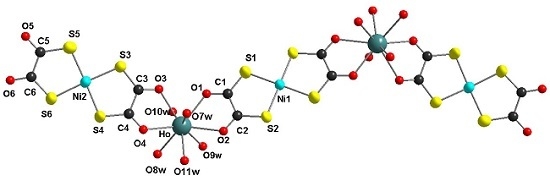
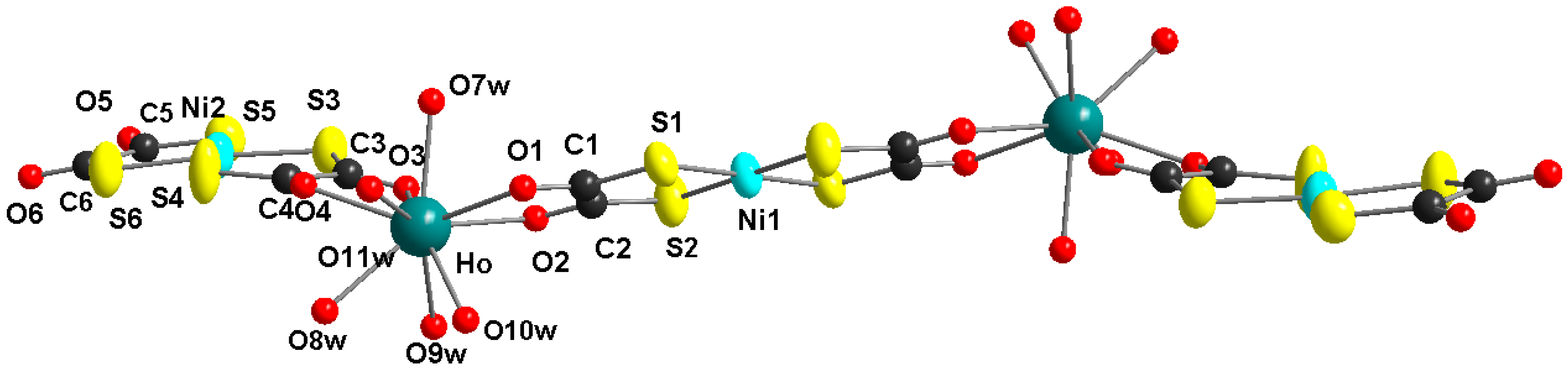
[µ2-O,O′,Oʺ,Oʺ′-Bis(1,2-dithiooxalato-S,S′)nickel(II)]bis[-O,O′-bis(1,2-dithiooxalato-S,S′)-nickel(II)pentaquaholmium(III)]hydrate, [Ho2Ni3(dto)6(H2O)10]
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Structures
2.2. IR Spectroscopy
3. Experimental Section
3.1. General Methods, Analytical and Physical Measurements
3.2. Synthesis of [Ho2Ni3 (dto)6(H2O)10]·10H2O
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| dto | 1,2-dithiooxalate |
| Ln | lanthanide |
References
- Sanz, J.L.; Ruiz, R.; Gleizes, A.; Lloret, F.; Faus, J.; Julve, M.; Borras-Almenar, J.J.; Journaux, Y. Crystal structure and magnetic properties of novel LnIIICuII (Ln = Gd, Dy, Ho) pentaanuclear complexes. Topology and ferromagnetic interaction in the LnIII–CuII pair. Inorg. Chem. 1996, 35, 7384–7393. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. A genuine example of a discrete bimetallic (Cu, Gd)–complex: structural determination and magnetic properties. Inorg. Chem. 1996, 35, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.-P. Experimental evidence of a ferromagnetic ground state (S=9/2) for a dinuclear Gd(III)–Ni(II) complex. Inorg. Chem. 1997, 36, 4284–4286. [Google Scholar] [CrossRef]
- Ramade, I.; Kahn, O.; Jeannnin, Y.; Robert, F. Design and magnetic properties of a magnetically isolated GdIIICuII pair. Crystal Structures of [Gd(hfa)3Cu(salen)], [Y(hfa)3Cu(salen)], [Gd(hfa)3Cu(salen)(Meim)], and [La(hfa)3H2OCu(salen)] [hfa = hexafluoroacetylacetonato, salen = N,N_-ethylenebis(salicylideneaminato), Meim = 1-Methylimidazole]. Inorg Chem. 1997, 36, 930–936. [Google Scholar] [CrossRef]
- Sanada, T.; Suzuki, T.; Kaizaki, S. Heterotrinuclear complexes containing d- and f-block elements: synthesis and structural characterization of novel lanthanide(III) nickel(II)-lanthanide(III) compounds bridged by oxamidate. J. Chem. Soc. Dalton Trans. 1998, 959–965. [Google Scholar] [CrossRef]
- Sanada, T.; Suzuki, T.; Yoshida, T.; Kaizaki, S. Heterodinuclear complexes containing d- and f-block elements: Synthesis, structural characterization, and metal–metal interactions of novel chromium(III)–lanthanide(III) compounds bridged by oxalate. Inorg. Chem. 1998, 37, 4712–4717. [Google Scholar] [CrossRef] [PubMed]
- Decurtins, S.; Gross, M.; Schmalle, H.W.; Ferlay, S. Molecular chromium(III)-lanthanide(III) compounds (Ln = La, Ce, Pr, and Nd) with a polymeric, ladder-lype architecture: A structural and magnetic study. Inorg. Chem. 1998, 37, 2443–2449. [Google Scholar] [CrossRef]
- Li, Y.-T.; Yan, C.-W.; Wang, H.D. Synthesis, characterization, and magnetic study of μ-oxamido-bridged copper(II)–lanthanide(III) heterobinuclear complexes. Polish J. Chem. 2000, 74, 1655–1663. [Google Scholar]
