Introduction
Pyrazolone ring systems represent an important class of compounds [
1] not only for their theoretical interest but also for their number of application in diverse area [
2,
3,
4,
5,
6]. 1-Phenyl pyrazolone derivatives have interesting pharmacological properties as analgesic [
7] antipyretic [
8] and antiinflamatory agents [
9]. The classes of chelating extractants which have received the most attention in recent years have the basic structure of pyrazole. Due to the above reasons we have take interest to synthesize this class of ligands [
10], and take interest of their spectroscopic as well as theoretical study. The electronic structure of the Schiff base ligand (III) was optimized using 6-311G basis set at HF level
ab-initio studies. The attempt was made to predict the coordinating atoms of the ligand. The electron correlation at MP2 level was also performed.
Experiment Section
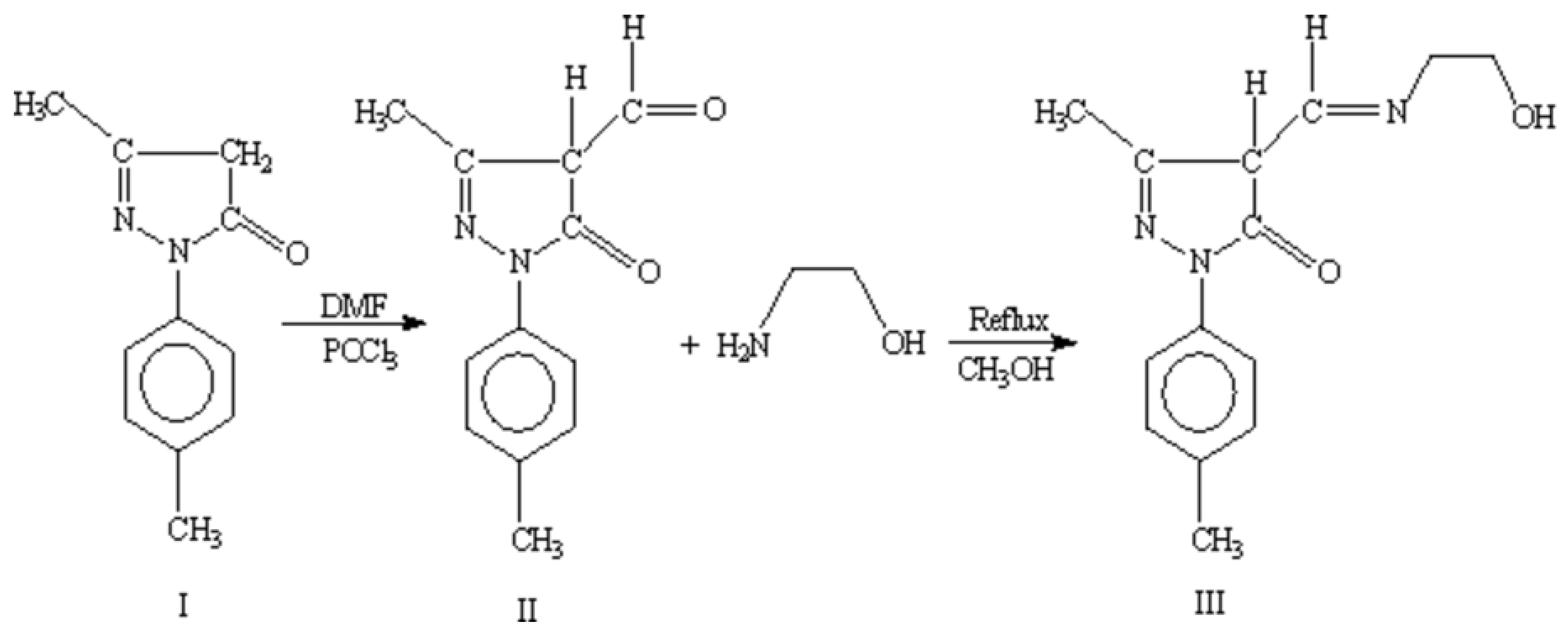
5-hydroxy-3-methyl-1-
p-tolyl-1
H-pyrazole-4-carbaldehyde(II) was prepared by condensation of 5-methyl-2-
p-tolyl-2
H-pyrazol-3-ol (I) with DMF and POCl
3 [
11]. 5-methyl-2-
p-tolyl-2
H-pyrazol-3-ol (I) (9.4 g, 0.05 mol) in DMF (10 cm
3, 0.05 mol) was cooled to 0 to 10°C in an ice bath. To this phosphoryl chloride (5.5 cm
3, 0.06 mol) was added dropwise at a rate to maintain the temperature between 10 and 20°C. After the addition was complete, the reaction mixture was heated on a steam bath for 2.5 hours then poured into 1L of ice water. The resulting mixture was allowed to stand overnight and product was collected by filtration, washed with water, and dried.
