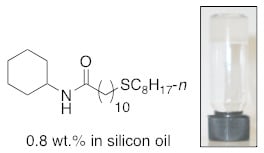
N-Cyclohexyl-11-(octylthio)undecanamide
Abstract
:Experimental
General
Gelation experiments and gel characterization
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Posner, T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573, and references therein. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Dondoni, A. The Emergence of Thiol-Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 8995–8997. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Lin, B.F.; Campos, L.M.; Dimitriou, M.D.; Hikita, S.T.; Treat, N.D.; Tirrell, M.V.; Clegg, D.O.; Kramer, E.J.; Hawker, C.J. A Versatile Approach to High-Throughput Microarrays Using Thiol-ene Chemistry. Nat. Chem. 2010, 2, 138–145, and references therein. [Google Scholar] [CrossRef] [PubMed]
- See Supplementary Files.
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Esch, J.V.; De Feyter, S.; De Schryver, F.; Kellogg, R.M.; Feringa, B.L. Self-assembly of bisurea compounds in organic solvents and on solid substrates. Chem. Eur. J. 1997, 3, 1238–1243. [Google Scholar] [CrossRef]

| Entry | Solvent | Initiatora | Time (min) | T (°C)b | Yield (%) |
| 1 | THF | −− | 60 | 76 | −c |
| 2 | Toluene | −− | 60 | 130 | −d |
| 3 | Toluene | AIBN | 5 | 130 | −e |
| 4 | Toluene | AIBN | 40 | 25 | 70f |
| 5 | Toluene | DMPA | 10 | 25 | 70f |
| 6 | THF | DMPA | 10 | 25 | 96f |
| 7 | THF | −− | 10 | 25 | −f,g |
| 8 | CH2Cl2 | DMPA | 10 | 25 | 95f |
| 9 | DMSO | DMPA | 10 | 25 | 68f |
| 10 | Acetone | DMPA | 10 | 25 | 78f |
| 11 | MeOH | DMPA | 10 | 25 | 83f |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schön, E.-M.; Díaz, D.D. N-Cyclohexyl-11-(octylthio)undecanamide. Molbank 2010, 2010, M689. https://doi.org/10.3390/M689
Schön E-M, Díaz DD. N-Cyclohexyl-11-(octylthio)undecanamide. Molbank. 2010; 2010(3):M689. https://doi.org/10.3390/M689
Chicago/Turabian StyleSchön, Eva-Maria, and David D. Díaz. 2010. "N-Cyclohexyl-11-(octylthio)undecanamide" Molbank 2010, no. 3: M689. https://doi.org/10.3390/M689