Dihydropyrimidinones (DHPMs) have attracted considerable interest due to the promising biological and pharmacological properties as calcium channel blockers, antihypertensive agents, and anticancer drugs associated with this heterocyclic scaffold.
1,2 Thus, the synthesis of such a heterocyclic nucleus is of considerable importance, and quite a number of synthetic modifications based on the Biginelli three-component condensation reaction of aldehyde, β-ketoester, and urea have been developed.
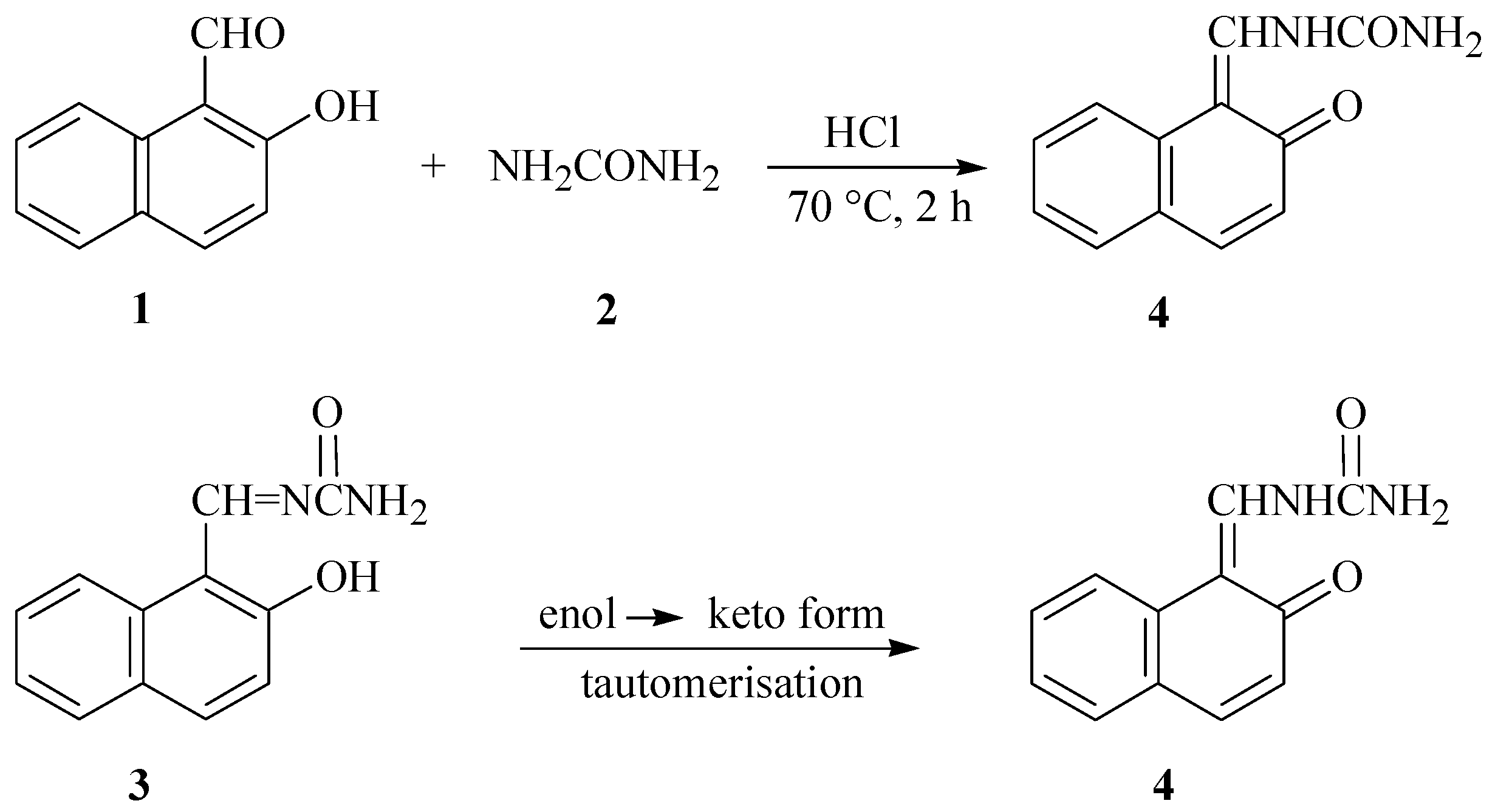
3In continuation of our green chemistry programme towards the synthesis of DHPM heterocyclic compounds, we found an unexpected product, namely 1-[(2-oxonaphthalen-1(2
H)-ylidene)methyl]urea
4 of the Biginelli reaction of 2-hydroxy-1-naphthaldehyde with ethyl benzoylacetate and urea under hydrochloric acid-catalyzed and solvent-free conditions (
Scheme 1).
