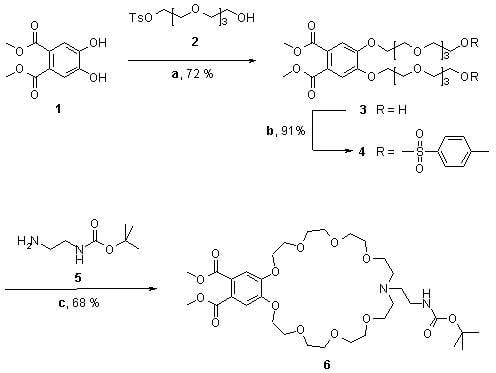
Aza-27-crown-9 Amino Acid
Abstract
:1. Introduction
2. Synthesis
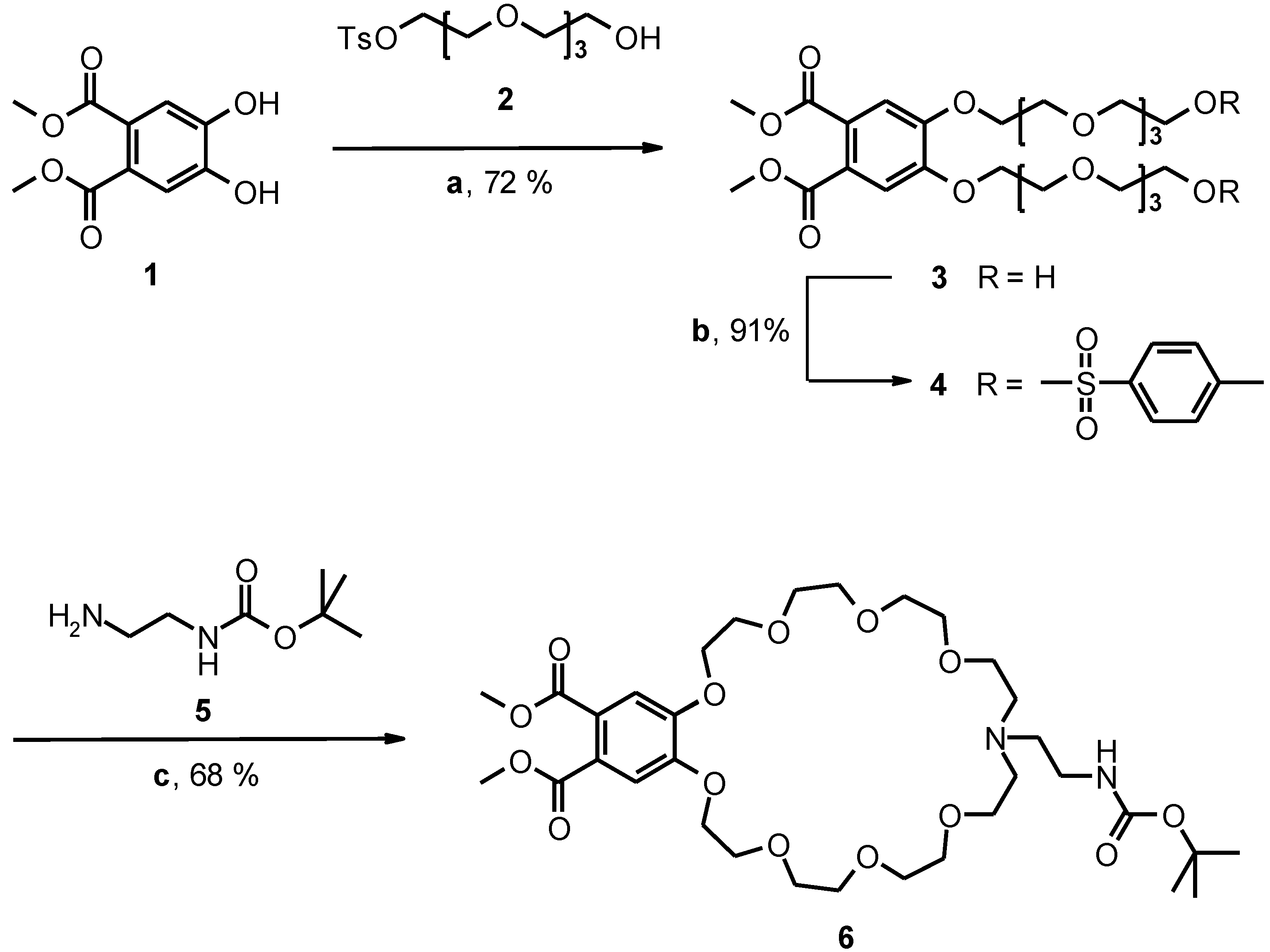
3. Experimental
Dimethyl 13-(2-(tert-butoxycarbonylamino)ethyl)-3,5,6,8,9,11,12,13,14,15,17,18,20,21,23,24-hexadecahydro-2H-benzo[k][1,4,7,10,13,16,19,22,25]octaoxaazacycloheptacosine-27,28-dicarboxylate (6)
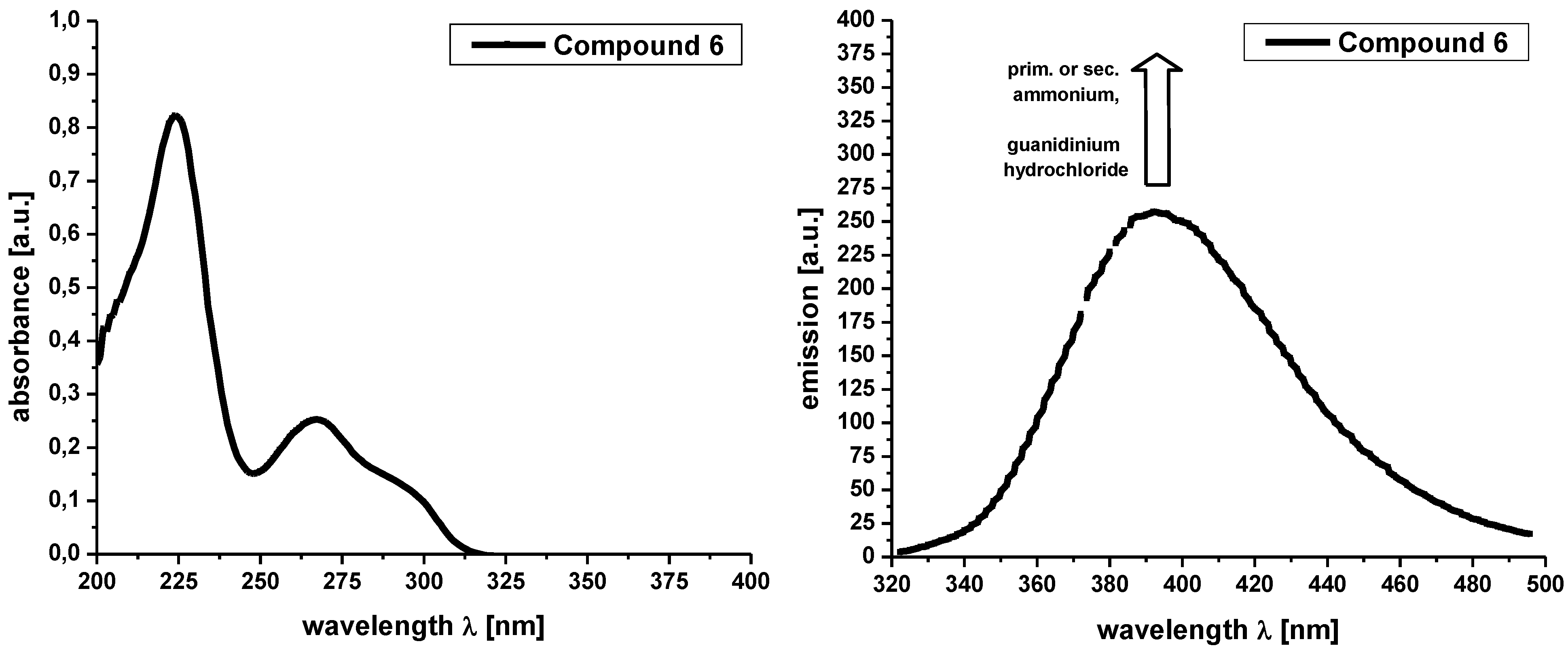
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
References and Notes
- Gokel, G.W. Crown Ethers and Cryptands; Royal Society of Chemistry: Cambridge, UK, 1991. [Google Scholar]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S. Thermodynamic and Kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 1991, 91, 1721–2085. [Google Scholar] [CrossRef]
- Izatt, R.M.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- Späth, A.; König, B. Modular synthesis of di- and tripeptides of luminescent crown ether aminocarboxylic acids. Tetrahedron 2009, 65, 690–695. [Google Scholar] [CrossRef]
- Mandl, C.P.; Koenig, B. Luminescent crown ether amino acids-selective binding to N-terminal lysine in peptides. J. Org. Chem. 2005, 70, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Mandl, C.; Miltschitzky, S.; König, B. A luminescent receptor with affinity for N-terminal histidine in peptides in aqueous solution. J. Am. Chem. Soc. 2005, 127, 3362–3365. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, S.; Riechers, A.; Späth, A.; König, B. Utilizing reversible copper(II) peptide coordination in a sequence-selective luminescent receptor. Chem. Eur. J. 2008, 14, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Kyba, E.P.; Helgeson, R.C.; Madan, K.; Gokel, G.W.; Tarnowski, T.L.; Moore, S.S.; Cram, D.J. Host-guest complexation. 