Diphenyl(2-propoxyethyl)phosphine
Abstract
:1. Introduction
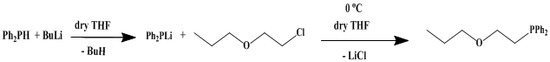
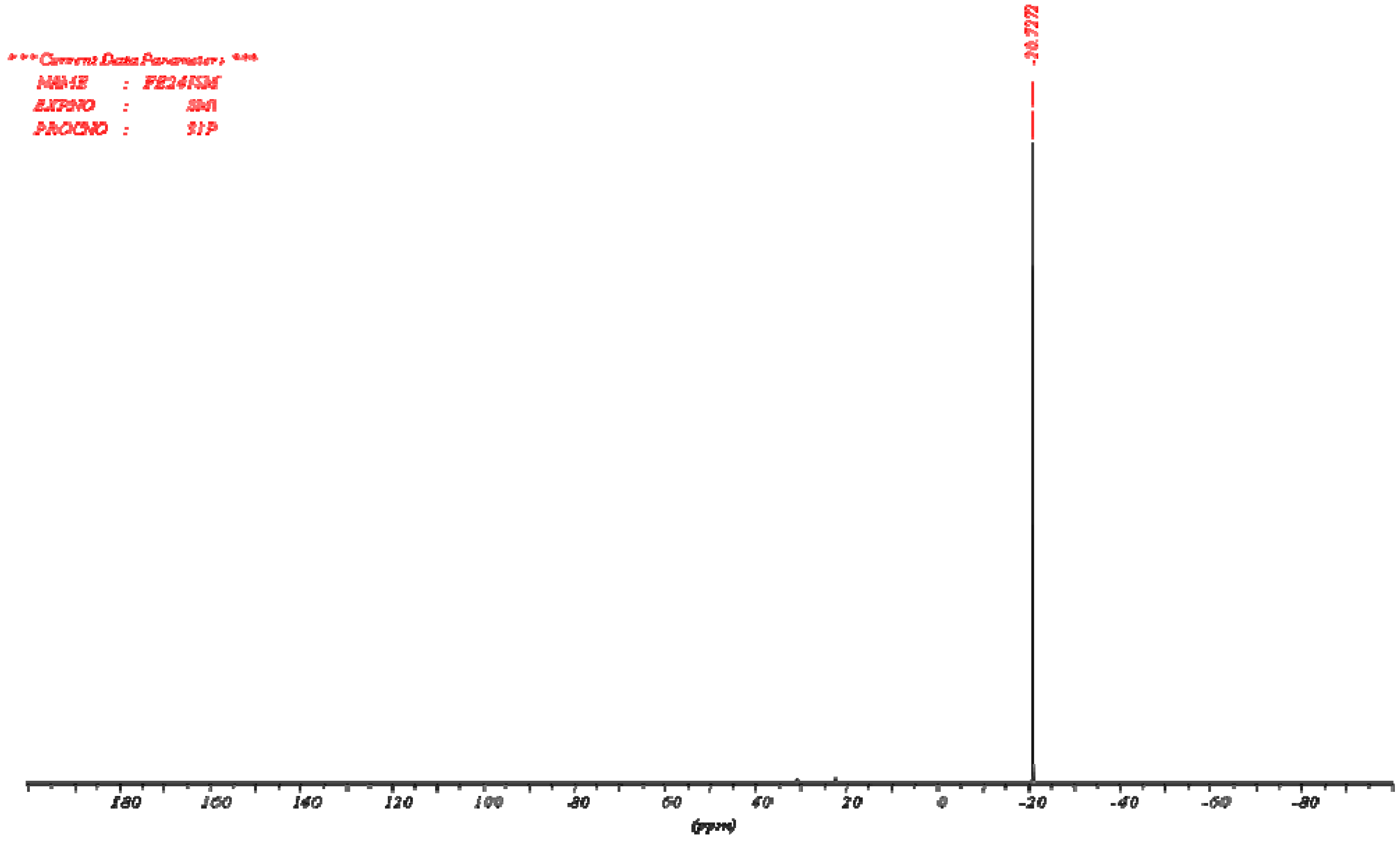
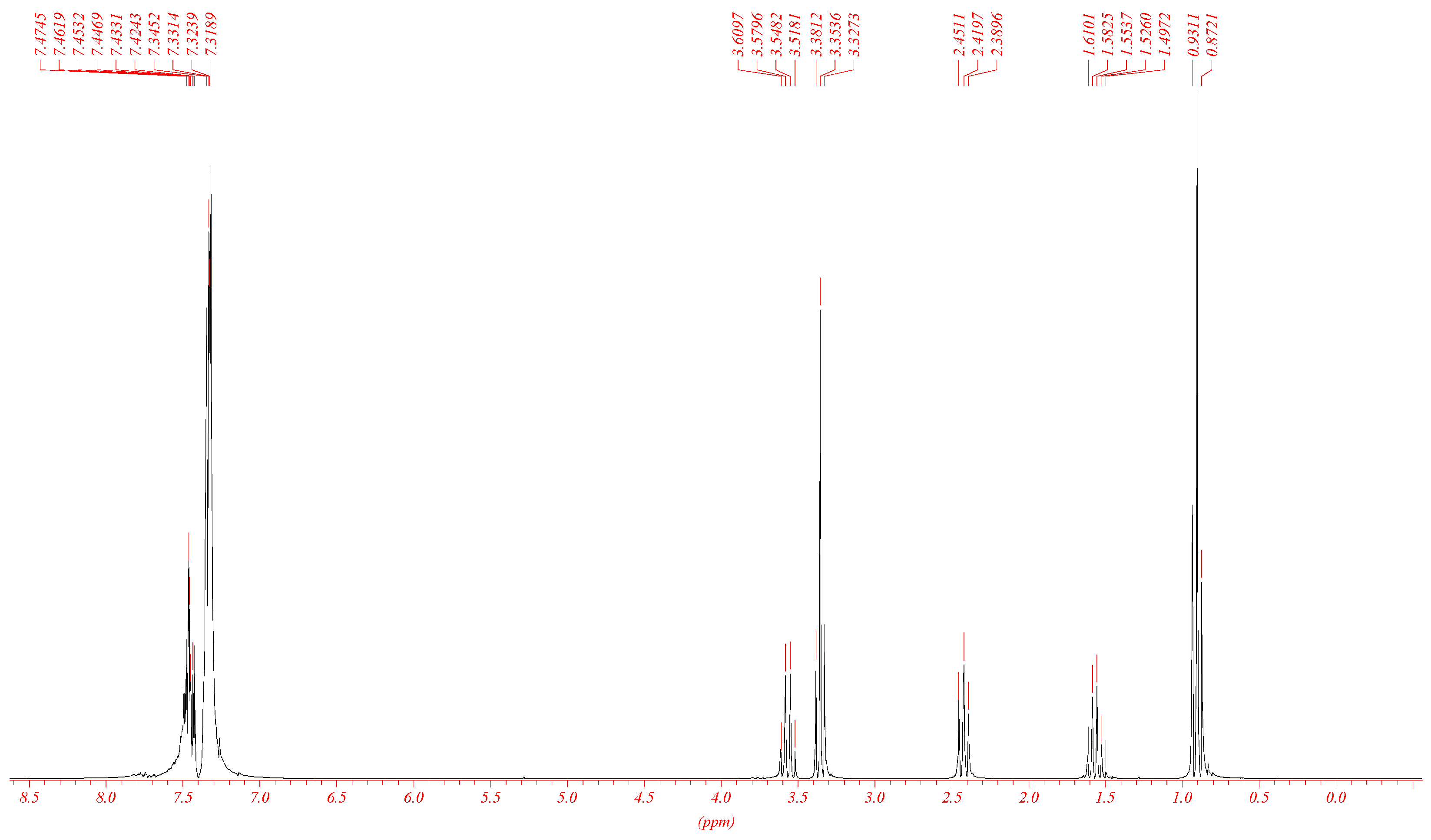
2. Result and Discussion
3. Experimental
3.1. Preparation
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Angell, S.; Rogers, C.; Zhang, Y.; Wolf, M.; Jones, W. Hemilabile coordination complexes for sensing applications. Coord. Chem. Rev. 2006, 250, 1829–1841. [Google Scholar] [CrossRef]
- Ruiz, J.; Flor, T.; Bayon, J. A chiral diphosphine containing hemilabile ether donor groups and its use in rhodium asymmetric hydroboration of styrene. Inorg. Chem. Comm. 1999, 484–486. [Google Scholar] [CrossRef]
- Kuriyama, M.; Nagai, K.; Yamada, K.; Miwa, Y.; Taga, T.; Tomioka, K. Hemilabile Amidomonophosphine Ligand Rhodium(I) Complex-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to Cycloalkenones. J. Am. Chem. Soc. 2002, 124, 8932–8939. [Google Scholar] [CrossRef] [PubMed]
