N1-{4-[2-(Methylthio)-1H-imidazol-5-yl]pyridin-2-yl}benzene-1,4-diamine
Abstract
:1. Introduction
2. Results and Discussion
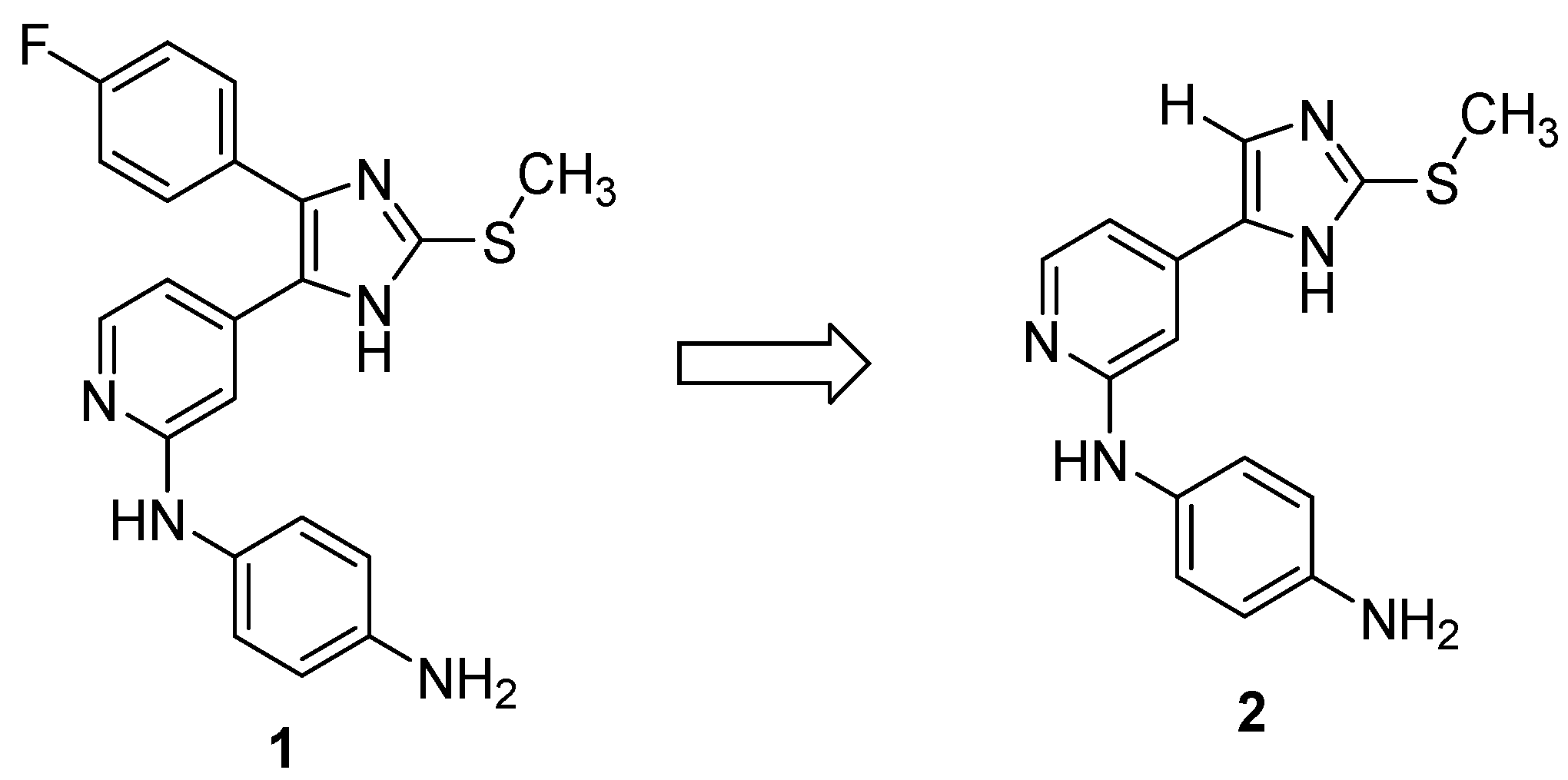
2.1. Chemistry
2.2. Biological Activity
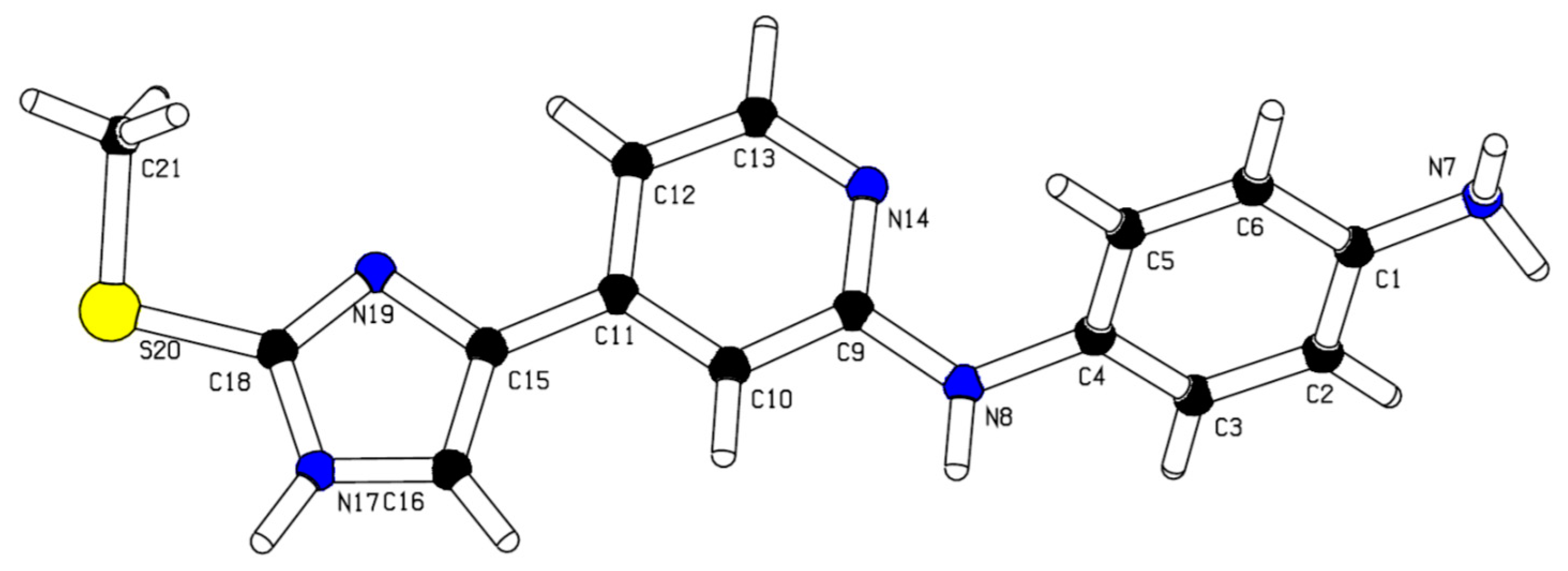
2.3. X-Ray Structure
3. Materials and Methods
3.1. General
3.2. Chemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koch, P.; Ansideri, F. 2-Alkylsulfanyl-4(5)-aryl-5(4)-heteroarylimidazoles: An Overview on Synthetic Strategies and Biological Activity. Arch. Pharm. 2017, 350, e1700258. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Bäuerlein, C.; Jank, H.; Laufer, S. Targeting the ribose and phosphate binding site of p38 mitogen-activated protein (MAP) kinase: Synthesis and biological testing of 2-alkylsulfanyl-, 4(5)-aryl-, 5(4)-heteroaryl-substituted imidazoles. J. Med. Chem. 2008, 51, 5630–5640. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Hauser, D.; Stegmiller, T.; Bracht, C.; Ruff, K.; Schattel, V.; Albrecht, W.; Koch, P. Tri- and tetrasubstituted imidazoles as p38alpha mitogen-activated protein kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6671–6675. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Koch, P. Towards the improvement of the synthesis of novel 4(5)-aryl-5(4)-heteroaryl-2-thio-substituted imidazoles and their p38 MAP kinase inhibitory activity. Org. Biomol. Chem. 2008, 6, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.A.; Hauser, D.R.J.; Domeyer, D.M.; Kinkel, K.; Liedtke, A.J. Design, synthesis, and biological evaluation of novel tri- and tetrasubstituted imidazoles as highly potent and specific ATP-mimetic inhibitors of p38 MAP kinase: Focus on optimized interactions with the enzyme’s surface-exposed front region. J. Med. Chem. 2008, 51, 4122–4149. