Effects of Land Cover Pattern Along Urban-Rural Gradient on Bird Diversity in Wetlands
Abstract
1. Introduction
2. Materials and Methods
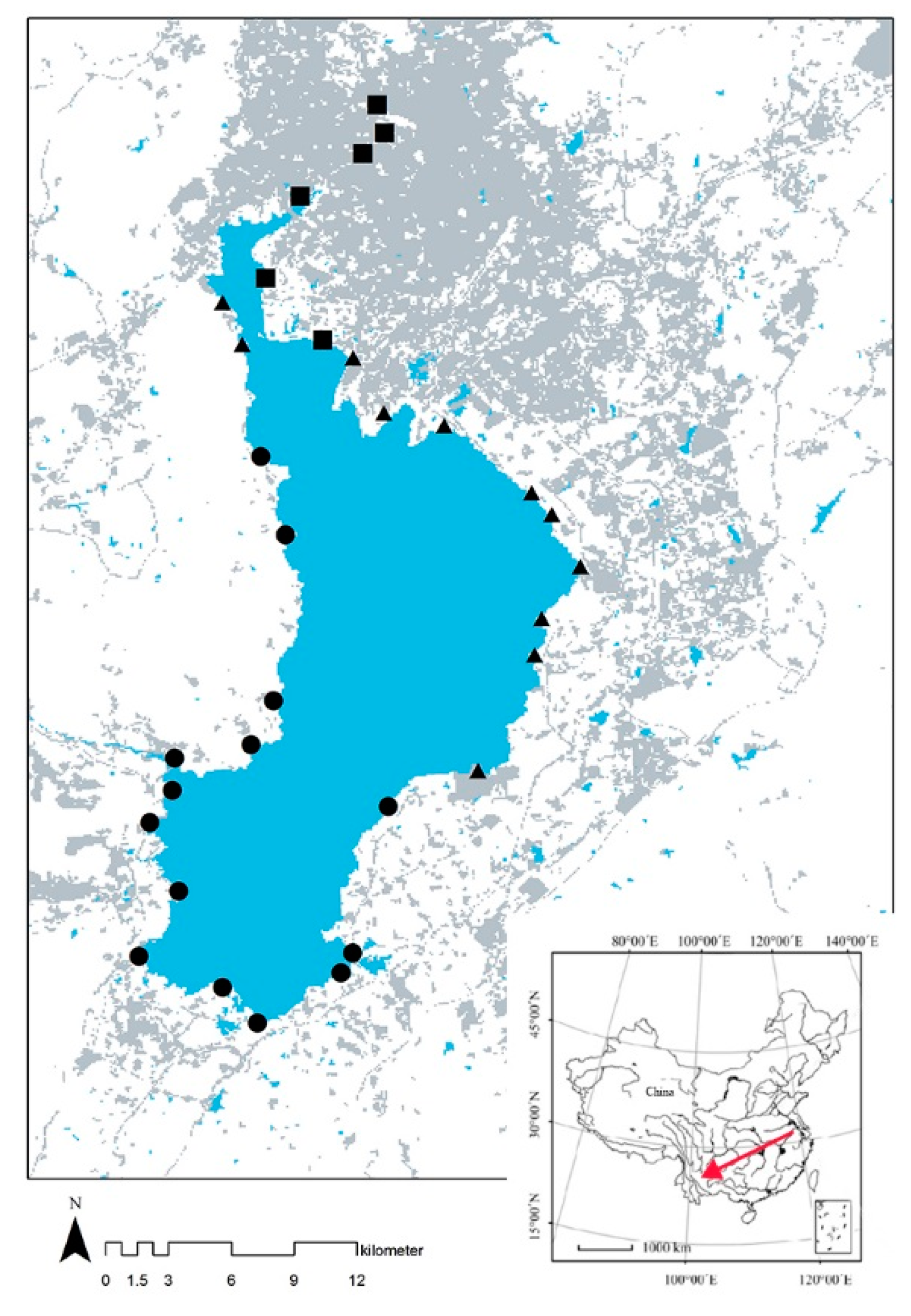
2.1. Study Area
2.2. Bird Survey and Diversity Indices
2.3. Land Cover and Plant Community Survey
2.4. Statistical Analysis
3. Result
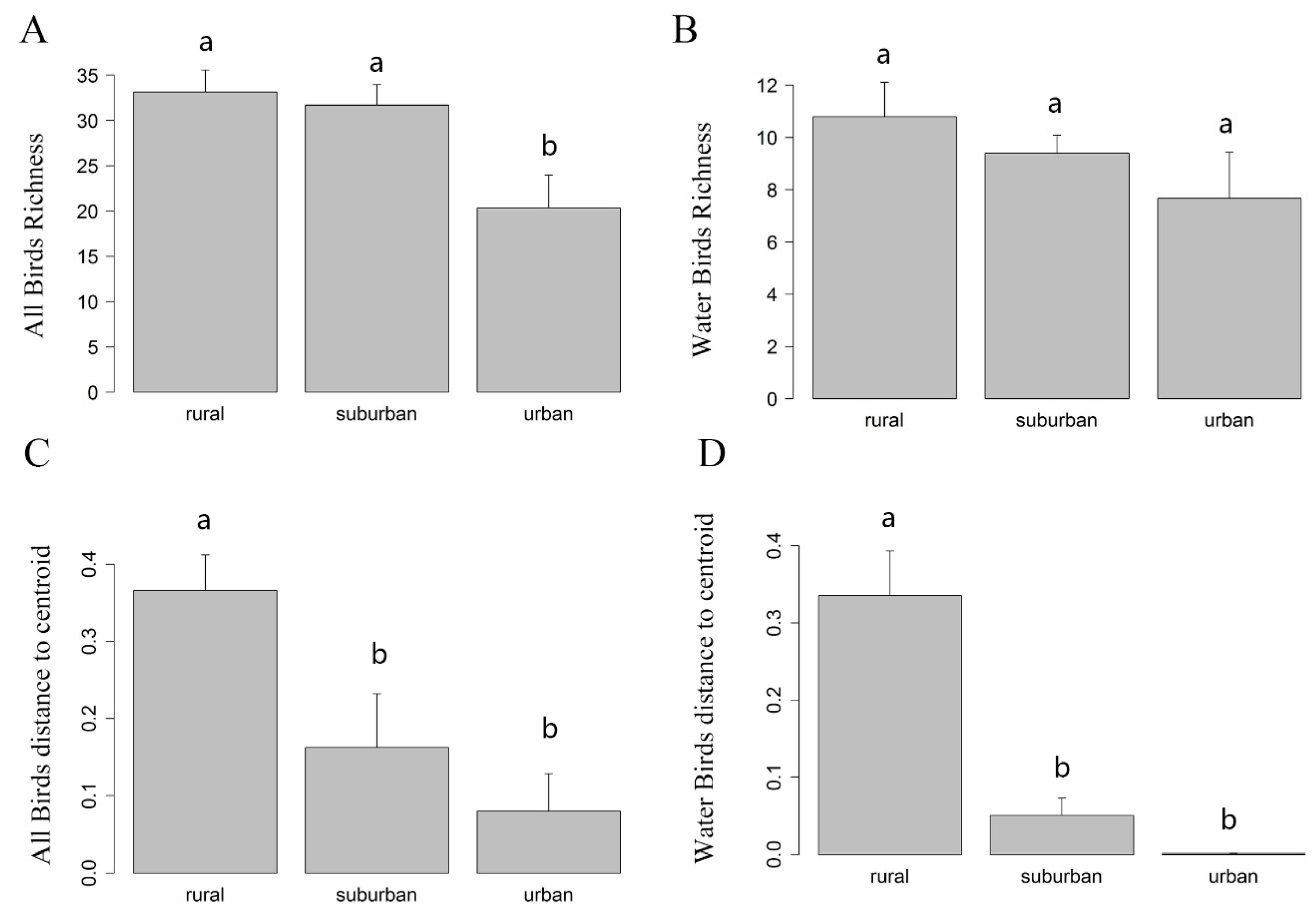
3.1. Differences of α Diversity in Urban-Rural Gradient
3.2. The Differences of β Diversities in Urban-Rural Gradient
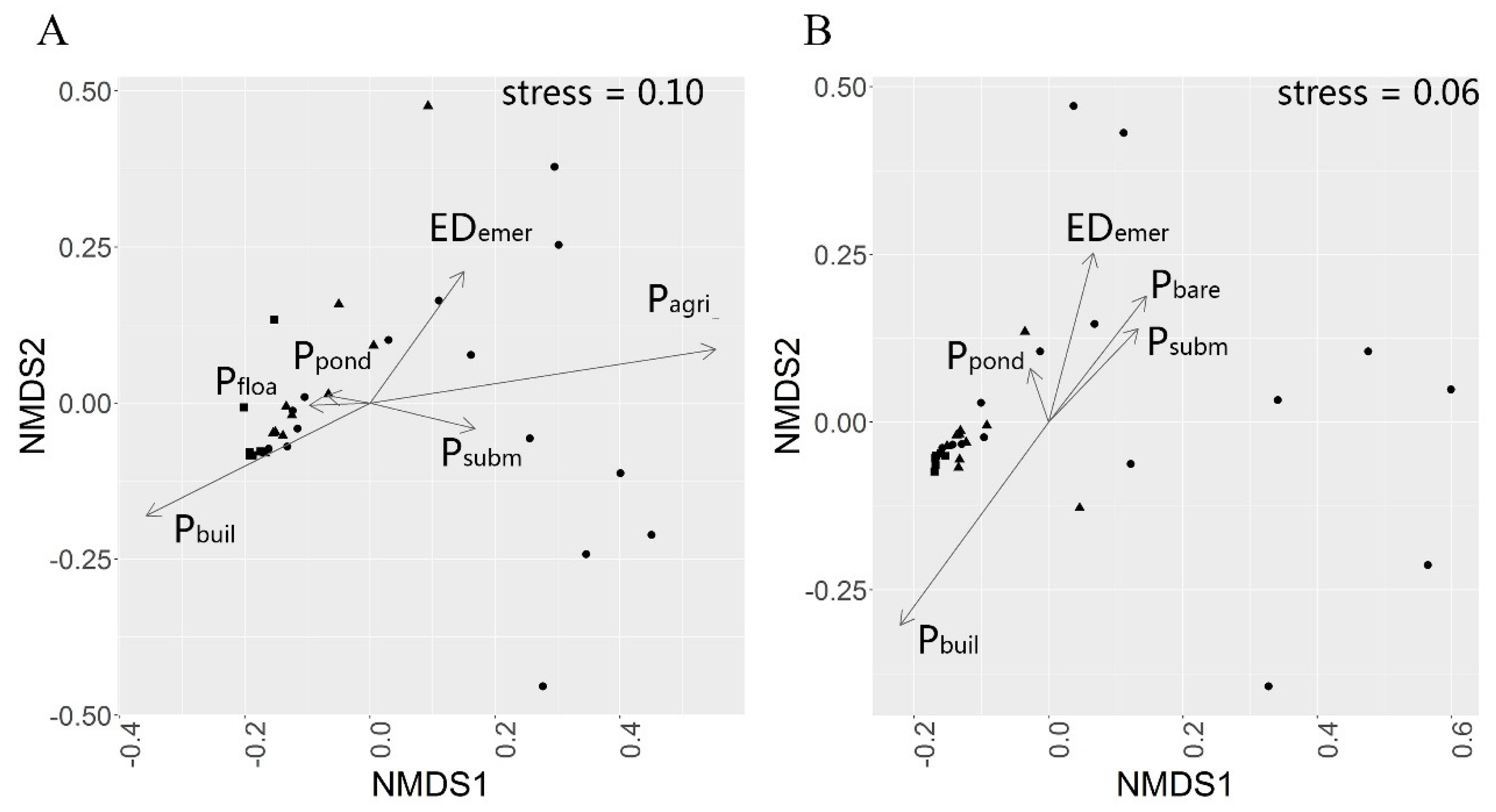
3.3. Effects of Land Cover Patterns on Bird Species α Diversities
3.4. Effects of Land Cover Patterns on Bird Community Dispersion in Urban-Rural Gradient
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Differences in the Shannon-Wiener Index of the Urban-Rural Gradient
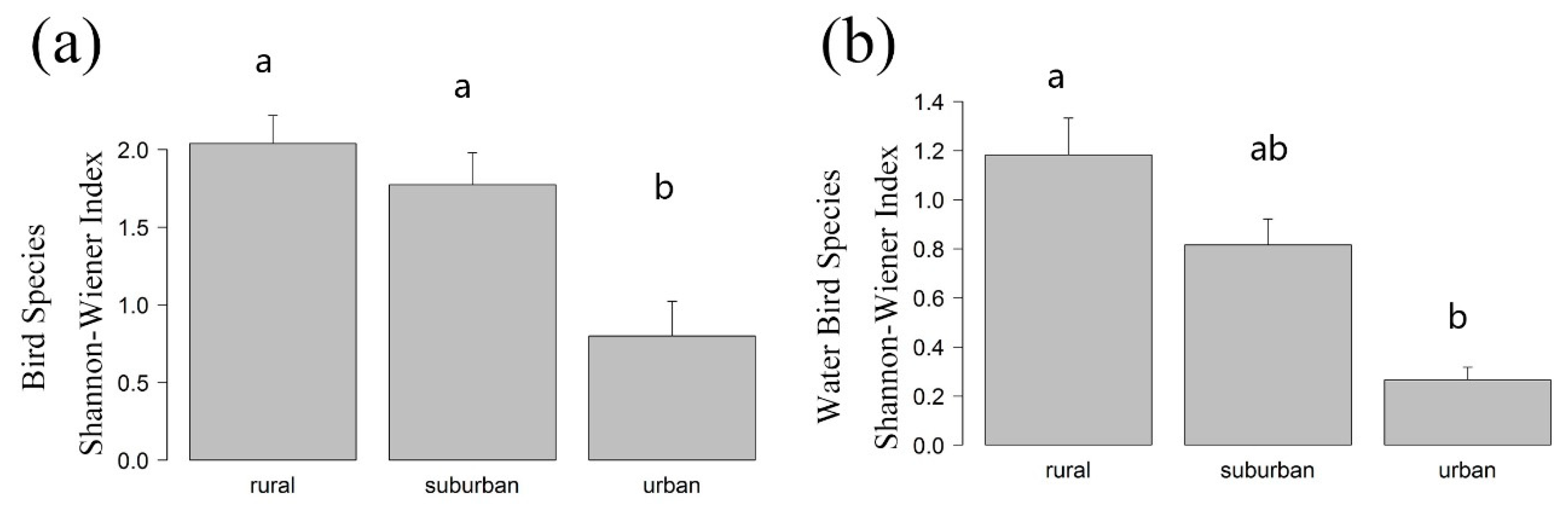
| Season | Type | Species Richness | Shannon-Wiener Index | ||
|---|---|---|---|---|---|
| Range | Mean ± S.E. | Range | Mean ± S.E. | ||
| Summer | All birds | 4–34 | 19.32 ± 1.32 | 0.42–2.98 | 1.97 ± 0.12 |
| Water birds | 0–15 | 5.00 ± 0.55 | 0–1.95 | 0.75 ± 0.09 | |
| Winter | All birds | 6–40 | 22.00 ± 1.35 | 0.10–2.71 | 1.27 ± 0.15 |
| Water birds | 1–15 | 7.74 ± 0.61 | 0–1.89 | 0.59 ± 0.10 | |
Appendix B
References
- Hu, S.; Niu, Z.; Chen, Y.; Li, L.; Zhang, H. Global wetlands: Potential distribution, wetland loss, and status. Sci. Total Environ. 2017, 586, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res. 2014, 65, 934–941. [Google Scholar] [CrossRef]
