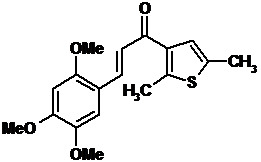
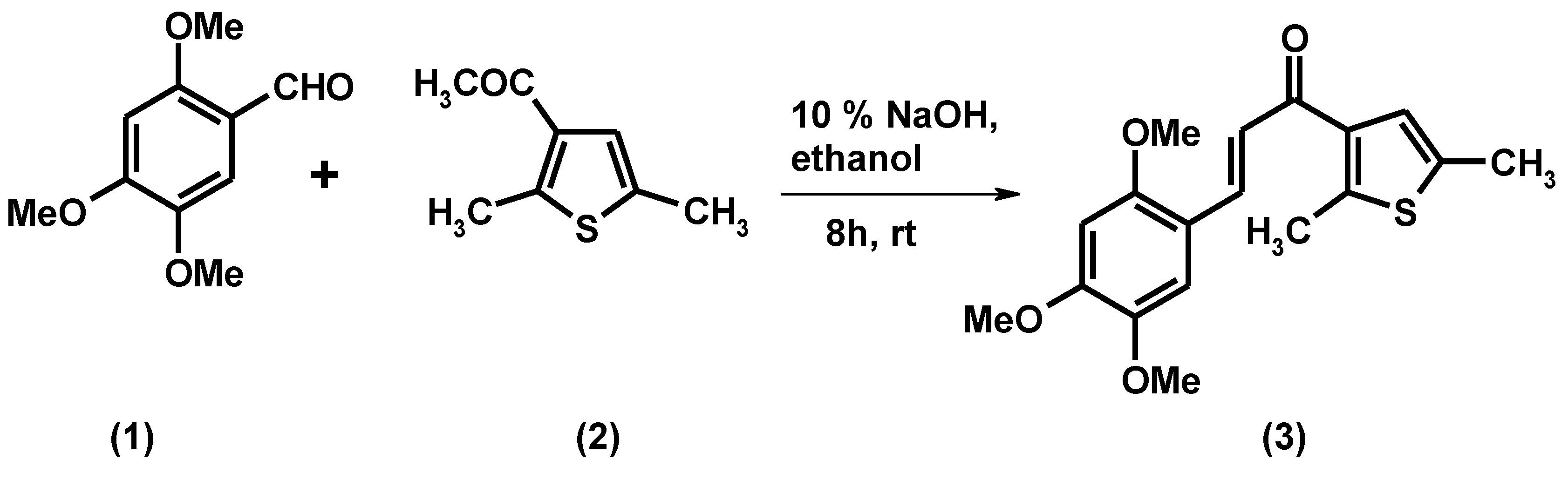
1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one
Abstract
:Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References
- Lin, M.; Zhou, Y.; Flavin, M.T.; Zhou, L.; Nie, W.; Chen, F. Chalcones and flavonoids as anti-Tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. [Google Scholar] [CrossRef]
- Wu, X.; Tiekink, E.R.T.; Kostetski, I.; Kocherginsky, N.; Tan, A.L.C.; Khoo, S.B.; Wilairat, P.; Go, M.L. Antiplasmodial activity of ferrocenyl chalcones: Investigations into the role of ferrocene. Eur. J. Pharm. Sci. 2006, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.Y.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Lee, K.H. Antitumor agents. Part 202: Novel 2′-amino chalcones: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2000, 10, 699–701. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Patil, S.A.; Korbad, B.L.; Nile, S.H.; Khobragade, C.N. Synthesis and biological evaluation of β-chloro vinyl chalcones as inhibitors of TNF-α and IL-6 with antimicrobial activity. Eur. J. Med. Chem. 2010, 45, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.C.; Bariwal, J.B.; Upadhyay, K.D.; Naliapara, Y.T.; Joshi, S.K.; Pannecouque, C.C.; Clercq, E.D.; Shah, A.K. Improved and rapid synthesis of new coumarinyl chalcone derivatives and their antiviral activity. Tetrahedron Lett. 2007, 48, 8472–8474. [Google Scholar] [CrossRef]
- Vogel, S.; Barbic, M.; Jurgenliemk, G. Heilmann, Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem. 2010, 45, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Forejtnikova, H.; Lunerova, K.; Kubinova, R.; Jankovska, D.; Marek, R.; Kares, R.; Suchy, V.; Vondracek, J.; Machala, M. Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology 2005, 208, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Kadota, S. Neosappanone A, a xanthine oxidase (XO) inhibitory dimeric methanodibenzoxocinone with a new carbon skeleton from Caesalpinia sappan. Tetrahedron Lett. 2004, 45, 8519–8522. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. 1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank 2010, 2010, M692. https://doi.org/10.3390/M692
Asiri AM, Khan SA. 1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank. 2010; 2010(3):M692. https://doi.org/10.3390/M692
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2010. "1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one" Molbank 2010, no. 3: M692. https://doi.org/10.3390/M692
APA StyleAsiri, A. M., & Khan, S. A. (2010). 1-(2,5-Dimethyl-3-thienyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one. Molbank, 2010(3), M692. https://doi.org/10.3390/M692