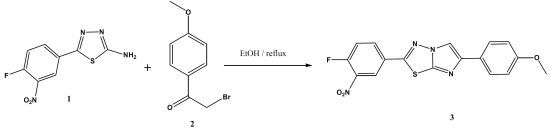
2-(4-Fluoro-3-nitrophenyl)-6-(4-methoxyphenyl)imidazo[2,1-b]-1,3,4-thiadiazole
Abstract
:Introduction
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References
- Demirbas, A.; Sahin, D.; Demirbas, N.; Karaoglu, S.A. Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Solak, N.; Rollas, S. Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1,3,4-thiadiazoles and their Schiff bases. ARKIVOC 2006, 12, 173–181. [Google Scholar]
- Mullican, M.D.; Wilson, M.W.; Connor, D.T.; Kostlan, C.R.; Schrier, D.J.; Dyer, R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally active, nonulcerogenic antiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Connor, D.T.; Sercel, A.D.; Sorenson, R.J.; Doubleday, R.; Unangst, P.C.; Roth, B.D.; Beylin, V.G.; Gilbertson, R.B.; Chan, K.; et al. Synthesis, Structure−Activity Relationships, and in vivo Evaluations of Substituted Di-tert-butylphenols as a Novel Class of Potent, Selective, and Orally Active Cyclooxygenase-2 Inhibitors. 1. Thiazolone and Oxazolone Series. J. Med. Chem. 1999, 42, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Mathew, V.; Keshavayya, J.; Vaidya, V.P.; Giles, D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem. 2007, 42, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, M.; Myers, P.L.; Saville, J.F.; Smith, A.C.B.; Stilling, M.R.; Tulloch, I.F.; Walter, D.S.; Welbourne, A.P. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 1. Hydrazines. J. Med. Chem. 1986, 29, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, P.L.; Smith, A.C.; Stilling, M.R.; Tulloch, I.F.; Walter, D.S. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 4. Amidines. J. Med. Chem. 1988, 31, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Myers, M.; Gadie, B.; Nelson, A.J.; Pape, R.; Saville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive thiadiazoles. 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4-thiadiazoles with vasodilator activity. J. Med. Chem. 1988, 31, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Myers, M.; Gadie, B.; Hale, S.A.; Horsley, A.; Nelson, A.J.; Pape, R.; Saville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive thiadiazoles. 2. Vasodilator activity of some 2-aryl-5-guanidino-1,3,4-thiadiazoles. J. Med. Chem. 1988, 31, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Nazulewicz, A.; Pelczynska, M.; Switalska, M.; Jaroszewicz, I.; Opolski, A. Synthesis and antiproliferative activity of some 5-substituted 2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2006, 41, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Rao, B.S.; Gonsalves, R.; Sarojini, B.K.; Shridhara, K. Synthesis of some new biologically active thiadiazolotriazinones—Part III. Il Farmaco 2002, 57, 693–696. [Google Scholar] [CrossRef]
- Radi, M.; Crespan, E.; Botta, G.; Falchi, F.; Maga, G.; Manetti, F.; Corradi, V.; Mancini, M.; Santucciv, M.A.; Schenone, S.; et al. Discovery and SAR of 1,3,4-thiadiazole derivatives as potent Abl tyrosine kinase inhibitors and cytodifferentiating agents. Bioorg. Med. Chem. Lett. 2008, 18, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.B.; Kulkarni, M.V.; Rasal, V.P.; Biradar, S.S.; Vinay, M.D. Synthesis and anti-inflammatory evaluation of methylene bridged benzofuranyl imidazo[2,1-b][1,3,4]thiadiazoles. Eur. J. Med. Chem. 2008, 43, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kundapur, U.; Sarojini, B.K.; Narayana, B. 2-(4-Fluoro-3-nitrophenyl)-6-(4-methoxyphenyl)imidazo[2,1-b]-1,3,4-thiadiazole. Molbank 2012, 2012, M778. https://doi.org/10.3390/M778
Kundapur U, Sarojini BK, Narayana B. 2-(4-Fluoro-3-nitrophenyl)-6-(4-methoxyphenyl)imidazo[2,1-b]-1,3,4-thiadiazole. Molbank. 2012; 2012(4):M778. https://doi.org/10.3390/M778
Chicago/Turabian StyleKundapur, Umesha, Balladka Kunhanna Sarojini, and Badiadka Narayana. 2012. "2-(4-Fluoro-3-nitrophenyl)-6-(4-methoxyphenyl)imidazo[2,1-b]-1,3,4-thiadiazole" Molbank 2012, no. 4: M778. https://doi.org/10.3390/M778