- Cohen, P.; Potchter, O.; Schnell, I. The impact of an urban park on air pollution and noise levels in the Mediterranean city of Tel-Aviv, Israel. Environ. Pollut. 2014, 195, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Song, J.; Yuan, H. Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: An overview. Environ. Int. 2009, 35, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Braga, E.S.; Bonetti, C.; Burone, L.; Bonetti, J. Eutrophication and bacterial pollution caused by industrial and domestic wastes at the Baixada Santista estuarine system - Brazil. Mar. Pollut. Bull. 2000, 40, 165–173. [Google Scholar] [CrossRef]
- Verzijden, M.N.; Ripmeester, E.A.P.; Ohms, V.R.; Snelderwaard, P.; Slabbekoorn, H. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J. Exp. Biol. 2010, 213, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Hamer, A.J.; McDonnell, M.J. Amphibian ecology and conservation in the urbanising world: A review. Biol. Conserv. 2008, 141, 2432–2449. [Google Scholar] [CrossRef]
- Sewell, S.R.; Catterall, C.P. Bushland modification and styles of urban development: Their effects on birds in south-east Queensland. Wildl. Res. 1998, 25, 41–63. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Boerger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.L.H.; Hardy, P.B. Loss rates of butterfly species with urban development. A test of atlas data and sampling artefacts at a fine scale. Biodivers. Conserv. 