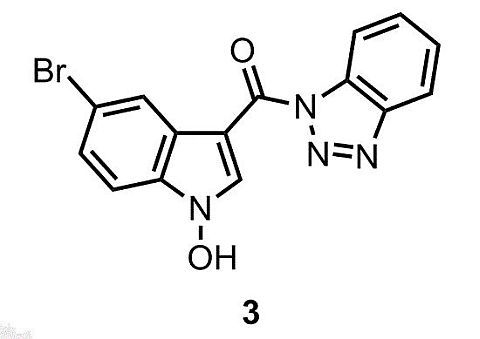
(1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone †
Abstract
:Experimental
1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inman, M.; Moody, C.J. Indole synthesis—something old, something new. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Palmisano, G.; Penoni, A.; Sisti, M.; Tibiletti, F.; Tollari, S.; Nicholas, K.M. Synthesis of indole derivatives with biological activity by reactions between unsaturated hydrocarbons and N-aromatic precursors. Curr. Org. Chem. 2010, 14, 2409–2441. [Google Scholar] [CrossRef]
- Krüger, K.; Tillack, A.; Beller, M. Catalytic synthesis of indoles from alkynes. Adv. Synth. Catal. 2008, 350, 2153–2167. [Google Scholar]
- Penoni, A.; Nicholas, K.M. A novel and direct synthesis of indoles via catalytic reductive annulation of nitroaromatics with alkynes. Chem. Commun. 2002, 484–485. [Google Scholar] [CrossRef]
- Penoni, A.; Volkman, J.; Nicholas, K.M. Regioselective synthesis of indoles via reductive annulation of nitrosoaromatics with alkynes. Org. Lett. 2002, 4, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Penoni, A.; Palmisano, G.; Zhao, Y.-L.; Houk, K.N.; Volkman, J.; Nicholas, K.M. On the mechanism of nitrosoarene-alkyne cycloaddition. J. Am. Chem. Soc. 2009, 131, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Penoni, A.; Palmisano, G.; Broggini, G.; Kadowaki, A.; Nicholas, K.M. Efficient synthesis of N-methoxyindoles via alkylative cycloaddition of nitrosoarenes with alkynes. J. Org. Chem. 2006, 71, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Ieronimo, G.; Mondelli, A.; Tibiletti, F.; Maspero, A.; Palmisano, G.; Galli, S.; Tollari, S.; Masciocchi, N.; Nicholas, K.M.; Tagliapietra, S.; et al. A simple, efficient, regioselective and one-pot preparation of N-hydroxy- and N-O-protected hydroxyindoles via cycloaddition of nitrosoarenes with alkynes. Synthetic scope, applications and novel by-products. Tetrahedron 2013, 69, 10906–10920. [Google Scholar] [CrossRef]
- Tibiletti, F.; Simonetti, M.; Nicholas, K.M.; Palmisano, G.; Parravicini, M.; Imbesi, F.; Tollari, S.; Penoni, A. One-pot synthesis of meridianins and meridianin analogues via indolization of nitrosoarenes. Tetrahedron 2010, 66, 1280–1288. [Google Scholar] [CrossRef]
- Ragaini, F.; Rapetti, A.; Visentin, E.; Monzani, M.; Caselli, A.; Cenini, S. Synthesis of indoles by intermolecular cyclization of unfunctionalized nitroarenes and alkynes, catalyzed by palladium-phenanthroline complexes. J. Org. Chem. 2006, 71, 3748–3753. [Google Scholar] [CrossRef] [PubMed]
- Ragaini, F.; Ventriglia, F.; Hagar, M.; Fantauzzi, S.; Cenini, S. Synthesis of indoles by intermolecular cyclization of unfunctionalized nitroarenes and alkynes: One-step synthesis of the skeleton of fluvastatin. Eur. J. Org. Chem. 2009, 2009, 2185–2189. [Google Scholar] [CrossRef]
- Murru, S.; Gallo, A.A.; Srivastava, R.S. Gold-catalyzed synthesis of 3-arylindoles via annulation of nitrosoarenes and alkynes. ACS Catal. 2011, 1, 29–31. [Google Scholar] [CrossRef]
- Murru, S.; Gallo, A.A.; Srivastava, R.S. Copper-catalyzed direct synthesis of 3-arylindoles. Eur. J. Org. Chem. 2011, 2011, 2035–2038. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Zhang, Y.; Singh, S.K. Efficient conversion of carboxylic acids into N-acylbenzotriazoles. Synthesis 2003, 2795–2798. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rachwal, S. Synthesis of heterocycles mediated by benzotriazole. 1. Monocyclic systems. Chem. Rev. 2010, 110, 1564–1610. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Rachwal, S. Synthesis of heterocycles mediated by benzotriazole. 2. Bicyclic systems. Chem. Rev. 2011, 111, 7063–7120. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Dathi, M.D.; Saifuddin, M.; Kundu, B. Engineering of indole-based tethered biheterocyclic alkaloid meridianin into β-carboline-derived tetracyclic polyheterocycles via amino functionalization/6-endo cationic π-cyclization. Beilstein J. Org. Chem. 2012, 8, 1901–1908. [Google Scholar] [CrossRef] [PubMed]

© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tibiletti, F.; Penoni, A.; Palmisano, G.; Maspero, A.; Nicholas, K.M.; Vaghi, L. (1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone. Molbank 2014, 2014, M829. https://doi.org/10.3390/M829
Tibiletti F, Penoni A, Palmisano G, Maspero A, Nicholas KM, Vaghi L. (1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone. Molbank. 2014; 2014(3):M829. https://doi.org/10.3390/M829
Chicago/Turabian StyleTibiletti, Francesco, Andrea Penoni, Giovanni Palmisano, Angelo Maspero, Kenneth M. Nicholas, and Luca Vaghi. 2014. "(1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone" Molbank 2014, no. 3: M829. https://doi.org/10.3390/M829
APA StyleTibiletti, F., Penoni, A., Palmisano, G., Maspero, A., Nicholas, K. M., & Vaghi, L. (2014). (1H-Benzo[d][1,2,3]triazol-1-yl)(5-bromo-1-hydroxy-1H-indol-3-yl)methanone. Molbank, 2014(3), M829. https://doi.org/10.3390/M829