(E)-1-(2-Aminophenyl)-3-(4-chlorophenyl)prop-2-en-1-one
Abstract
:1. Introduction
2. Results and Discussion
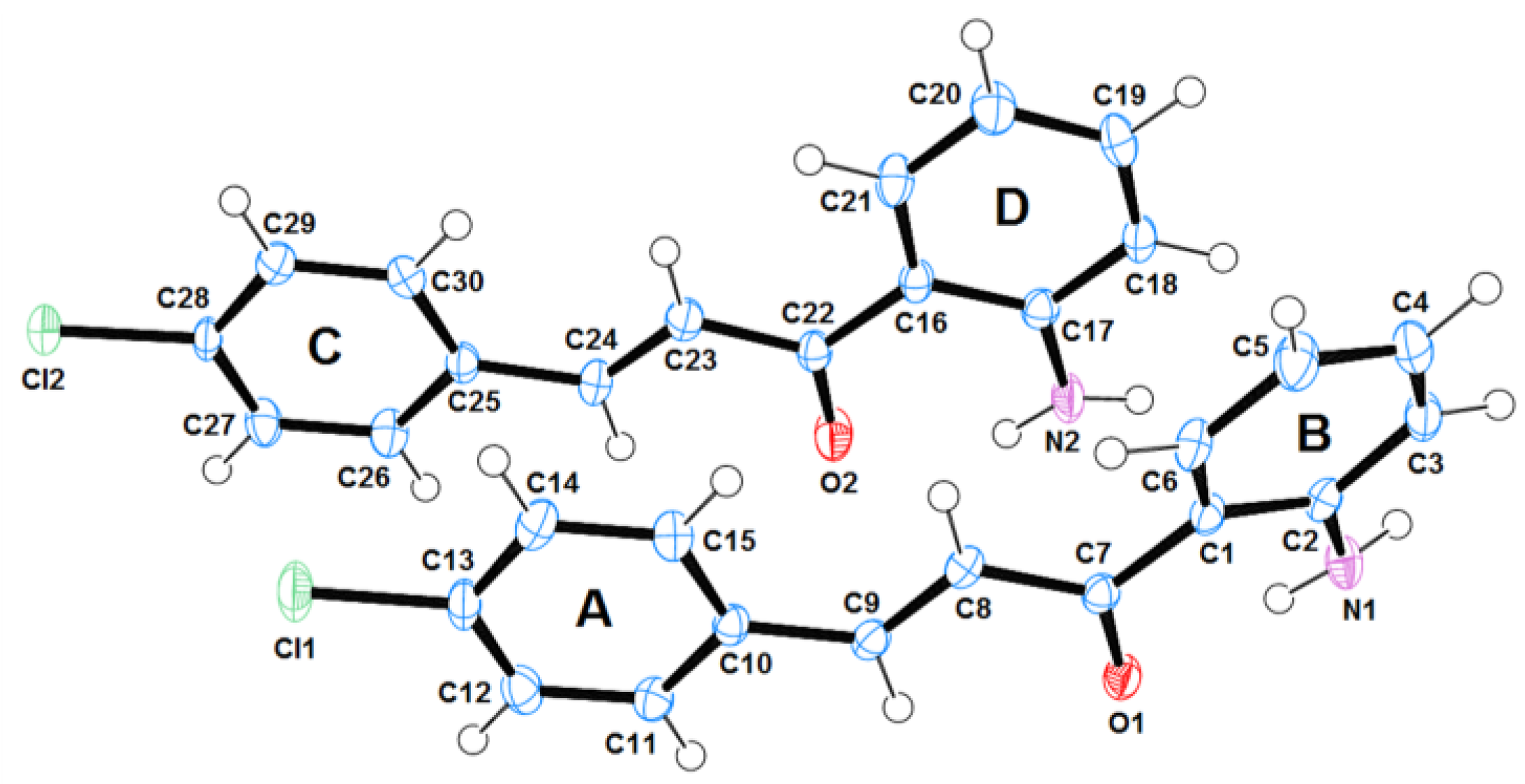
2.1. Structural Analysis
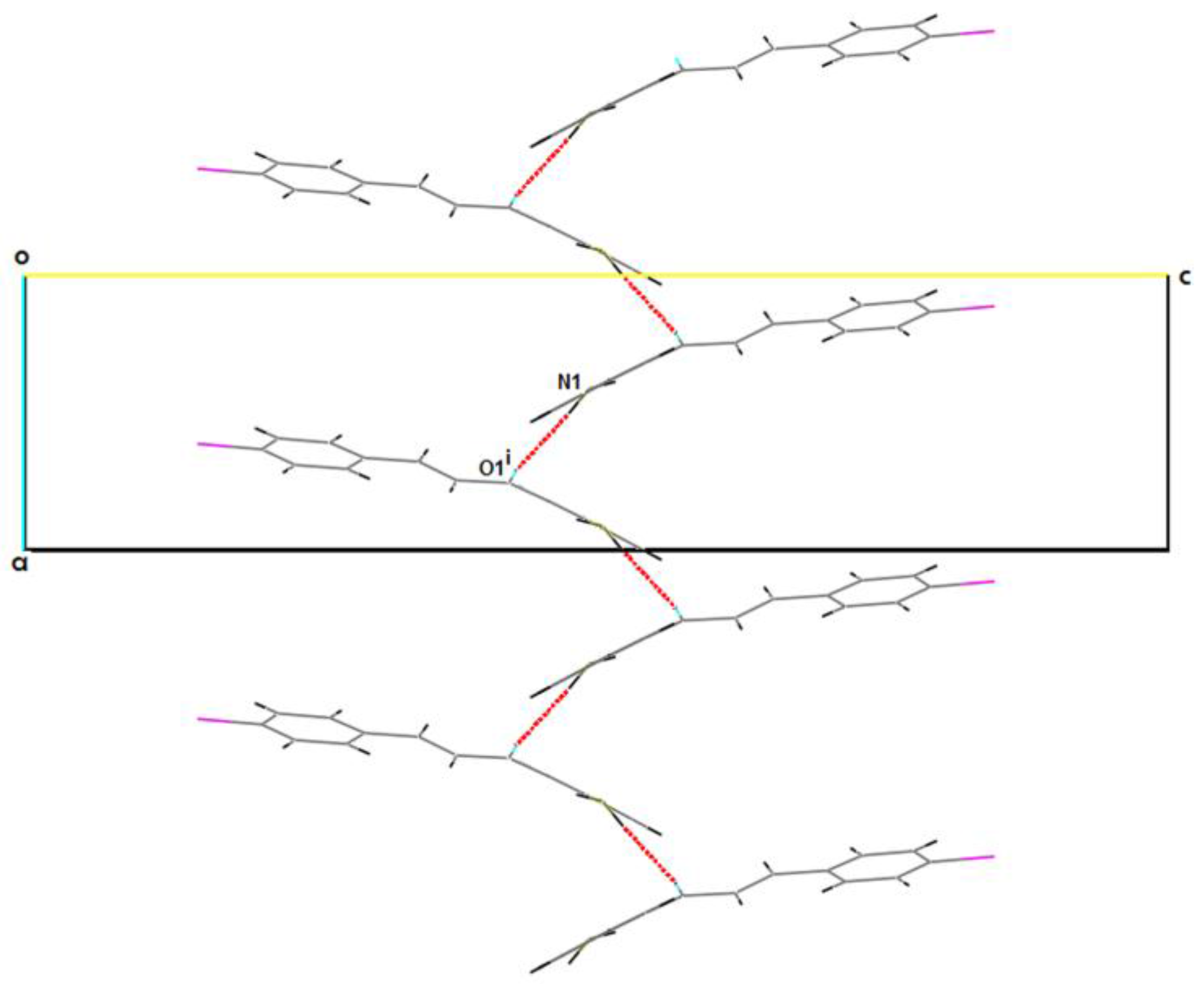
2.2. Supramolecular Features
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (E)-1-(2-Aminophenyl)-3-(4-chlorophenyl)prop-2-en-1-one (3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Lin, Y.M.; Zhou, Y.; Flavin, M.T.; Zhou, I.M.; Nie, W.; Chen, F.C. Chalcones and flavonoids as anti-tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. [Google Scholar] [CrossRef]
- Won, S.-J.; Liu, C.-T.; Tsao, L.-T.; Weng, J.-R.; Ko, H.-H.; Wang, J.-P.; Lin, C.-N. Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. Eur. J. Med. Chem. 2005, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wilairat, P.; Go, M.-L. Antimalarial alkoxylated and hydroxylated chalones: Structure-sctivity relationship analysis. J. Med. Chem. 2001, 44, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Ávila, H.; de Albino-Smânia, E.F.; Delle-Monache, F.; Smânia-Júnior, A. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Muthu-Kumar, T.; Doble, M. Antifungal activity, mechanism and QSAR studies on chalcones. Chem. Biol. Drug Des. 2009, 74, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
- Elarfi, M.J.; Al-Difar, H.A. Synthesis of some heterocyclic compounds derived from chalcones. Sci. Revs. Chem. Commun. 2012, 2, 103–107. [Google Scholar]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar]
- Abonia, R.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Meier, H.; Lotero, E. An Amberlyst-15® mediated synthesis of new functionalized dioxoloquinolinone derivatives. Open Org. Chem. J. 2008, 2, 26–34. [Google Scholar] [CrossRef]
- Abonia, R.; Cuervo, P.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. A simple two-step sequence for the synthesis of novel 3-aryl[1,3]dioxolobenzo[f]pyrrolo[1,2-a]azepin-11-ones from 6-amino-3,4-methylenedioxyacetophenone. Eur. J. Org. Chem. 2008, 4684–4689. [Google Scholar] [CrossRef]
- Lee, J.I.; Youn, J.S. A novel synthesis of 2-aryl-4-quinolones from 2-aminobenzoic acids. Bull. Korean Chem. Soc. 2008, 29, 1853–1856. [Google Scholar]
- This compound is commercially available from ChemSRC (China) under the CAS number 78396–06–2.