- Valls, E.; Suades, J.; Mathieu, R. Synthesis of New Hemilabile Amphiphilic Phosphines Complexing Properties toward Ruthenium(II) and Catalytic Activity for Hydrogenation of Prenal. Organometallics 1999, 18, 5475–5483. [Google Scholar] [CrossRef]
- Jimenez, V.; Perez-Torrente, J.; Bartolome, I.; Oro, A. Convenient Methods for the Synthesis of a Library of Hemilabile Phosphines. Synthesis 2009, 1916–1922. [Google Scholar] [CrossRef]
- Lindner, E.; Pautz, S.; Fawzi, R.; Steimann, M. Behavior of (Ether-phosphine)ruthenium(II) Complexes [C6Me6)RuH(PO)][BF4] Containing Reactive RuO and RuH Bonds toward Various Small Molecules and Their Application in Ring-Opening Metathesis Polymerization. Organometallics 1998, 17, 3006–3014. [Google Scholar] [CrossRef]
- Warad, I. Supported and Non-Supported Ruthenium(II)/Phosphine/[3-(2-Aminoethyl)-aminopropyl]trimethoxysilane Complexes and Their Activities in the Chemoselective Hydrogenation of trans-4-Phenyl-3-butene-2-al. Molecules 2010, 15, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Warad, I.; Lindner, E.; Eichele, K.; Mayer, A.H. Cationic Diamine(ether–phosphine)ruthenium(II) Complexes as Precursors for the Hydrogenation of trans-4-phenyl-3-butene-2-one. Inorg. Chim. Acta 2004, 357, 1847–1853. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Eichele, K.; Warad, I.; Mayer, H.A.; Lindner, E.; Jiang, Z.; Schurig, V.Z. Bis(methoxyethyldimethylphosphine)ruthenium(II) Complexes as Transfer Hydrogenation Catalysts. Anorg. Allg. Chem. 2003, 629, 1308–1315. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Nuri, M. Diphenyl(2-propoxyethyl)phosphine. Molbank 2010, 2010, M709. https://doi.org/10.3390/M709
Al-Nuri M. Diphenyl(2-propoxyethyl)phosphine. Molbank. 2010; 2010(4):M709. https://doi.org/10.3390/M709
Chicago/Turabian StyleAl-Nuri, Mohammed. 2010. "Diphenyl(2-propoxyethyl)phosphine" Molbank 2010, no. 4: M709. https://doi.org/10.3390/M709
APA StyleAl-Nuri, M. (2010). Diphenyl(2-propoxyethyl)phosphine. Molbank, 2010(4), M709. https://doi.org/10.3390/M709