N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine
Abstract
:1. Introduction
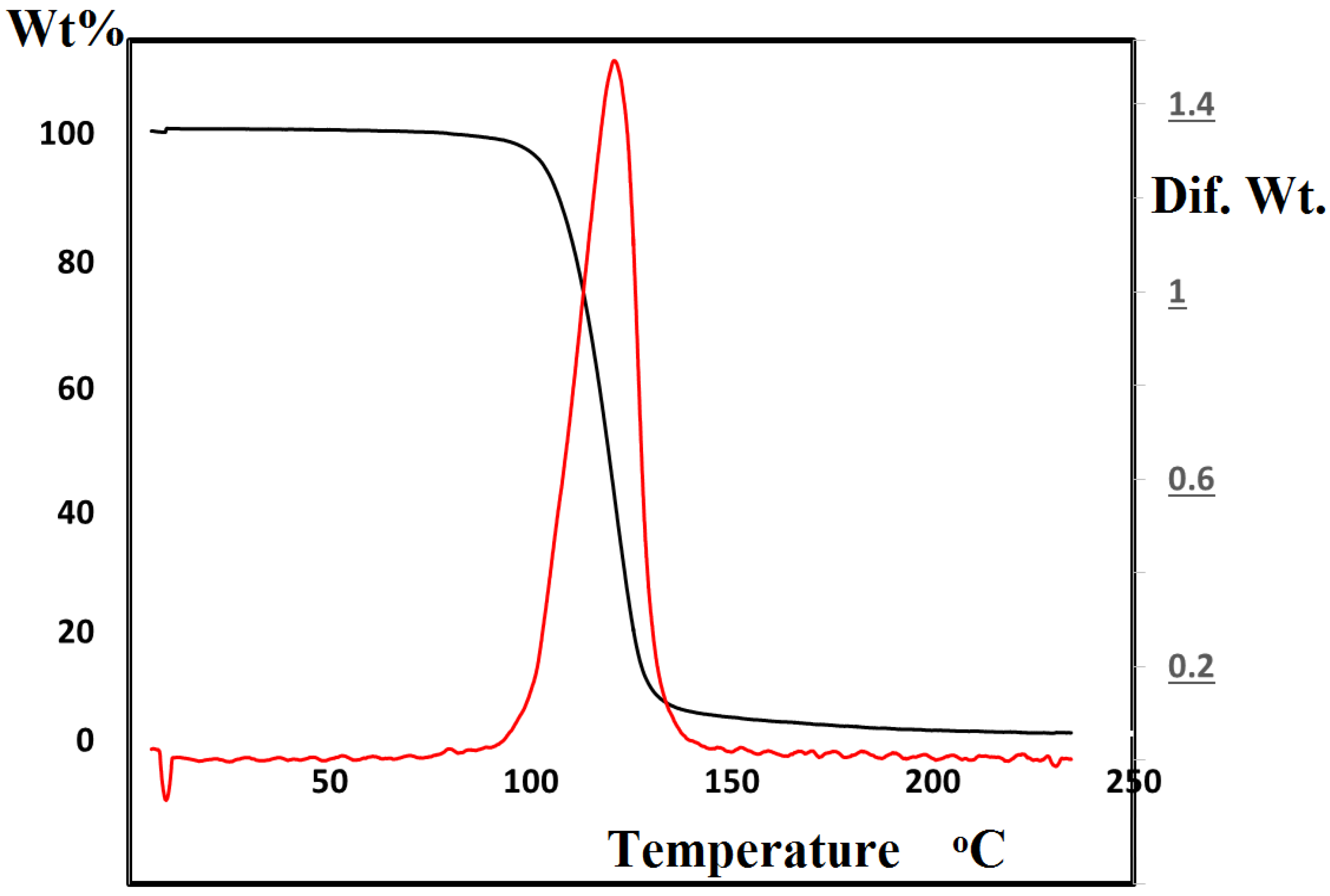
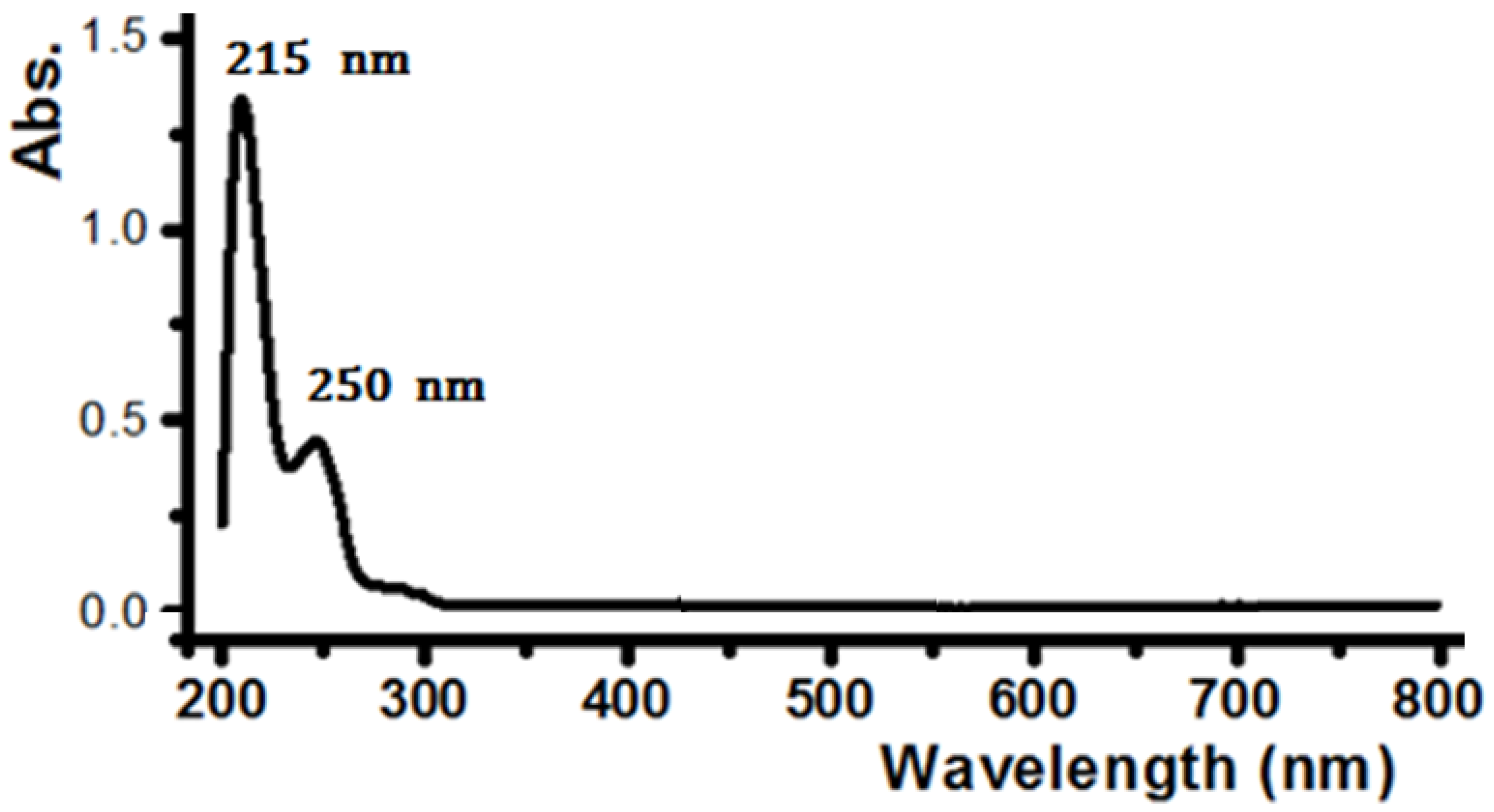
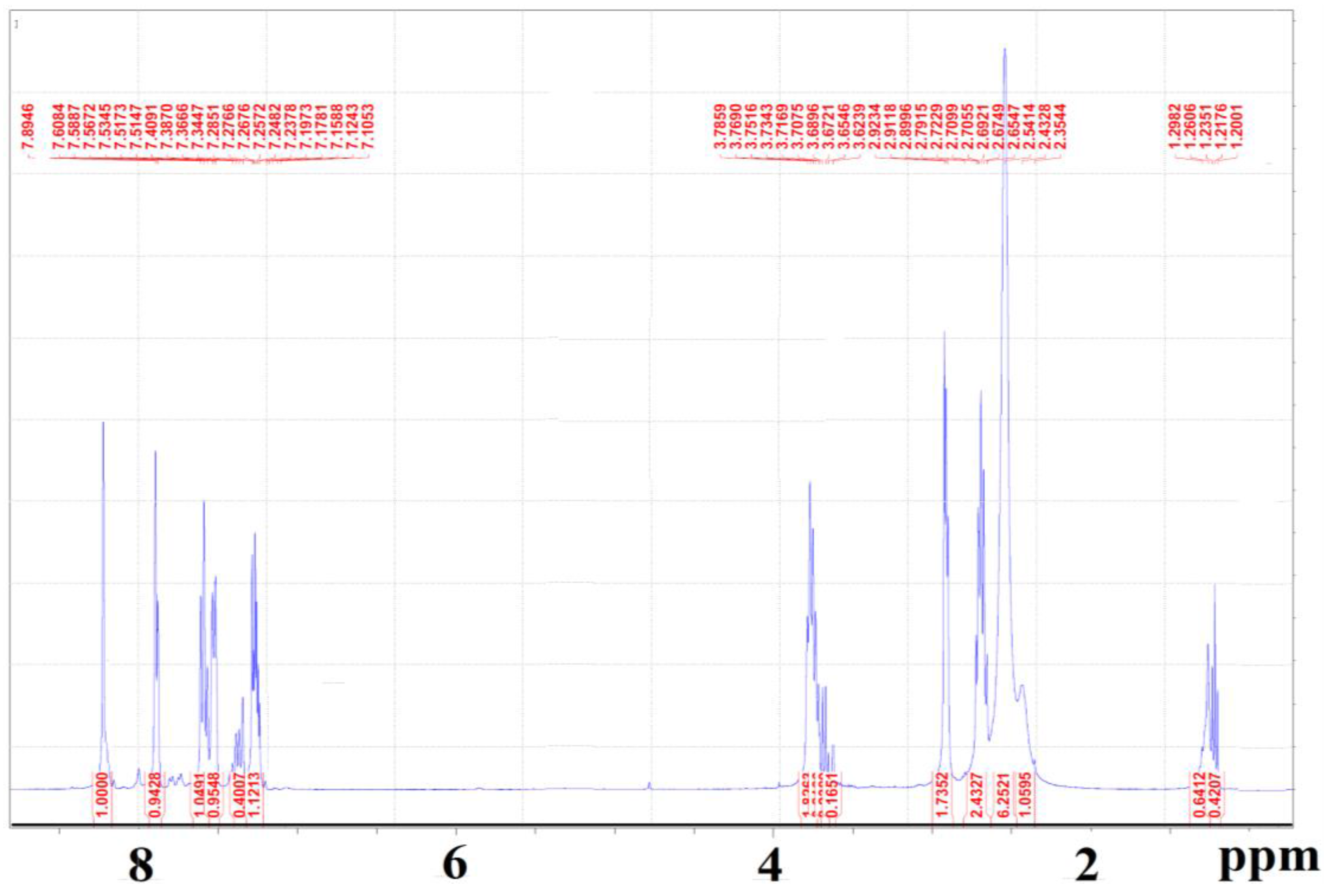
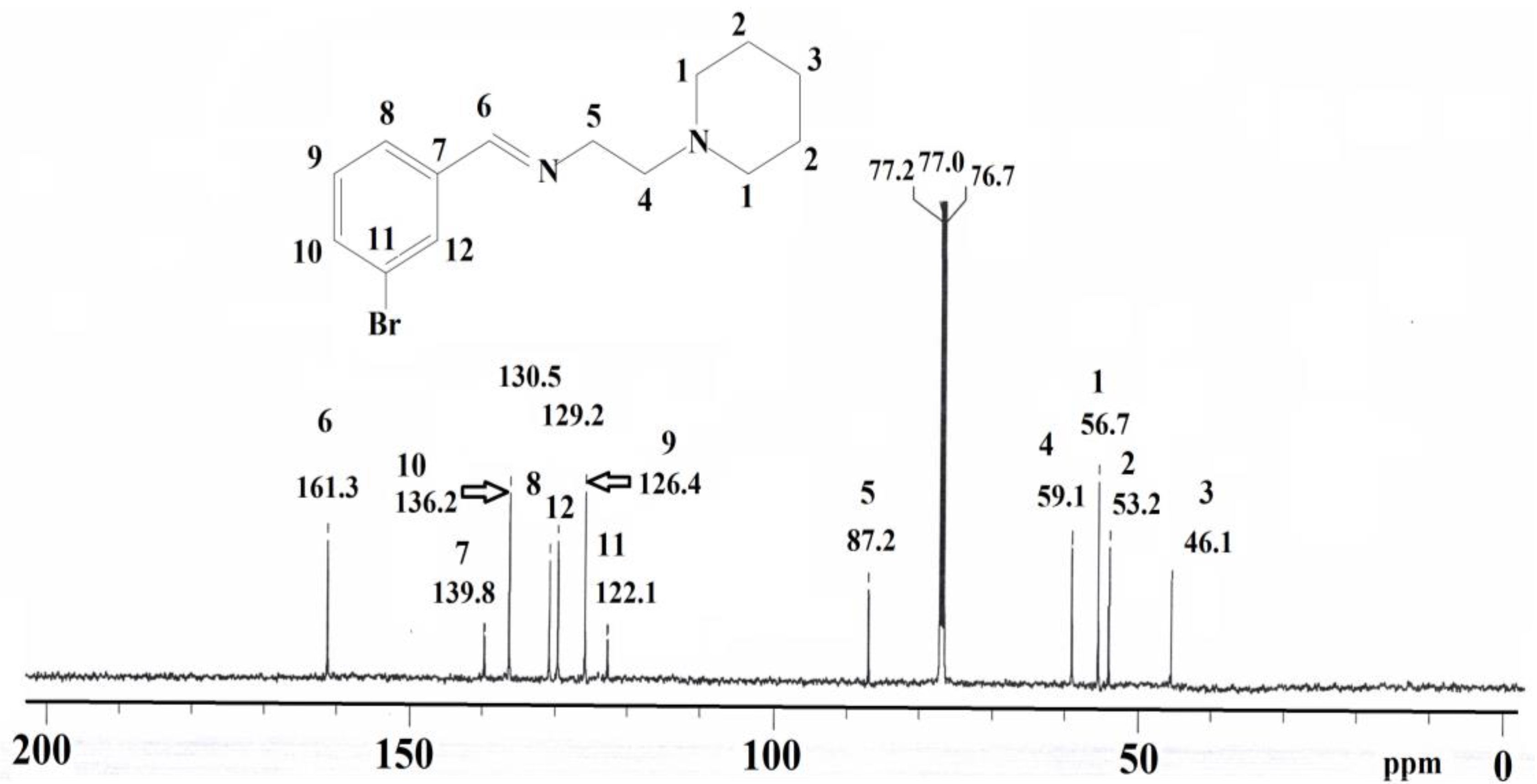
2. Results and Discussion
3. Experimental Section
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ispir, E. The synthesis, characterization, electrochemical character, catalytic and antimicrobial activity of novel azo-containing Schiff bases and their metal complexes. Dyes Pigm. 2009, 82, 13–19. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Kumar, A.; Upadhyay, S.; Mishra, P.C. Synthesis, characterization, structural optimization using density functional theory and superoxide ion scavenging activity of some Schiff bases. J. Mol. Struct. 2008, 873, 5–16. [Google Scholar] [CrossRef]