4-[(2-Hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol- 3-one(III) Schiff base was synthesized by refluxing methenolic solutions of ethanolamine (0.61 g, 0.01 mol) in 10 cm3 with 5-hydroxy-3-methyl-1-p-tolyl-1H-pyrazole-4-carbaldehyde (II) (2.16 g, 0.01 mol) in 20 cm3 methenol. A solid mass separated, was collected and washed by ether. Recrystallization was done with ethanol and the dried over CaCl2.
Results and Discussion
Schiff base ligand 4-[(2-hydroxy-ethylamino)-methylene]-5-methyl-2-
p-tolyl-2,4-dihydro-pyrazol-3-one (III) characterized by the spectroscopic data, as shown below. Schiff base ligand (III) shows tautomeric form imine-ol and amine-one, (
Figure 1) these forms are active in solution and in solid state respectively [
12]. Also the attempt to carried out the
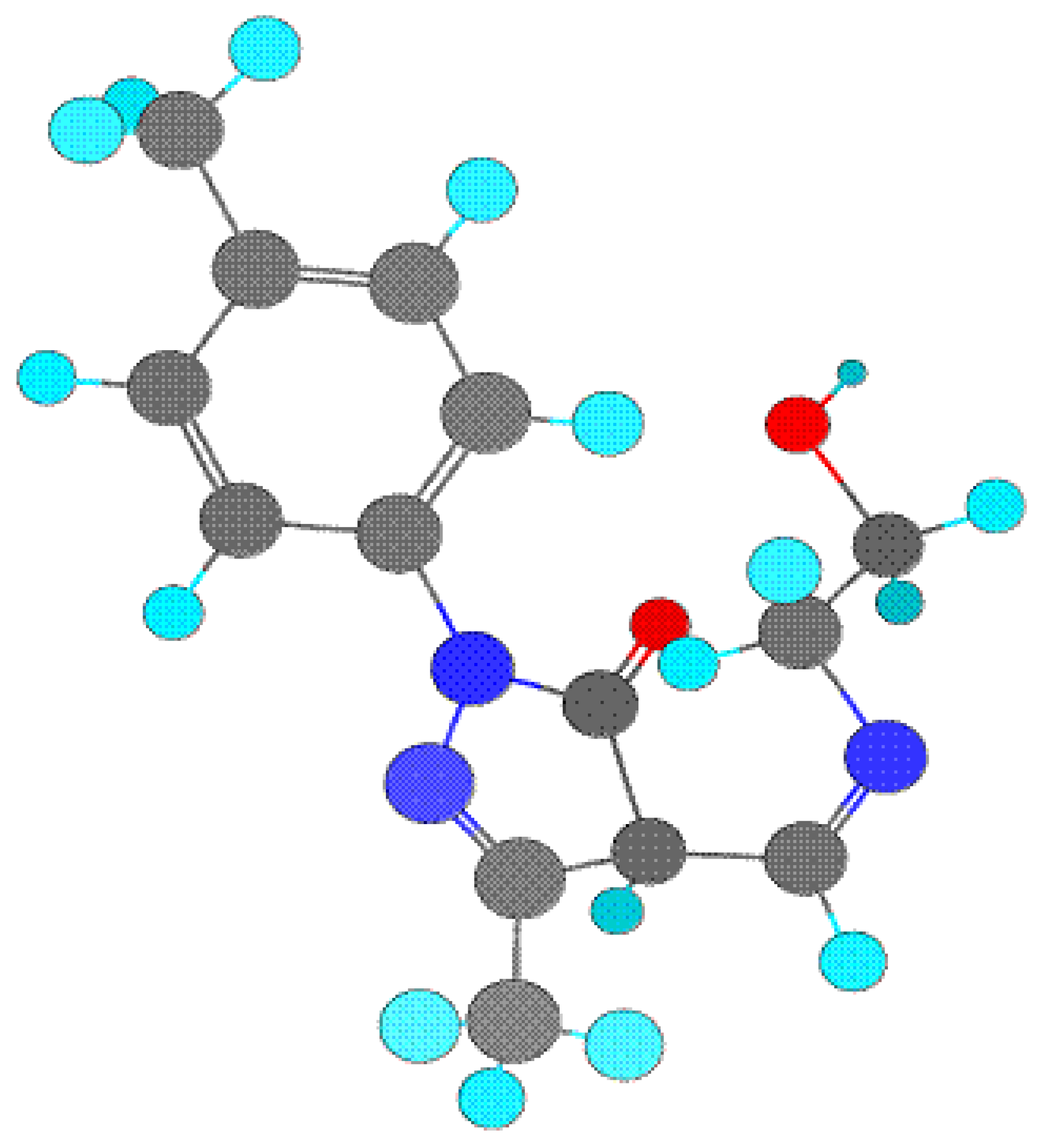
ab-initio study of this ligand, the full geometry optimization of schiff base ligand (III) has been carried out in cartesian coordinates by quasi-Newton-Raphson gradient method using the restricted Hartree-Fock (RHF) approximation with help of Gamess program package [
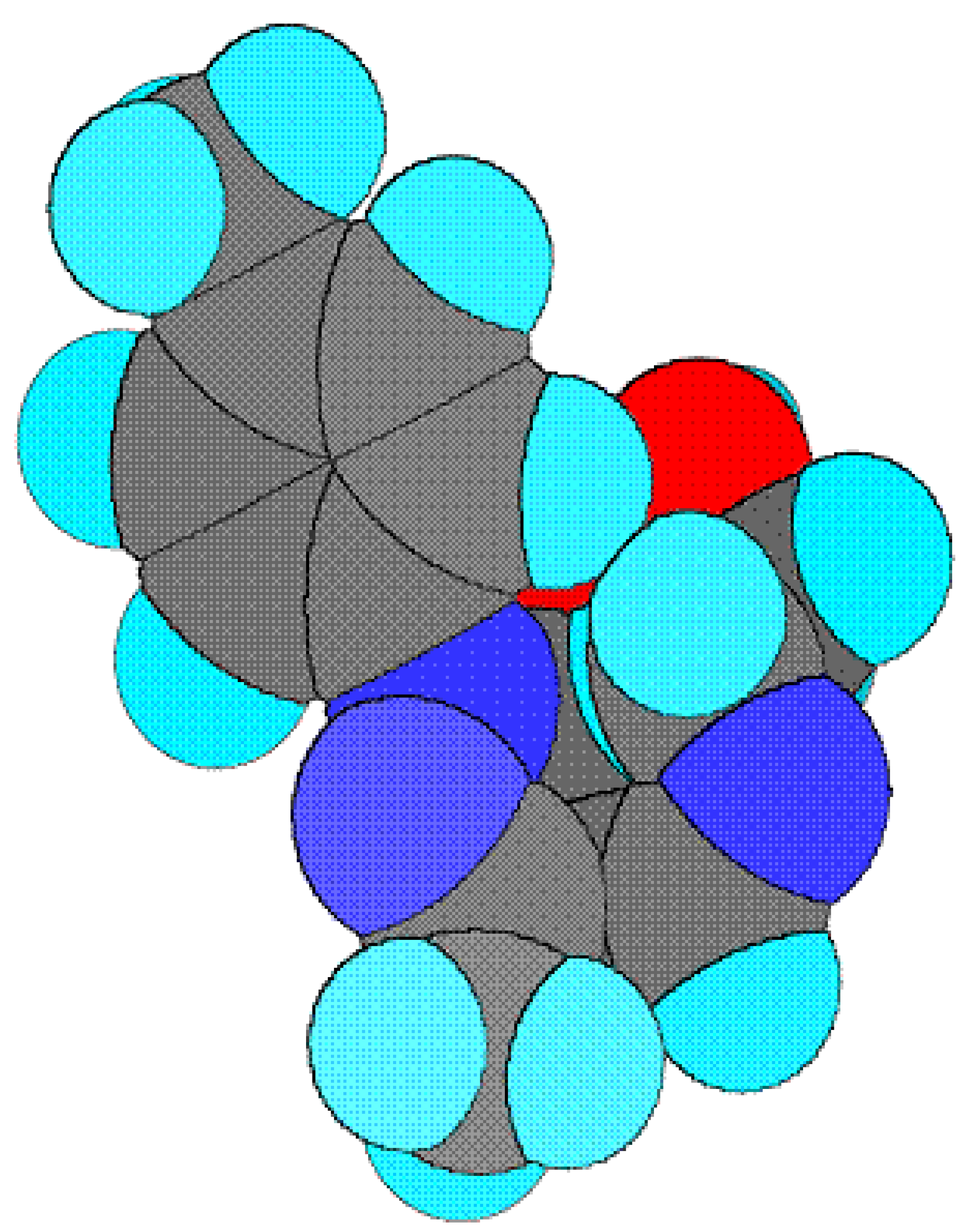