2001, 10, 1831–1837. [Google Scholar] [CrossRef]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, Competition, and Biodiversity Loss in Urban Ecosystems. Bioscience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Moffatt, S.F.; McLachlan, S.M.; Kenkel, N.C. Impacts of land use on riparian forest along an urban-rural gradient in southern Manitoba. Plant Ecol. 2004, 174, 119–135. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an “urban exploiter”? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- McKinney, R.A.; Raposa, K.B.; Cournoyer, R.M. Wetlands as habitat in urbanizing landscapes: Patterns of bird abundance and occupancy. Landsc. Urban Plan. 2011, 100, 144–152. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Diego Ibanez-Alamo, J.; Jokimaki, J.; Mand, R.; Tryjanowski, P.; Moller, A.P. Evidence of evolutionary homogenization of bird communities in urban environments across Europe. Glob. Ecol. Biogeogr. 2016, 25, 1284–1293. [Google Scholar] [CrossRef]
- Clergeau, P.; Croci, S.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Dinetti, M. Avifauna homogenisation by urbanisation: Analysis at different European latitudes. Biol. Conserv. 2006, 127, 336–344. [Google Scholar] [CrossRef]
- Blair, R.B. Creating a homogeneous avifauna. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer US: Boston, MA, USA, 2001; pp. 459–486. ISBN 978-1-4615-1531-9. [Google Scholar]
