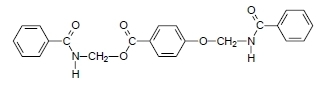
(Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate
Abstract
:Experimental
(Benzoylamino)methyl 4-[(benzoylamino)methoxy]benzoate (3)
Procedure A
Procedure B
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Rapior, S.; Breheret, S.; Talou, T.; Pélissier, Y.; Bessière, J.-M. The anise-like odor of Clitocybe odora, Lentinellus cochleatus and Agaricus essettei. Mycologia 2002, 94, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Binder, R.G.; Flath, R.A. Volatile components of pineapple guava. J. Agric. Food Chem. 1989, 37, 734–736. [Google Scholar] [CrossRef]
- Hardy, P.J.; Michael, B.J. Volatile components of feijoa fruits. Phytochemistry 1970, 9, 1355–1357. [Google Scholar] [CrossRef]
- Shaw, G.J.; Ellingham, P.J.; Birch, E.J. Volatile constituents of feijoa-headspace analysis of intact fruit. J. Sci. Food Agr. 1983, 34, 743–747. [Google Scholar] [CrossRef]
- Ceballos, L.; Pino, J.A.; Quijano-Celis, C.E.; Dago, A. Optimization of a HS-SPME/GC-MS Method for determination of volatile compounds in some Cuban unifloral honeys. J. Food Quality 2010, 33, 507–528. [Google Scholar] [CrossRef]
- Wang, H.; Pullambhatla, M.; Guilarte, T.R.; Mease, R.C.; Pomper, M.G. Synthesis of [125I]iodoDPA-713: A new probe for imaging inflammation. Biochem. Biophys. Res. Commun. 2009, 389, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Chandra, B.K.; Ramadasu, G.; Gangaiah, L.; Madhusudhan, G.; Mukkanti, K. A new route for the synthesis of (R)-glyceraldehyde acetonide: A key chiral building block. Indian J. Chem. B 2010, 49, 260–263. [Google Scholar]
- Perriot, R.; Breme, K.; Meierhenrich, U.J.; Carenini, E.; Ferrando, G.; Baldovini, N. Chemical composition of French mimosa absolute oil. J. Agric. Food Chem. 2010, 58, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Panda, H. Perfumes and flavours technology handbook; Asia Pacific Business Press Inc: Delhi, India, 2010. [Google Scholar]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Simple method for benzamidomethylation of phenols in water solution. Synth. Commun. 1999, 29, 3451–3458. [Google Scholar] [CrossRef]
- Popovski, E.; Mladenovska, K. (Benzoylamino)methyl 4-hydroxybenzoate. Molbank 2010, 2010, M658. [Google Scholar] [CrossRef]
- Popovski, E.; Klisarova, L.; Vikic-Topic, D. Benzamidomethylation with (benzamidomethyl)triethylammonium chloride 2. A simple method for benzamidomethylation of thiols, amines and carboxylic acids. Molecules 2000, 5, 927–936. [Google Scholar] [CrossRef]

© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Popovski, E.; Mladenovska, K.; Panovska, A.P. (Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank 2011, 2011, M711. https://doi.org/10.3390/M711
Popovski E, Mladenovska K, Panovska AP. (Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank. 2011; 2011(1):M711. https://doi.org/10.3390/M711
Chicago/Turabian StylePopovski, Emil, Kristina Mladenovska, and Ana Poceva Panovska. 2011. "(Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate" Molbank 2011, no. 1: M711. https://doi.org/10.3390/M711
APA StylePopovski, E., Mladenovska, K., & Panovska, A. P. (2011). (Benzoylamino)methyl 4-[(Benzoylamino)methoxy]benzoate. Molbank, 2011(1), M711. https://doi.org/10.3390/M711