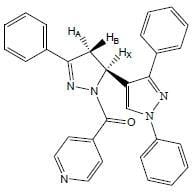
[5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone
Abstract
:1. Introduction
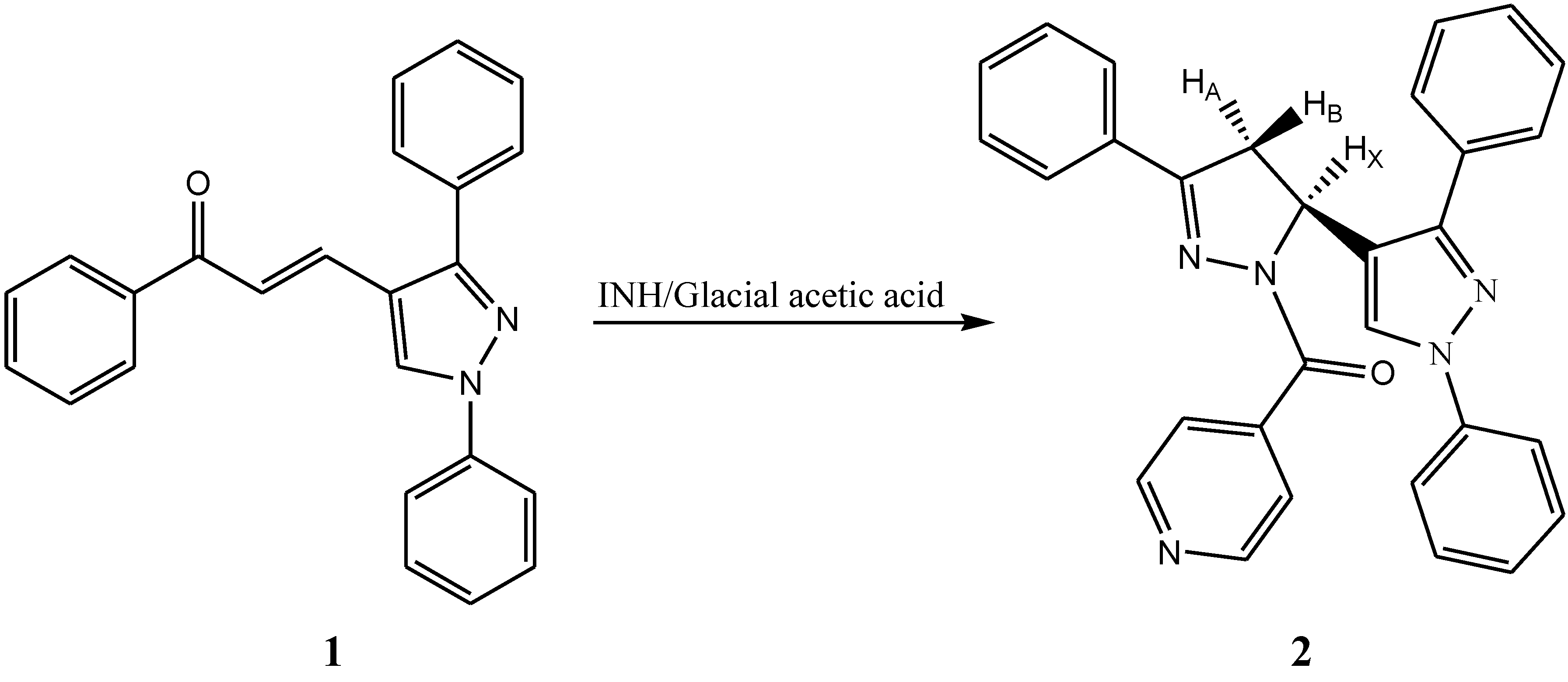
2. Results & Discussion
3. Experimental
Synthesis of [5-(1,3-diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References
- Shamsuzzaman; Khan, M.S.; Alam, M. Synthesis, characterization and antimicrobial activity of new steroidal cholest-5-en-7-one derivatives fused with substituted pyrazoline ring. J. Chil. Chem. Soc. 2009, 54, 372–374. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.A.; Park, W.S.; Kang, S.K.; Kim, K.Y.; Kim, K.R.; Rhee, S.D.; Bae, M.A.; Kang, N.S.; Sohn, S.K.; Kim, S.G.; Lee, J.O.; Lee, D.H.; Cheon, H.G.; Kim, S.S.; Ahn, J.H. Synthesis and biological evaluation of pyrazoline analogues with β-amino acyl group as dipeptidyl peptidase IV inhibitors. Eur. J. Med. Chem. 2008, 43, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Gokhan-Kelekci, N.; Koyunoglu, S.; Yabanoglu, S.; Yelekci, K.; Ozgen, O.; Ucar, G.; Erol, K.; Kendi, E.; Yesilada, A. New pyrazoline bearing 4(3H)-quinqzolinone inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg. Med. Chem. 2009, 17, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Gzella, A.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Chevallet, P.; Kilic; Fatma, S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Bhat, A.R.; Athar, F.; Azam, A. Bis-pyrazolines; Synthesis, characterization and antiamoebic activity as inhibitors of growth of Entamoeba histolytica. Eur. J. Med. Chem. 2009, 44, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rathish, I.G.; Javed, K.; Ahmad, S.; Bano, S.; Alam, M.S.; Pillai, K.K.; Singh, S.; Bagchi, V. Synthesis and anti-inflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg. Med. Chem. Lett. 2009, 19, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Shaharyar, M.; Siddiqui, A.A.; Ali, M.A.; Dharmarajan, S.; Perumal, Y. Synthesis and in vitro antimycobacterial activity of N1- nicotinoyl-3-(4'-hydroxy-3'-methyl phenyl )-5-[(sub)phenyl]-2-pyrazoline. Bioorg. Med. Chem. Lett. 2006, 16, 3947–3949. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Khan, S.A.; Chawla, G.; Kumar, S. N'-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene] 2/4-substituted hydrazides: synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kumar, V.; Singh, S.P. Syntheis of some new 1-(6-fluorobenzothiazol-2-yl)-3-(4-fluoro-phenyl)-5-arylpyrazolines and their iodine (III) mediated oxidation to corresponding pyrazoles. Indian J. Chem. 2007, 46B, 1332–1336. [Google Scholar]
- Revanasiddappa, B.C.; Rao, N.R.; Subrahmanyam, E.V.S.; Satyanarayana, D. Synthesis and biological evaluation of some novel 1,3,5-trisubstituted pyrazolines. E-J. Chem. 2010, 7, 295–298. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaushik, D.; Nagpal, U.; Verma, T.; Madan, K. [5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone. Molbank 2011, 2011, M714. https://doi.org/10.3390/M714
Kaushik D, Nagpal U, Verma T, Madan K. [5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone. Molbank. 2011; 2011(1):M714. https://doi.org/10.3390/M714
Chicago/Turabian StyleKaushik, Darpan, Upasana Nagpal, Tarawanti Verma, and Kapish Madan. 2011. "[5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone" Molbank 2011, no. 1: M714. https://doi.org/10.3390/M714
APA StyleKaushik, D., Nagpal, U., Verma, T., & Madan, K. (2011). [5-(1,3-Diphenyl-1H-pyrazol-4-yl)-3-phenyl-4,5-dihydropyrazol-1-yl](pyridin-4-yl)methanone. Molbank, 2011(1), M714. https://doi.org/10.3390/M714