1. Concept and illustration. J. Am. Chem. Soc. 1977, 99, 2564–2571. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Vierling, P.; Hayward, R.C. Stable and selective guanidinium and imidazolium complexes of a macrocyclic receptor molecule. J. Chem. Soc. Chem. Commun. 1979, 296–298. [Google Scholar] [CrossRef]
- Stolwijk, T.B.; Grootenhuis, P.D.J.; van der Wal, P.D.; Sudhölter, E.J.R.; Reinhoudt, D.N.; Harkema, S.; Uiterwijk, J.W.H.M.; Kruise, L. Crown ether mediated transport of guanidinium thiocyanate through a bulk liquid membrane and the correlation with the complex stability constants. J. Org. Chem. 1986, 51, 4891–4898. [Google Scholar] [CrossRef]
- de Boer, J.A.A.; Uiterwijk, J.W.H.M.; Geevers, J.; Harkema, S.; Reinhoudt, D.N. Macrocyclic receptor molecules for guanidinium cations. Preparation, X-ray structures, and kinetic stabilities of 1:1 complexes of guanidinium perchlorate with benzo-27-crown-9, dibenzo-27-crown-9, and dibenzo-30-crown-10. J. Org. Chem. 1983, 48, 4821–4830. [Google Scholar] [CrossRef]
- Van Staveren, C.J.; Hertog, H.J.; Reinhoudt, D.N.; Uiterwijk, J.W.H.M.; Kruise, L.; Harkema, S. Syntheses and crystal structures of encapsulated guanidinium complexes of 27-membered macrocyclic ligands. J. Chem. Soc. Chem. Commun. 1984, 1409–1411. [Google Scholar] [CrossRef]
- Gawley, R.E.; Mao, H.; Haque, M.M.; Thorne, J.B.; Pharr, J.S. Visible fluorescence chemosensor for saxitoxin. J. Org. Chem. 2007, 72, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Thorne, J.B.; Pharr, J.S.; Gawley, R.E. Effect of crown ether ring size on binding and fluorescence response to saxitoxin in anthracylmethyl monoazacrown ether chemosensors. Can. J. Chem. 2006, 84, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Murai, S.; Ryu, I. Two types of indirect cyclodimerization of biacetyl via its enol silyl ethers. Chem. Lett. 1977, 1219–1222. [Google Scholar] [CrossRef]