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.; Hauser, D.R.J.; Unger, A.; Albrecht, W.; Laufer, S.A. 2-Acylaminopyridin-4-ylimidazoles as p38 MAP Kinase Inhibitors: Design, Synthesis, and Biological and Metabolic Evaluations. Chemmedchem 2009, 4, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Juchum, M.; Kelter, G.; Fiebig, H.; Laufer, S. Lung Cancer: EGFR Inhibitors with Low Nanomolar Activity against a Therapy-Resistant L858R/T790M/C797S Mutant. Angew. Chem. Int. Ed. 2016, 55, 10890–10894. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Lategahn, J.; Juchum, M.; Döring, E.; Keul, M.; Engel, J.; Tumbrink, H.L.; Rauh, D.; Laufer, S. Trisubstituted Pyridinylimidazoles as Potent Inhibitors of the Clinically Resistant L858R/T790M/C797S EGFR Mutant: Targeting of Both Hydrophobic Regions and the Phosphate Binding Site. J. Med. Chem. 2017, 60, 5613–5637. [Google Scholar] [CrossRef] [PubMed]
- Halekotte, J.; Witt, L.; Ianes, C.; Krüger, M.; Bührmann, M.; Rauh, D.; Pichlo, C.; Brunstein, E.; Luxenburger, A.; Baumann, U.; Knippschild, U.; Bischof, J.; Peifer, C. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1delta and Their Structural Relation to p38alpha MAPK. Molecules 2017, 22, 522. [Google Scholar] [CrossRef] [PubMed]
- Muth, F.; El-Gokha, A.; Ansideri, F.; Eitel, M.; Döring, E.; Sievers-Engler, A.; Lange, A.; Boeckler, F.M.; Lämmerhofer, M.; Koch, P.; Laufer, S.A. Tri- and Tetrasubstituted Pyridinylimidazoles as Covalent Inhibitors of c-Jun N-Terminal Kinase 3. J. Med. Chem. 2017, 60, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Ansideri, F.; Macedo, J.T.; Eitel, M.; El-Gokha, A.; Zinad, D.S.; Scarpellini, C.; Kudolo, M.; Schollmeyer, D.; Boeckler, F.M.; Blaum, B.S.; Laufer, S.A.; Koch, P. Structural optimization of a pyridinylimidazole scaffold: shifting the selectivity from p38α mitogen-activated protein kinase to c-Jun N-terminal kinase 3. ACS Omega 2018, 3, 7809–7831. [Google Scholar] [CrossRef] [PubMed]
- Ansideri, F.; Dammann, M.; Boeckler, F.M.; Koch, P. Fluorescence polarization-based competition binding assay for c-Jun N-terminal kinases 1 and 2. Anal. Biochem. 