In comparative experiments, reactions of 2-hydroxy-1-naphthaldehyde 1 and urea 2 with or without ethyl benzoylacetate under hydrochloric-acid-catalyzed and solvent or solvent-free conditions afforded 4 as the only product. With ethyl or methyl acetoacetate, however, 4 turned out to be a minor product besides the major product of the Biginelli reaction.
It may be rationalized that 4 is the tautomer of 3 that resulted from dehydration condensation reaction of 2-hydroxy-1-naphthaldehyde and urea.
This novel reaction may find application in the synthesis of naphthalenylmethylureas which may be bioactively interesting substances.
Experimental procedure
Method A
A mixture of 2-hydroxy-1-naphthaldehyde 1 (0.86 g, 5 mmol), ethyl benzoylacetate (1.06 g, 5.5 mmol), urea 2 (0.33 g, 5.5 mmol), and hydrochloric acid (2 drops) was heated at 70˚C for 2 h. Upon cooling, ethyl acetate-petroleum (1:1, v/v) was added and a yellow precipitate was obtained, then filtered and recrystallized from ethanol to afford the title compound 4 as yellow crystals, 0.65 g, yield 60.7%, m.p. 179.3-180.8 ˚C.
Structural Characterization
1H NMR (Bruker Avance 300 MHz, DMSO-d6): δH 13.22 (d, J = 10.5 Hz, 1 H, N-H), 9.01 (d, J = 10.5 Hz, 1 H, N-H), 7.97 (d, J = 8.2 Hz, 2 H), 7.81 (d, J = 9.6 Hz, 1 H, Ar-H-4), 7.63 (d, J = 7.6 Hz, 1 H), 7.50-6.58 (m, 3 H), 6.60 (d, J = 9.6 Hz, 1 H, Ar-H-3) ppm. 13C NMR (75 MHz, DMSO-d6): δC 184.0, 154.3, 147.1, 141.9, 134.5, 129.9, 129.6, 126.9, 126.7, 124.9, 119.9, 108.3 ppm. IR (Bruker Tensor 27, KBr): νmax 3426 (N-H), 1741 (C=O), 1620 (N-C=O), 1584 cm-1. MS (Agilent 5973N MSD, EI, 70 eV): m/z (%) 214 (M+, 44), 170 (100), 115 (50). Elemental Anal. (Perkin Elmer PE 2400 II HONS) calcd for C12H10N2O2: C 67.28, H 4.71, N 13.08; found: C 67.26, H 4.60, N 13.04.
Method B
A mixture of 2-hydroxy-1-naphthaldehyde 1 (0.86 g, 5 mmol), urea 2 (0.33 g, 5.5 mmol), and hydrochloric acid (2 drops) was heated at 100˚C for 1 h. Upon cooling, ethyl acetate-petroleum (1:1, v/v) was added and a yellow precipitate was obtained, then filtered and recrystallized from ethanol to give rise to 4 as yellow crystals, 0.49 g, yield 45.5%, m.p. 179.2-180.5 ˚C.
Moreover, other conditions such as solvent-free catalyzed with ammonium chloride and in ethanol catalyzed with hydrochloric acid have also been investigated and 4 was also obtained.