1-Phenylpyrazolo[4',3':5,6]pyrano[3,2-c]pyridine-4(1H)-thione
Abstract
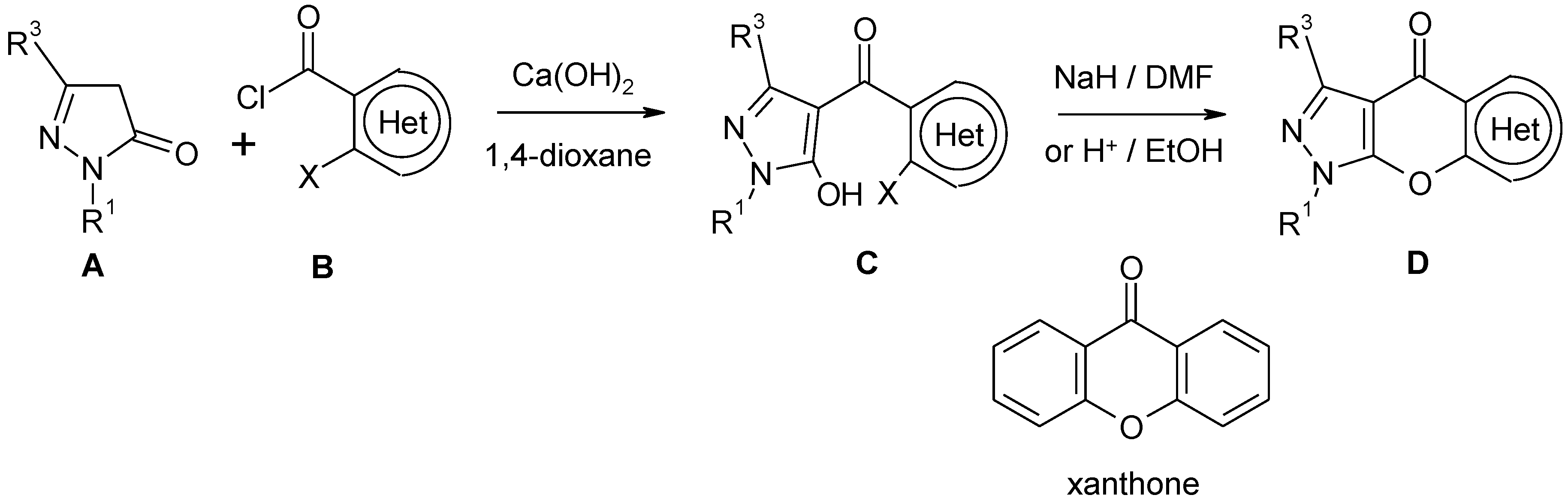
:Experimental
1-Phenylpyrazolo[4',3':5,6]pyrano[3,2-c]pyridin-4(1H)-thione (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Eller, G.A.; Wimmer, V.; Haring, A.W.; Holzer, W. An Efficient Approach to Heterocyclic Analogues of Xanthone: A Short Synthesis of all possible Pyrido[5,6]pyrano[2,3-c]pyrazol-4(1H)-ones. Synthesis 2006, 4219–4229. [Google Scholar] [CrossRef]
- Eller, G.A.; Haring, A.W.; Datterl, B.; Zwettler, M.; Holzer, W. Tri- and Tetracyclic Heteroaromatic Systems: Synthesis of Novel Benzo-, Benzothieno- and Thieno-Fused Pyrano[2,3-c]pyrazol-4(1H)-ones. Heterocycles 2007, 71, 87–104. [Google Scholar] [CrossRef]
- Eller, G.A.; Holzer, W. A Convenient Approach to Heterocyclic Building Blocks: Synthesis of Novel Ring Systems Containing a [5,6]Pyrano[2,3-c]pyrazol-4(1H)-one Moiety. Molecules 2007, 12, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Eller, G.A.; Datterl, B.; Holzer, W. Pyrazolo[4’,3’:5,6]pyrano[2,3-b]quinoxalin-4(1H)-one: Synthesis and Characterization of a Novel Tetracyclic Ring System. J. Heterocycl. Chem. 2007, 44, 1139–1143. [Google Scholar] [CrossRef]
- Eller, G.A.; Wimmer, V.; Holzer, W. Synthesis of Novel Polycyclic Ring Systems Containing two Pyrano[2,3-c]pyrazol-4(1H)-one Moieties. Khim. Geterotsikl. Soedin. 2007, 1251–1255, (Chem. Heterocycl. Comp. 2007, 43, 1060–1064.). [Google Scholar] [CrossRef]
- Eller, G.A.; Habicht, D.; Holzer, W. Synthesis of a Novel Pentacycle: 8-Methyl-10-phenylpyrazolo[4’,3’:5,6]pyrano[3,2-c][1,10]phenanthrolin-7(10H)-one. Khim. Geterotsikl. Soedin. 2008, 884–890, (Chem. Heterocycl. Comp. 2008, 44, 709–714.). [Google Scholar] [CrossRef]
- Eller, G.A.; Zhang, Q.; Habicht, D.; Datterl, B.; Holzer, W. Synthesis and NMR Data of Pyrazolo[4’,3’:5,6]pyrano[2,3-b]pyrazin-4(1H)-ones: Derivatives of a Novel Tricyclic Ring System. Acta Chim. Slov. 2009, 56, 521–526. [Google Scholar]
- Jensen, B.S. The Synthesis of 1-Phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668–1670, (Chem. Abstr. 1962, 56, 66890.). [Google Scholar] [CrossRef]
- Williams, A.C.; Camp, N. Product class 4: Benzopyranones and benzopyranthiones. Sci. Synth. 2003, 14, 347–638. [Google Scholar] and references cited therein.