- Kühn, I.; Klotz, S. Urbanization and homogenization—Comparing the floras of urban and rural areas in Germany. Biol. Conserv. 2006, 127, 292–300. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and ConservationThe impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Fortel, L.; Henry, M.; Guilbaud, L.; Guirao, A.L.; Kuhlmann, M.; Mouret, H.; Rollin, O.; Vaissiere, B.E. Decreasing Abundance, Increasing Diversity and Changing Structure of the Wild Bee Community (Hymenoptera: Anthophila) along an Urbanization Gradient. PLoS ONE 2014, 9, e104679. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Fairbrass, A.J.; Falk, S.J.; Hale, J.D.; Matthews, T.J. Changing Bee and Hoverfly Pollinator Assemblages along an Urban-Rural Gradient. PLoS ONE 2011, 6, e23459. [Google Scholar] [CrossRef]
- Clucas, B.; Marzluff, J.M. A cross-continental look at the patterns of avian species diversity and composition across an urbanisation gradient. Wildl. Res. 2015, 42, 554–562. [Google Scholar] [CrossRef]
- Turner, M.G. Landscape ecology in North America: Past, present, and future. Ecology 2005, 86, 1967–1974. [Google Scholar] [CrossRef]
- Thornton, D.H.; Branch, L.C.; Sunquist, M.E. The influence of landscape, patch, and within-patch factors on species presence and abundance: A review of focal patch studies. Landsc. Ecol. 2011, 26, 7–18. [Google Scholar] [CrossRef]
- Colwell, M.A. Shorebird ecology, conservation, and management; Univ of California Press: Berkley, CA, USA, 2010; pp. 134–138. [Google Scholar]
