophyllia natans (upper left), were dislodged or displaced. Sponges were stripped off the Red Mangrove roots (photos, C. Rogers).
The north side of Water Creek was less damaged than the south side, for unclear reasons, possibly because of the angle of the storm waves. Some large reef-building corals (notably Siderastrea siderea) in very shallow water remained intact (link to video #3 https://youtu.be/FO8cYYUs5F0). A few stretches of the shoreline still have prop roots with colorful sponges and other organisms growing on them.
The loss of shade in the mangroves and over the entire island of St. John is one of the most conspicuous consequences of the hurricanes. As a result of the damage to the mangroves, much more sunlight now reaches the shallow nearshore waters. Research shows that Hurricane Hole has been functioning as a refuge from some aspects of climate change by providing variable water temperatures and lower light levels [3].
3. Potential for Recovery
It is difficult to estimate how long it might take for this ecosystem to recover. The word “recovery” is often used without clarity as to just what is meant. Coral cover could return to pre-disturbance levels but with a different coral community composition—for example, “weedier”, smaller species could replace the major framework builders (“ecosystem engineers”—sensu [6]). Coral cover is hard to quantify in the mangroves where one cannot swim over the substrate and use a point-count or transect method. Recovery is largely a reflection of the re-growth of surviving corals (intact and partly dead) and settlement and persistence of new coral recruits. Coral larvae settle out of the water column on to hard substrate, including rocks and prop roots, calcify, and start new coral colonies. Given the worldwide decline of coral reefs (e.g., [7]), it is likely that recruitment levels are declining. However, the relationship between recruitment rates and reef recovery is complicated and not fully understood [8]. In Hurricane Hole, most of the recovery would probably depend on new recruits as many corals have been buried or smashed. In addition, recovery may now be slower than in the past because warming temperatures are slowing growth of massive corals [9].
Estimating time of recovery for corals growing in Hurricane Hole is constrained by a lack of information on: (1) growth rates of corals in general, and in particular in shaded conditions and how these will change given the loss of the mangrove canopy along some portions of the shoreline; (2) time for Red Mangroves to recruit and grow or grow from existing seedlings; (3) the source (local or distant?) of the coral larvae available to settle and grow; (4) the ability of damaged areas “upstream” of Hurricane Hole to provide larvae; (5) subsequent mortality from new and more frequent bleaching events [10] and disease outbreaks; (6) a lack of understanding of how corals might become more resistant to stressors [11]; and (7) a lack of information on future changes in the prevailing environmental factors such as temperature and irradiance, as well as the on frequency/intensity of future storms.
Before the storms of 2017, the most abundant corals were Agaricia agaricites growing on prop roots. The largest corals, generally found on the bottom were of the following species: Colpophyllia natans, Orbicella annularis, Diploria labyrinthiformis, and Siderastrea siderea. Most of the species observed before the storms survived, although no individuals of the rare coral Mycetophyllia spp. have been seen since. Siderastrea siderea colonies appear to have increased in relative abundance. Close examination of the scientific literature reveals very limited data on Caribbean and western Atlantic coral growth rates [12,13,14]. For several species, notably many of the ones that do not reach a large size, no data are available. Madin et al. 2016 [14] cite 167 studies pertaining to coral growth rates. Of these, only a small number contain original data on growth rates for Caribbean corals, and most are for Orbicella annularis. For many species no data at all are available. Examining the ranges for all species for which data are available reveals that very few ever exceed 1 cm/year linear extension rate. Unless some of the coral colonies are growing faster in Hurricane Hole, these rates indicate that recovery to some of the largest colonies would take a minimum of a few decades.
Sponges are a very significant component of the diversity in Hurricane Hole, with about 60 species documented. Some are important food items for endangered Hawksbill Sea Turtles (Eretmochelys imbricata). Most of the sponges in Hurricane Hole are typically associated with reefs rather than mangroves, and little is known of how quickly these might regenerate or settle from recruits in this unusual habitat [15].