13]. The present compound contains too many hetero atoms. Thus it is interesting to know that out of these which one are the coordinating atoms. The lowdin atomic charges on N (schiff base N) and O (enolic O of pyrazoline) are -0.243106 and -0.435565, respectively, which are highest negative charges among all hetero atoms suggesting that these atoms are coordinating to metals. The lowdin charge on O (O of ethanolamine) is -0.416095 which is comparable with O but the stereochemistry of this oxygen is such that it is away from the bite site and hence coordination through this oxygen is not possible. The bond-stick and space filling diagram of schiff base ligand shown in
figure 2 and
3 respectively.
5-Hydroxy-3-methyl-1-p-tolyl-1H-pyrazole-4-carbaldehyde (II)
Yellow crystal obtained, yield 76%
Melting point: 184 °C
FT-IR (KBr) υmax(cm-1): 3400-3300m (OHhydrogen bonded), 2809-2728vs (C–Haldehydic , doublet), 1674s (C=O), 1598vw (C=Ncyclic ).
1H-NMR (300 MHz, CDCl3): δ= 9.65 (s, 1H, CHO), 8.45 (s (enolic), 1H, OH), 7.2-7.7 (m, 4H, Ph), 2.2 (s, 3H, ph-CH3), 1.2 (s, 3H, CH3).