- Bara, M.; Segura, L.N. Effect of Air Temperature and Water Depth on Bird Abundance: A Case Study of Rallidae and Anatidae in the Northeastern Algerian Garaet Hadj Tahar. Pak. J. Zool. 2019, 51, 211–217. [Google Scholar] [CrossRef]
- Xia, S.; Wang, Y.; Lei, G.; Liu, Y.; Lei, J.; XiuboYu; Wen, L.; Zhou, Y. Restriction of Herbivorous Waterbird Distributions in the Middle and Lower Yangtze River Floodplain in View of Hydrological Isolation. Wetlands 2017, 37, 79–88. [Google Scholar] [CrossRef]
- Petersen, K.L.; Westmark, A.S. Bird Use of Wetlands in a Midwestern Metropolitan Area in Relation to Adjacent Land Cover. Am. Midl. Nat. 2013, 169, 221–228. [Google Scholar] [CrossRef]
- Rosselli, L.; Stiles, F.G. Wetland habitats of the Sabana de Bogota Andean Highland Plateau and their birds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 303–317. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Borges-Martins, M.; Zilio, F. Bird diversity in an urban ecosystem: The role of local habitats in understanding the effects of urbanization. Iheringia Ser. Zool. 2018, 108, e2018017. [Google Scholar] [CrossRef]
- Whited, D.; Galatowitsch, S.; Tester, J.R.; Schik, K.; Lehtinen, R.; Husveth, J. The importance of local and regional factors in predicting effective conservation - Planning strategies for wetland bird communities in agricultural and urban landscapes. Landsc. Urban Plan. 2000, 49, 49–65. [Google Scholar] [CrossRef]
- Paz Silva, C.; Garcia, C.E.; Estay, S.A.; Barbosa, O. Bird Richness and Abundance in Response to Urban Form in a Latin American City: Valdivia, Chile as a Case Study. PLoS ONE 2015, 10, e0138120. [Google Scholar]