- Ceylan, G.; Keose, M.; Tümer, M.; Demirtas, I.; Yaglioglu, A.S.; McKee, V. Structural characterization of some Schiff bases compounds: Investigation of their electrochemical, photoluminescence, thermal and anticancer activity properties. J. Lumin. 2013, 143, 623–634. [Google Scholar]
- Asiri, A.M.; Khan, S.A.; Marwani, H.M.; Sharma, K. Synthesis, spectroscopic and physicochemical investigations of environmentally benign heterocyclic Schiff bases derivatives as antibacterial agents on the bases of in vitro and density functional theory. J. Photochem. Photobiol. B Biol. 2013, 120, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Akhter, Z.; Ahmad, I.; Ahmed, S.; Ismail, S.; Mirza, B.; McKee, V.; Bolte, M. Synthesis, characterization, biological and electrochemical evaluation of novel ether based ON donor bidentate Schiff bases. J. Mol. Strut. 2016, 1116, 84–92. [Google Scholar] [CrossRef]
- Resayes, S.; Warad, I.; Choudhary, M.; Wahab, A.; Rasheed, S. Heterocyclic Schiff’s Bases as Novel and New Antiglycation Agents. U.S. Patent US 2014/0221429A1, 7 August 2014. [Google Scholar]
- Abdoh, M.; Warad, I.; Naveen, S.; Lokanathd, N.; Salghi, R. Crystal structure of (1E,1’E)-N,N’-(ethane-1,2-diyl)bis[(pyridin-2-yl)-methanimine]. Acta Crystallogr. 2015, E71, o431–o435. [Google Scholar]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Haddad, S. Design and structural studies of diimine/CdX2 (X = Cl, I) complexes based on 2,2-dimethyl-1,3-diaminopropane ligand. J. Mol. Strut. 