- Li, Y.-T.; Yan, C.-W.; Zhang, J. Synthesis, characterization, and magnetic properties of copper(II)-lanthanide(III) heterobinuclear complexes bridged by N,N-oxamidobis(3,5-dibromobenzoato)cuprate(II). Crystal Growth Design 2003, 3, 481–485. [Google Scholar] [CrossRef]
- Shiga, T.; Ohba, M.; Okawa, H. Structure and magnetism of a trinuclear CuII–GdIII–CuII complex derived from one-pot reaction with 2,6-di(acetoacetyl)pyridine. Inorg. Chem. Commun. 2003, 6, 15–18. [Google Scholar] [CrossRef]
- Kido, T.; Ikuta, Y.; Sunatsuki, Y.; Ogawa, Y.; Matsumoto, N.; Re, N. Nature of copper(II)–lanthanide(III) magnetic interactions and generation of a large magnetic moment with magnetic anisotropy of 3d–4f cyclic cylindrical tetranuclear complexes [CuIILLnIII(hfac)2]2, (H3L = 1-(2-Hydroxybenzamido)-2-(2-hydroxy-3-methoxybenzylideneamino)ethane and Hhfac = hexafluoroacetylacetone, LnIII = Eu, Gd, Tb, Dy). Inorg. Chem. 2003, 42, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Sorace, L.; Casellato, U.; Tomasin, P.; Vigato, P.A. d- or f-Mononuclear and related heterodinuclear complexes with [1+1] asymmetric compartmental macrocycles. Eur. J. Inorg. Chem. 2004, 2004, 3887–3900. [Google Scholar] [CrossRef]
- Koner, R.; Lee, G.-H.; Wang, Y.; Wei, H.-H.; Mohanta, S. Two new diphenoxo-bridged discrete dinuclear CuIIGdIII compounds with cyclic diimino moieties: Synthesis, structure, and magnetic properties. Eur. J. Inorg. Chem. 2005, 2005, 1500–1505. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, B.; Yu, A.; Song, H.-B.; Cheng, P.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P. Construction of 3d-4f mixed-metal complexes based on a binuclear oxovanadium unit: synthesis, crystal structure, EPR, and magnetic properties. Inorg. Chem. 2006, 45, 3949–3957. [Google Scholar] [CrossRef] [PubMed]
- Merca, A.; Müller, A.; Slageren, J.V.; Läge, M.; Krebs, B. Systematic study of the interaction between VIV centres and lanthanide ions MIII in well defined {VIV2MIII}{AsIIIW9O33}2 Sandwich Type Clusters: Part 1. J. Clust. Sci. 2007, 18, 711–719. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of lanthanides in molecular materials with transition-metal ions and organic radicals. Chem. Rev. 2002, 102, 2369–2387, and reverences therein. [Google Scholar] [CrossRef] [PubMed]
- Tanase, S.; Reedijk, J. Chemistry and magnetism of cyanido-bridged d-f assemblies. Coord. Chem. Rev. 2006, 250, 2501–2510, and references therein. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A tetranuclear 3d-4f single molecular magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2004, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Trombé, J.C.; Gleizes, A.; Galy, J. Synthese et etude cristallochimique de dithio-oxalates mixtes hydrates de nickel et d elements de terres rares (t.r. (III) = Y, La, Ce, Nd, Sm, Eu, Gd, Dy, Er, Yb). Structure cristalline de Ni3Eu2(O2C2S2)6(H2O)10 xH2O (10<x<12). R. Acad. Sc. Paris 1982, 294-II, 1369–1372. [Google Scholar]