The unusual environmental conditions in Hurricane Hole, particularly the shading by Red Mangrove trees fringing the shorelines, likely influence settlement and growth of corals. Prop roots were the substrate for many coral colonies, and the density/number of prop roots has declined with the uprooting and destruction of Red Mangrove trees. A few of the larger coral species (e.g., Orbicella faveolata) settle on the prop roots, typically near the base, and then grow into large colonies. Many settle and become established on nearby substrate.
Corals that survived the storms may or may not be more resistant to anticipated future stressors such as increasing sea water temperature [11]. A recent comprehensive analysis of coral species traits groups corals into four categories, the largest being “stress tolerant” [16]. However, the emphasis was on low light and high sedimentation primarily, rather than other stressors such as storms. Not all stressors are inherently similar, and storm damage differs greatly from diseases or thermal stress. Consequently, this stress-tolerant category must be interpreted carefully.
The Red Mangrove, the dominant species along the shores of the bays in Hurricane Hole, does not re-sprout from branches or trunks as do other tree species [17,18,19]. Initial recovery will likely depend more on regrowth of surviving seedlings than establishment of new propagules; some of the Red Mangrove seedlings that are still alive perhaps were submerged under the water during the storms and therefore escaped being killed by the wind [20].
The shade once provided by the canopy of Red Mangrove trees in Hurricane Hole seemed to allow recruitment and growth of many organisms that would not otherwise have persisted. The trees were reducing the light reaching nearshore corals and other marine life by over 70% [3]. As noted above, much more solar radiation now reaches the shallow water nearshore. Hurricane Hole may have been functioning as a refuge from some aspects of climate change by providing variable water temperatures and lower light levels [3]. Corals, and even sponges, grow slowly. Coral growth rates (generally less than 1 cm/year) at least for some species have declined with rising sea water temperatures and decreasing pH [9,21]. Coral growth tends to decrease with depth at least for some species [22], but the growth rates of corals in shallow waters (less than 1–2 m) such as in Hurricane Hole is not known. Some of the species, including Colpophyllia natans, are more encrusting or plate-like than on the reefs.
4. Discussion
Most studies of the effects of hurricanes on tropical ecosystems focus on either forests or coral reefs, that is, terrestrial or marine habitats, and Lugo et al. (2000) [23] compared the effects of Hurricane Hugo (1989) on coral reefs and rain forests. The mangrove/coral habitat in Hurricane Hole is an unusual ecosystem that integrates these very different systems, and rate of recovery is unpredictable. Red Mangroves could regrow over the next 10 to 20 years, and they are critical to the recovery of the coral communities which will be delayed while the trees regrow. This dependence on “living” substrate (i.e., prop roots) differs from the typical case where hard substrate is the limiting factor.
Recovery of the coral communities in the mangroves will likely depend more on coral recruitment and subsequent growth than on regrowth of partially dead or fragmented corals because relatively few partially dead colonies remain, and because the faster-growing branching species (such as the acroporids) that characterize reefs that have been documented to recover quickly are not growing in this area.
No comprehensive inventory of even the primary marine organisms besides the corals and the fish has been compiled. There may have been loss of species that have not even been described yet. For example, in 2014 a new genus of worm was discovered here [24].
The marine organisms in Hurricane Hole will not likely be growing under the same environmental conditions as before the storms, and the current conditions could reduce growth rates, recruitment levels and survival of new recruits. The small bays of Hurricane Hole shielded this habitat from the destructive forces of previous hurricanes, but the power of Irma and Maria may have tipped the balance, establishing a new baseline for quantifying biological responses to tropical storms. Additionally, changing climatic conditions are predicted to increase the intensity and perhaps also the frequency of major storms [25]. Repeated bleaching events could lower the resilience of corals to the point they no longer survive [10].