13C-NMR (75 MHz, CDCl3): δ= 17.4, 20.9 (CH3); 120.3, 129.4, 133.3, 137.8 (benzene ring) ; 60.8, 155.6, (heterocyclic ring), 169.89 (C=O), 199.5 (H –C=O) aldehyic
MS (LC-MS): (Calcd. for m/z [M]+ 216 ) 217 [M+1]+
Elemental analysis: Calculated for C12H12N2O2: C 66.3; H 5.6; N 12.9; found: C 66.1; H 5.3; N 12.9%.
4-[(2-hydroxy-ethylamino)-methylene]-5-methyl-2-p-tolyl-2,4-dihydro-pyrazol-3-one (III)
Brown crystal obtained, yield 82%
Melting point: 157 °C
FT-IR (KBr) υmax(cm-1): 3294m (O–H), 2946-2873m (C–H), 1646 (C=N), 1676s (C=O), 1598vs (C=Ncyclic).
1H-NMR (300 MHz, CDCl3): δ= 9.73 (s, 1H, –OHenol); 7.93 (s, 1H, –HC=N) ; 7.87 (m, 2H J = 8.4, ortho- to pyrazoline ring); 7.34 (d, 2H J = 8.4, m- to pyrazoline ring); 5.12 (s, 1H, –OH); 3.56 (t, 2H J = 4.8, NCH2); 3.48 (t, 2H J = 4.8, CH2O); 2.32 (s, 3H, CH3); 1.23 (s ,3H, CH3).
13C-NMR (75 MHz, CDCl3): δ= 17.3, 20.9 (CH3); 56.0, 64.2 (CH2); 120.3, 129.4, 133.3, 137.8 (benzene ring) ; 43.6, 155.6, (heterocyclic ring), 169.89 (C=O).
Elemental analysis: Calculated for C14H17N3O2: C 64.85; H 6.6; N 16.21 Found: C 64.9; H 6.3; N 16.2%.
MS (LC-MS): (Calcd. for m/z [M]+ 259.13) 260 [M+1]+