- Trombé, J.C.; Gleizes, A.; Galy, J. Structural evolution within a series of hydrated bis(dithiooxalato)nickelate(II) of rare earth (RE)2(H2O)2nNi3(S2C2O2)6, xH2O. Inorg. Chim. Acta 1984, 87, 129–141. [Google Scholar] [CrossRef]
- Siebold, M.; Kelling, A.; Schilde, U.; Strauch, P. Heterobimetallische 3d-4f Komplexe mit Bis(1,2-dithiooxalato)nickelat(II) als planarem Brückenbaustein. Z. Naturforsch. 2005, 60b, 1149–1157. [Google Scholar]
- Siebold, M.; Eidner, S.; Kelling, A.; Kumke, M.U.; Schilde, U.; Strauch, P. Pentanuclear heterobimetallic 3d-4f complexes – structure and luminescence. Z. anorg. allg. Chem. 2006, 632, 1963–1965. [Google Scholar] [CrossRef]
- Siebold, M.; Korabik, M.; Schilde, U.; Mrozinski, J.; Strauch, P. Pentanuclear heterobimetallic 3d-4f complexes of Ln2M3-type – structure and magnetism. Chem. Papers 2008, 62, 487–495. [Google Scholar] [CrossRef]
- Siebold, M.; Strauch, P. Series of Pentanuclear Heterobimetallic 3d-4f-Complexes Revisited. In Advances in Coordination, Bioinorganic and Inorganic Chemistry; Melnik, M., Šima, J., Tatarko, M., Eds.; Slovak Technical University Press: Bratislava, Slovakia, 2005; pp. 399–410. [Google Scholar]
- Czernuszewicz, R.S.; Nakamoto, K.; Strommen, D.P. Resonance raman spectra and vibrational assignments of “red” and “black” forms of potassium bis(dithiooxalato) nickel(II). J. Am. Chem. Soc. 1982, 104, 1515–1521. [Google Scholar] [CrossRef]
- X-Area; vers. 1.26; Stoe & Cie GmbH: Darmstadt, Germany, 2004.
- Sheldrick, G.M. SHELXS-97; Program for Crystal Structure Solution; University Göttingen: Göttingen Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014/7; Program for the Refinement of Crystal Structures; University Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Matz, C.; Mattes, R. Darstellung und Struktur von Kalium-1,2-diselenooxalat. Z. Naturforsch. 1978, 88b, 461–462. [Google Scholar]
- Wenzel, B.; Wehse, B.; Schilde, U.; Strauch, P. 1,2-Dithioquadratato- und 1,2-Dithiooxalatoindate(III). Z. anorg. allg. Chem. 2004, 630, 1469–1476. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, J.; Kelling, A.; Schilde, U.; Strauch, P. [µ2-O,O′,Oʺ,Oʺ′-Bis(1,2-dithiooxalato-S,S′)nickel(II)]bis[-O,O′-bis(1,2-dithiooxalato-S,S′)-nickel(II)pentaquaholmium(III)]hydrate, [Ho2Ni3(dto)6(H2O)10]. Molbank 2016, 2016, M895. https://doi.org/10.3390/M895
König J, Kelling A, Schilde U, Strauch P. [µ2-O,O′,Oʺ,Oʺ′-Bis(1,2-dithiooxalato-S,S′)nickel(II)]bis[-O,O′-bis(1,2-dithiooxalato-S,S′)-nickel(II)pentaquaholmium(III)]hydrate, [Ho2Ni3(dto)6(H2O)10]. Molbank. 2016; 2016(2):M895. https://doi.org/10.3390/M895
Chicago/Turabian StyleKönig, Jana, Alexandra Kelling, Uwe Schilde, and Peter Strauch. 2016. "[µ2-O,O′,Oʺ,Oʺ′-Bis(1,2-dithiooxalato-S,S′)nickel(II)]bis[-O,O′-bis(1,2-dithiooxalato-S,S′)-nickel(II)pentaquaholmium(III)]hydrate, [Ho2Ni3(dto)6(H2O)10]" Molbank 2016, no. 2: M895. https://doi.org/10.3390/M895