One of the great challenges to recovery is that sponges, and particularly corals, grow slowly. It is possible that the surviving sponges and corals could produce larvae to replenish this area with additional larvae coming from nearby sites in the British Virgin Islands (although they too sustained severe damage). No detailed information is available on the current patterns and connectivity among St. John and surrounding islands (see [26]). The full recovery to anything resembling the uniquely diverse, colorful coral/mangrove ecosystem that existed in Hurricane Hole before the 2017 hurricanes will take time, possibly decades, and, given the unique nature of this ecosystem, is not guaranteed.
The highly destructive storms offer the opportunity to learn more about how mangroves, corals and other marine communities respond to natural disasters and changing climate and more about the different responses of various reef-building corals to a severely altered environment. Hurricane Hole may no longer have the characteristics that allowed it to function as a coral reef refuge and a nursery before the storms [3]. Red Mangroves bridge the land and the sea. In the coral reef literature there is frequent reference to the large, coral reef-building coral species as “ecosystem engineers” [6]. These species are the architects of the “true” coral reefs [27]. However, Red Mangroves could be considered ecosystem engineers also particularly in Hurricane Hole where they provide the “substrate” for the high biodiversity of corals, sponges, tunicates, seaweeds, anemones, and other organisms. Extensive new settlement and growth both above and below the water will be required for this extraordinary ecosystem to recover.
Funding
Salary for the author was funded by the U.S. Geological Survey.
Acknowledgments
Thanks to Kristen Hart (USGS) for comments on an earlier version of this paper. My thanks also to Breanna Williams and Betsy Boynton (Cherokee Nation Technologies, contracted to the USGS) for preparing Figure 1, and Betsy Boynton for preparing Figure 2. Special thanks to Andy From (USGS) and Miguel Canals-Silander (University of Puerto Rico) for helpful discussions and interpretation, and for Figure 4 and Figure 5, respectively. Addressing the comments from three anonymous reviewers improved the paper substantially. Thomas Kelley with the National Park Service assisted in getting me to the study sites in Hurricane Hole, and I appreciate the ongoing collaboration with NPS. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The author declares no conflict of interest.
References
- Browning, T.N.; Sawyer, D.E.; Brooks, G.R.; Larson, R.A.; Ramos-Scharron, C.E.; Canals-Silander, M. Widespread deposition in a coastal bay following three major 2017 hurricanes (Irma, Jose, and Maria). Sci. Rep. 2019, 9, 7107. [Google Scholar] [CrossRef] [PubMed]
- Miller, J. (National Park Service, St. John, VI, USA). Personal communication, 2018.
- Yates, K.K.; Rogers, C.S.; Herlan, J.J.; Brooks, G.R.; Smiley, N.A.; Larson, R.A. Diverse coral communities in mangrove habitats suggest a novel refuge from climate change. Biogeosciences 2014, 11, 4321–4337. [Google Scholar] [CrossRef]
- Rogers, C.S. A unique coral community in the mangroves of Hurricane Hole, St. John, US Virgin Islands. Diversity 2017, 9, 29. [Google Scholar] [CrossRef]
- Doyle, T.W.; Smith, T.J., III; Robblee, M.B. Wind damage effects of Hurricane Andrew on mangrove communities along the southwest coast of Florida, USA. J. Coast. Res. 1995, 21, 159–168. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Connolly, S.M.; Marshall, D.J.; Cohen, A.L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Adjeroud, M.; Kayal, M.; Penin, L. Importance of recruitment processes in the dynamics and resilience of coral reef assemblages. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 549–569. [Google Scholar]
- Lough, J.M.; Canin, N.E. Perspectives on massive coral growth rates in a changing ocean. Biol. Bull. 2014, 226, 187–202. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Darling, E.S.; Cote, I.M. Seeking resilience in marine ecosystems. Science 2018, 359, 986–987. [Google Scholar] [CrossRef]
- Huston, M. Variation in coral growth rates with depth in Discovery Bay, Jamaica. Coral Reefs 1985, 4, 19–25. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Anderson, K.D.; Hoogenboom, M.O.; Widman, E.; Baird, A.H.; Pandolfi, J.M.; Edmunds, P.J.; Lough, J.M. Spatial, temporal and taxonomic variation in coral growth—Implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 2015, 53, 215–295. [Google Scholar]
- Madin, J.S.; Anderson, K.D.; Andreasen, M.H.; Bridge, T.C.L.; Cairns, S.D.; Connolly, S.R.; Darling, E.S.; Diaz, M.; Falster, D.S.; Franklin, E.C.; et al. The coral trait database, a curated database of trait information for coral species from the global oceans. Sci. Data 2016, 3, 160017. [Google Scholar] [CrossRef] [PubMed]
- Wulff, J. (Florida State University, Tallahassee, FL, USA). Personal communication, 2018.