2014, 1062, 167–173. [Google Scholar] [CrossRef]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Khan, M.; Ahmad, P.; Al-Nuri, M.; Jodeh, S.; Husein, A.; Haddad, S.; et al. Structural studies on Cd(II) complexes incorporating di-2-pyridyl ligand and the X-ray crystal structure of the chloroform solvated DPMNPH/CdI2 complex. Inorg. Chem. Commum. 2014, 43, 155–161. [Google Scholar] [CrossRef]
- Azam, M.; Warad, I.; Al-Resayes, S.; Alzaqri, Z.; Khan, M.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Strut. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Warad, I.; Al-Noaimi, M.; Haddad, S.; Al-Demeri, Y.; Hammouti, B.; Ben Hadda, T. Rac-(E,E)-N,N’-Bis(2-chlorobenzylidene)-cyclohexane-1,2-diamine. Acta Crystallogr. 2013, E69, o1442–o1445. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warad, I.; Al-Demeri, Y.; Al-Nuri, M.; Suleiman, M.; Al-Ali, A.; Amereih, S. N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine. Molbank 2016, 2016, M903. https://doi.org/10.3390/M903
Warad I, Al-Demeri Y, Al-Nuri M, Suleiman M, Al-Ali A, Amereih S. N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine. Molbank. 2016; 2016(3):M903. https://doi.org/10.3390/M903
Chicago/Turabian StyleWarad, Ismail, Yasmin Al-Demeri, Mohammmed Al-Nuri, Mohammed Suleiman, Anas Al-Ali, and Sameer Amereih. 2016. "N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine" Molbank 2016, no. 3: M903. https://doi.org/10.3390/M903