- Huemer, V.; Eller, G.A.; Holzer, W. Heterocyclic analogues of xanthiones: 5,6-Fused 3-methyl-1-phenylpyrano[2,3-c]pyrazol-4(1H)thiones – synthesis and NMR (1H, 13C, 15N) data. Magn. Reson. Chem. 2010. [Google Scholar] [CrossRef] [PubMed]
- Cremlyn, R.J. An Introduction to Organosulfur Chemistry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Duus, F. Thiocarbonyl compounds. In Comprehensive Organic Chemistry; Barton, D.H.R., Ollis, W.D., Eds.; Pergamon Press: Oxford, UK, 1979; Volume 3, pp. 373–487. [Google Scholar]
- Cherkasov, R.A.; Kutyrev, G.A.; Pudovik, A.N. Tetrahedron report number 186: Organothiophosphorus reagents in organic synthesis. Tetrahedron 1985, 41, 2567–2624. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Scheibye, S.; Nilsson, N.H.; Lawesson, S.-O. Studies on organophosphorus compounds. XX. Syntheses of thioketones. Bull. Soc. Chim. Belg. 1978, 87, 223–228. [Google Scholar] [CrossRef]
- Jesberger, M.; Davis, T.P.; Barner, L. Application of Lawesson’s Reagent in Organic and Organometallic Synthesis. Synthesis 2003, 1929–1958. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course, 2nd ed.; Wiley–VCH: Weinheim, Germany, 1998; (Chem. Abstr. 1999, 131, 184497). [Google Scholar]
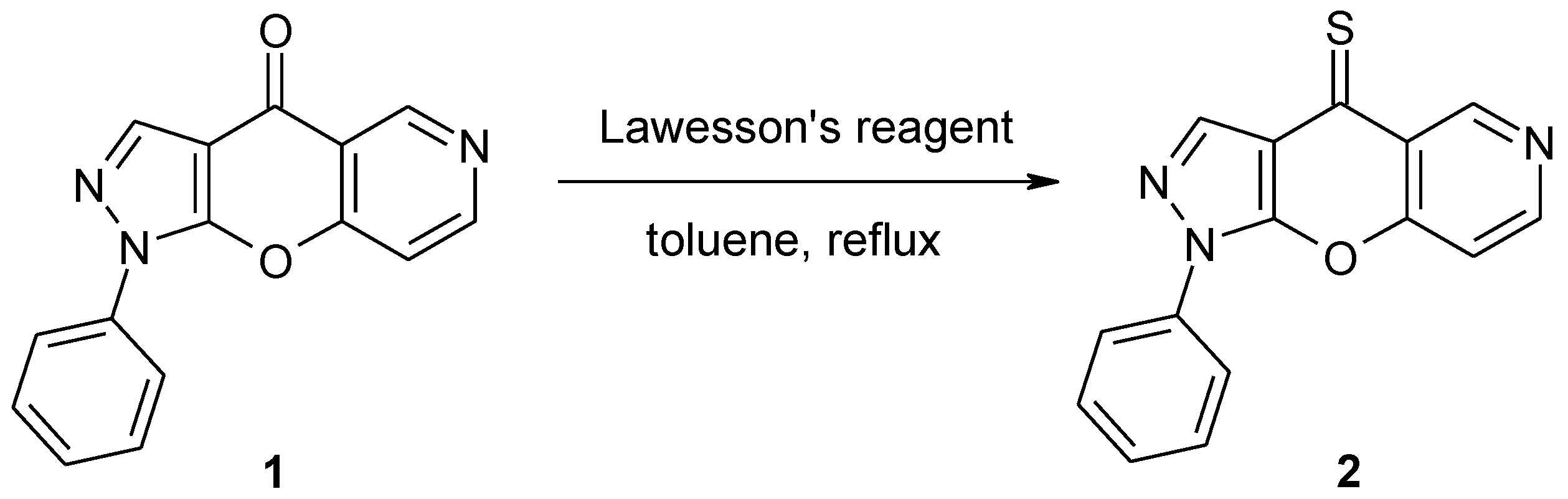
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huemer, V.; Holzer, W. 1-Phenylpyrazolo[4',3':5,6]pyrano[3,2-c]pyridine-4(1H)-thione. Molbank 2010, 2010, M678. https://doi.org/10.3390/M678
Huemer V, Holzer W. 1-Phenylpyrazolo[4',3':5,6]pyrano[3,2-c]pyridine-4(1H)-thione. Molbank. 2010; 2010(2):M678. https://doi.org/10.3390/M678
Chicago/Turabian StyleHuemer, Valerie, and Wolfgang Holzer. 2010. "1-Phenylpyrazolo[4',3':5,6]pyrano[3,2-c]pyridine-4(1H)-thione" Molbank 2010, no. 2: M678. https://doi.org/10.3390/M678