- Cuenú, F.; Abonía, R.; Cobo, J.; Glidewel, C. 1-(2-Aminophenyl)-3-(4-pyridyl)prop-2-en-1-one: A three-dimensional framework structure built from N—H_ _ _N, C—H_ _ _O and C—H_ _ _p(arene) hydrogen bonds. Acta Cryst. 2010, C66, o589–o592. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]


| Bond Lengths | |
|---|---|
| Cl(1)-C(13); 1.7391(18) Cl(2)-C(28); 1.7401(18) C(7)-C(8); 1.482(2) C(8)-C(9); 1.332(3) C(9)-C(10); 1.468(2) C(7)-O(1); 1.238(2) C(1)-C(7); 1.472(3) | C(2)-N(1); 1.372(3) C(16)-C(22); 1.467(3) C(22)-C(23); 1.483(3) C(23)-C(24); 1.325(3) C(24)-C(25); 1.468(2) C(17)-N(2); 1.353(3) |
| Angles | |
| C(7)-C(8)-C(9); 120.15(18) | C(22)-C(23)-C(24); 120.65(19) |
| Torsion angles | |
| C(2)-C(1)-C(7)-O(1); 22.9(3) | C(17)-C(16)-C(22)-O(2); 0.8(3) |
| C(8)-C(7)-C(1)-C(2); -154.66(17) | C(23)-C(22)-C(16)-C(17); -178.05(17) |
| C(10)-C(9)-C(8)-C(7); -177.55(17) | C(25)-C(24)-C(23)-C(22); -179.20(19) |
| D–H...A | D–H | H...A | D...A | D–H...A |
|---|---|---|---|---|
| N1–H2N...O1i | 0.89(3) | 2.09(4) | 2.982(3) | 176(3) |
| N2–H4N...N1i | 0.83(3) | 2.48(3) | 3.269(3) | 158(2) |
| C14–H14...O2ii | 0.95 | 2.40 | 3.293(3) | 157.3 |
| C29–H29...O1ii | 0.95 | 2.53 | 3.400(3) | 152.7 |
| N2–H3N...O2 | 0.89(3) | 1.95(3) | 2.633(3) | 133(3) |
| N1–H1N...O1 | 0.88(3) | 2.08(3) | 2.699(3) | 127(3) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abonia, R.; Cabrera, L.; Quiroga, J.; Insuasty, B.; Moreno-Fuquen, R.; Kennedy, A.R. (E)-1-(2-Aminophenyl)-3-(4-chlorophenyl)prop-2-en-1-one. Molbank 2016, 2016, M911. https://doi.org/10.3390/M911
Abonia R, Cabrera L, Quiroga J, Insuasty B, Moreno-Fuquen R, Kennedy AR. (E)-1-(2-Aminophenyl)-3-(4-chlorophenyl)prop-2-en-1-one. Molbank. 2016; 2016(4):M911. https://doi.org/10.3390/M911
Chicago/Turabian StyleAbonia, Rodrigo, Lorena Cabrera, Jairo Quiroga, Braulio Insuasty, Rodolfo Moreno-Fuquen, and Alan R. Kennedy. 2016. "(E)-1-(2-Aminophenyl)-3-(4-chlorophenyl)prop-2-en-1-one" Molbank 2016, no. 4: M911. https://doi.org/10.3390/M911