- Darling, E.S.; Alvarez-Filip, L.; Oliver, T.A.; McClanahan, T.R.; Cote, I.M. Evaluating life history strategies of reef corals from species traits. Ecol. Lett. 2012, 15, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Milbrandt, E.C.; Greenwalt-Boswell, J.M.; Sokoloff, P.D.; Bortone, S.A. Impact and response of southwest Florida mangroves to the 2004 hurricane season. Estuaries Coasts 2006, 29, 979–984. [Google Scholar] [CrossRef]
- Imbert, D. Hurricane disturbance and forest dynamics in east Caribbean mangroves. Ecosphere 2018, 9, e02231. [Google Scholar] [CrossRef]
- Krauss, K. (US Geological Survey, Lafayette, LA, USA). Personal communication, 2018.
- Manzello, D. Coral growth with thermal stress and ocean acidification. Coral Reefs 2010, 29, 749–758. [Google Scholar] [CrossRef]
- Groves, S.H.; Holstein, D.M.; Enochs, I.C.; Kolodzeij, G.; Manzello, D.P.; Brandt, M.E.; Smith, T.B. Growth rates of Porites astreoides and Orbicella franksi in mesophotic habitats surrounding St. Thomas, US Virgin Islands. Coral Reefs 2018, 37, 345–354. [Google Scholar] [CrossRef]
- Lugo, A.E.; Rogers, C.S.; Nixon, S.W. Hurricanes, coral reefs and rainforests: Resistance, ruin and recovery in the Caribbean. Ambio 2000, 29, 106–114. [Google Scholar] [CrossRef]
- Prentiss, N.; Vasilieiadou, K.; Faulwetter, S.; Arvanitidis, C. A new genus and species of Serpulidae (Annelida, Polychaeta, Sabellida) from the Caribbean Sea. Zootaxa 2014, 3900, 204–222. [Google Scholar] [CrossRef]
- Knutson, T.R.; McBride, J.L.; Chan, J.; Emanuel, K.; Holland, G.; Landsea, C.; Held, I.; Kossin, J.P.; Srivastava, A.K.; Sugi, M. Tropical cyclones and climate change. Nat. Geosci. 2010, 3, 157–163. [Google Scholar] [CrossRef]
- Edmunds, P.J.; McIlroy, S.E.; Adjeroud, M.; Ang, P.; Bergman, J.L.; Carpenter, R.C.; Coffroth, M.A.; Fujimura, A.G.; Hench, J.L.; Holbrook, S.J.; et al. Critical information gaps impeding understanding of the role of larval connectivity among coral reef islands in an era of global change. Front. Mar. Sci. 2018, 5, 290. [Google Scholar] [CrossRef]
- Rogers, C.S.; Miller, J. Measuring, interpreting, and responding to changes in coral reefs: A challenge for biologists, geologists, and managers. In Coral Reefs at the Crossroads; Hubbard, D., Rogers, C.S., Lipps, J., Stanley, G., Jr., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 277–292. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).