- House, H.O.; Czuba, L.J.; Gall, M.; Olmstead, H.D. Chemistry of carbanions. XVIII. Preparation of trimethylsilyl enol ethers. J. Org. Chem. 1969, 34, 2324–2336. [Google Scholar] [CrossRef]
- Drager, A.S.; O´Brien, D.F. Novel synthesis of liquid crystalline phthalocyanines. J. Org. Chem. 2000, 65, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Menzer, R.; Parsons, I.W.; Preece, J.A.; Stoddart, J.F.; Tolley, M.S.; White, A.J.P.; Williams, D.J. Bis[2]catenanes and a bis[2]rotaxane-model compounds for polymers with mechanically interlocked components. Chem. Eur. J. 1996, 2, 31–44. [Google Scholar]
- James, F.; Callahan, J.F.; Ashton-Shue, D.; Bryan, H.G.; Bryan, W.M.; Heckman, G.D.; Kinter, L.B.; McDonald, J.E.; Moore, M.L.; Schmidt, D.B.; Silvestri, J.S.; Stassen, F.L.; Sulat, L.; Yim, N.C.F.; Huffman, W.F. Structure-activity relationships of novel vasopressin antagonists containing C-terminal diaminoalkanes and (aminoalkyl)guanidines. J. Med. Chem. 1989, 32, 391–396. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Späth, A.; König, B. Aza-27-crown-9 Amino Acid. Molbank 2010, 2010, M660. https://doi.org/10.3390/M660
Späth A, König B. Aza-27-crown-9 Amino Acid. Molbank. 2010; 2010(1):M660. https://doi.org/10.3390/M660
Chicago/Turabian StyleSpäth, Andreas, and Burkhard König. 2010. "Aza-27-crown-9 Amino Acid" Molbank 2010, no. 1: M660. https://doi.org/10.3390/M660
APA StyleSpäth, A., & König, B. (2010). Aza-27-crown-9 Amino Acid. Molbank, 2010(1), M660. https://doi.org/10.3390/M660