- Albanese, G.; Davis, C.A. Characteristics within and around stopover wetlands used by migratory shorebirds: Is the neighborhood important? Condor 2015, 117, 328–340. [Google Scholar] [CrossRef]
- Shwartz, A.; Shirley, S.; Kark, S. How do habitat variability and management regime shape the spatial heterogeneity of birds within a large Mediterranean urban park? Landsc. Urban Plan. 2008, 84, 219–229. [Google Scholar] [CrossRef]
- Menon, M.; Devi, P.M.; Rangaswamy, M. Avifaunal Richness and Abundance along an Urban Rural Gradient with Emphasis on Vegetative and Anthropogenic Attributes in Tiruchirappalli, India. Landsc. Res. 2016, 41, 131–148. [Google Scholar] [CrossRef]
- Chong, K.Y.; Teo, S.; Kurukulasuriya, B.; Chung, Y.F.; Rajathurai, S.; Tan, H.T.W. Not all green is as good: Different effects of the natural and cultivated components of urban vegetation on bird and butterfly diversity. Biol. Conserv. 2014, 171, 299–309. [Google Scholar] [CrossRef]
- Jost, L.; Chao, A.; Chazdon, R.L. Compositional similarity and β (beta) diversity. Biol. Divers. Front. Meas. Assess. 2011, 66–84. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community ecology package. R package version 1.17-4. R-Proj. Org Acess. Em 2010, 23, 2010. [Google Scholar]
- Agisoft Agisoft PhotoScan Unser Manual: Professional Edition, Version 1.2. 2016. Available online: https://www.agisoft.com/pdf/photoscan-pro_1_2_en.pdf (accessed on 28 April 2019).