2017, 532, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Ansideri, F.; Lange, A.; El-Gokha, A.; Boeckler, F.M.; Koch, P. Fluorescence polarization-based assays for detecting compounds binding to inactive c-Jun N-terminal kinase 3 and p38 alpha mitogen-activated protein kinase. Anal. Biochem. 2016, 503, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Goettert, M.; Graeser, R.; Laufer, S.A. Optimization of a nonradioactive immunosorbent assay for p38alpha mitogen-activated protein kinase activity. Anal. Biochem. 2010, 406, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Goettert, M.; Luik, S.; Graeser, R.; Laufer, S.A. A direct ELISA assay for quantitative determination of the inhibitory potency of small molecules inhibitors for JNK3. J. Pharma. Biomed. Anal. 2011, 55, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Peifer, C.; Luik, S.; Thuma, S.; Herwey, Y.; Graeser, R.; Laufer, S.A. Development and optimization of a non-radioactive JNK3 assay. Comb. Chem. High Throughput Screen. 2006, 9, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Thuma, S.; Peifer, C.; Greim, C.; Herweh, Y.; Albrecht, A.; Dehner, F. An Immunosorbent, Nonradioactive p38 Map Kinase Assay Comparable to Standard Radioactive Liquid-Phase Assays. Anal. Biochem. 2005, 344, 135–137. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (nM) a | |
|---|---|---|
| p38α MAP kinase b | JNK3 c | |
| 1 | 17 | 24 |
| 2 | 3679 | 410 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gokha, A.; Ansideri, F.; Andreev, S.; Schollmeyer, D.; Laufer, S.; Koch, P. N1-{4-[2-(Methylthio)-1H-imidazol-5-yl]pyridin-2-yl}benzene-1,4-diamine. Molbank 2019, 2019, M1048. https://doi.org/10.3390/M1048
El-Gokha A, Ansideri F, Andreev S, Schollmeyer D, Laufer S, Koch P. N1-{4-[2-(Methylthio)-1H-imidazol-5-yl]pyridin-2-yl}benzene-1,4-diamine. Molbank. 2019; 2019(1):M1048. https://doi.org/10.3390/M1048
Chicago/Turabian StyleEl-Gokha, Ahmed, Francesco Ansideri, Stanislav Andreev, Dieter Schollmeyer, Stefan Laufer, and Pierre Koch. 2019. "N1-{4-[2-(Methylthio)-1H-imidazol-5-yl]pyridin-2-yl}benzene-1,4-diamine" Molbank 2019, no. 1: M1048. https://doi.org/10.3390/M1048