- Trimble ECognition Developer 8.7 Reference Book. 2011. Available online: http://www.ecognition.com/ (accessed on 28 April 2019).
- ESRI ArcGIS Desktop: Release 10. Environmental Systems Research Institute: Redlands, CA, USA, 2011.
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; For. Serv. Gen. Tech. Rep. PNW; USDA, Forest Service, Pacific Northwest Research Station: Washington, DC, USA, 1995; Volume 351. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 28 April 2019).
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002; pp. 75,131,323,324. ISBN 978-1-280-00948-8. [Google Scholar]
- Bickford, D.; Ng, T.H.; Qie, L.; Kudavidanage, E.P.; Bradshaw, C.J. Forest fragment and breeding habitat characteristics explain frog diversity and abundance in Singapore. Biotropica 2010, 42, 119–125. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Hinz, R.; Cunningham, V.J. On the Undecidability among Kinetic Models: From Model Selection to Model Averaging. J. Cereb. Blood Flow Metab. 2003, 23, 490–498. [Google Scholar] [CrossRef]
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Bartoń, K. Multi-Model Inference. R Package, Version 1.43.6. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 28 April 2019).
- Mazerolle, M.J. Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package, Version 2.2-1. Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 28 April 2019).
- Tylianakis, J.M.; Klein, A.-M.; Tscharntke, T. Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 2005, 86, 3296–3302. [Google Scholar] [CrossRef]
- Flohre, A.; Fischer, C.; Aavik, T.; Bengtsson, J.; Berendse, F.; Bommarco, R.; Ceryngier, P.; Clement, L.W.; Dennis, C.; Eggers, S.; et al. Agricultural intensification and biodiversity partitioning in European landscapes comparing plants, carabids, and birds. Ecol. Appl. 2011, 21, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Paz Silva, C.; Sepulveda, R.D.; Barbosa, O. Nonrandom filtering effect on birds: Species and guilds response to urbanization. Ecol. Evol. 2016, 6, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. Communities and Habitat Selection of Wetland Birds in the Lakeshore-Wetland Around the Dian Lake. Master’s Thesis, Yunnan University, Kunming, China, May 2014. [Google Scholar]
- Niemuth, N.D.; Estey, M.E.; Reynolds, R.E.; Loesch, C.R.; Meeks, W.A. Use of wetlands by spring-migrant shorebirds in agricultural landscapes of North Dakota’s Drift Prairie. Wetlands 2006, 26, 30–39. [Google Scholar] [CrossRef]
- Buler, J.J.; Moore, F.R.; Woltmann, S. A multi-scale examination of stopover habitat use by birds. Ecology 2007, 88, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Sushinsky, J.R.; Rhodes, J.R.; Possingham, H.P.; Gill, T.K.; Fuller, R.A. How should we grow cities to minimize their biodiversity impacts? Glob. Chang. Biol. 2013, 19, 401–410. [Google Scholar] [CrossRef] [PubMed]
| (Int) | Pagri | Pbuil | EDemer | Ppond | Psubm | Pfloa | AICc | ΔAICc | Weight |
|---|---|---|---|---|---|---|---|---|---|
| 28.20 | 0.217 | −0.192 | 0.071 | 218.6 | 0.00 | 0.290 | |||
| 30.58 | −0.265 | 0.463 | 2.570 | 219.5 | 0.89 | 0.186 | |||
| 30.43 | 0.212 | −0.266 | 0.424 | 220.0 | 1.48 | 0.138 | |||
| 29.63 | −0.206 | 0.633 | 2.041 | 220.1 | 1.51 | 0.136 | |||
| 31.13 | −0.229 | 0.070 | 220.1 | 1.54 | 0.134 | ||||
| 35.26 | −0.286 | 2.967 | −1.341 | 220.4 | 1.83 | 0.116 |
| (Int) | Pbare | Ppond | Psubm | EDemer | Pbuil | AICc | ΔAICc | Weight |
|---|---|---|---|---|---|---|---|---|
| 6.52 | 0.792 | 0.178 | 1.658 | 176.9 | 0.00 | 0.204 | ||
| 7.73 | 0.749 | 1.572 | 177.0 | 0.10 | 0.193 | |||
| 6.94 | 0.654 | 1.358 | 0.022 | 177.5 | 0.64 | 0.148 | ||
| 7.63 | 1.192 | 0.027 | 178.1 | 1.27 | 0.108 | |||
| 10.15 | 1.172 | −0.064 | 178.4 | 1.55 | 0.094 | |||
| 8.87 | 0.616 | 1.368 | −0.044 | 178.5 | 1.57 | 0.093 | ||
| 8.75 | 1.433 | 178.7 | 1.79 | 0.083 | ||||
| 9.11 | 0.167 | 1.238 | −0.066 | 178.8 | 1.93 | 0.078 |
| Parameter | Estimate | Unconditional SE | Confidence Interval | Relative Importance | Pr(>|z|) |
|---|---|---|---|---|---|
| (Intercept) | 30.357 | <0.001 | |||
| Pbuil | −0.234 | 0.085 | (−0.400, −0.068) | 1.00 | 0.008 |
| EDemer | 0.069 | 0.031 | (0.007, 0.130) | 0.56 | 0.036 |
| Psubm | 2.511 | 1.302 | (−0.042, 5.063) | 0.44 | 0.065 |
| Pagri | 0.215 | 0.108 | (0.004, 0.426) | 0.43 | 0.056 |
| Ppond | 0.446 | 0.216 | (0.023, 0.870) | 0.32 | 0.048 |
| Pfloa | −1.341 | 0.696 | (−2.704, 0.022) | 0.12 | 0.066 |
| Parameter | Estimate | Unconditional SE | Confidence Interval | Relative Importance | Pr(>|z|) |
|---|---|---|---|---|---|
| (Intercept) | 7.878 | <0.001 | |||
| Psubm | 0.722 | 0.656 | (0.138, 2.708) | 1.00 | 0.037 |
| Pbare | 0.175 | 0.371 | (−0.005, 1.448) | 0.64 | 0.063 |
| Ppond | 1.423 | 0.109 | (−0.039, 0.389) | 0.28 | 0.126 |
| Pbuil | 0.024 | 0.040 | (−0.137, 0.021) | 0.26 | 0.168 |
| EDemer | −0.058 | 0.016 | (−0.006, 0.055) | 0.26 | 0.137 |
| Statistic Value | Pbuil | Psubm | EDemer | Ppond |
|---|---|---|---|---|
| F-value | 19.337 | 3.189 | 3.450 | 3.780 |
| p-value | <0.001 | 0.056 | 0.046 | 0.035 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Q.; Liao, C.; Wu, Z.; Guan, W.; Yang, W.; Tang, Y.; Wu, G. Effects of Land Cover Pattern Along Urban-Rural Gradient on Bird Diversity in Wetlands. Diversity 2019, 11, 86. https://doi.org/10.3390/d11060086
Mao Q, Liao C, Wu Z, Guan W, Yang W, Tang Y, Wu G. Effects of Land Cover Pattern Along Urban-Rural Gradient on Bird Diversity in Wetlands. Diversity. 2019; 11(6):86. https://doi.org/10.3390/d11060086
Chicago/Turabian StyleMao, Qian, Chencan Liao, Zhaolu Wu, Wenbo Guan, Wenda Yang, Yuqin Tang, and Gang Wu. 2019. "Effects of Land Cover Pattern Along Urban-Rural Gradient on Bird Diversity in Wetlands" Diversity 11, no. 6: 86. https://doi.org/10.3390/d11060086
APA StyleMao, Q., Liao, C., Wu, Z., Guan, W., Yang, W., Tang, Y., & Wu, G. (2019). Effects of Land Cover Pattern Along Urban-Rural Gradient on Bird Diversity in Wetlands. Diversity, 11(6), 86. https://doi.org/10.3390/d11060086





