Antiplatelet Effect and Selective Binding to Cyclooxygenase (COX) by Molecular Docking Analysis of Flavonoids and Lignans
Abstract
:1. Introduction
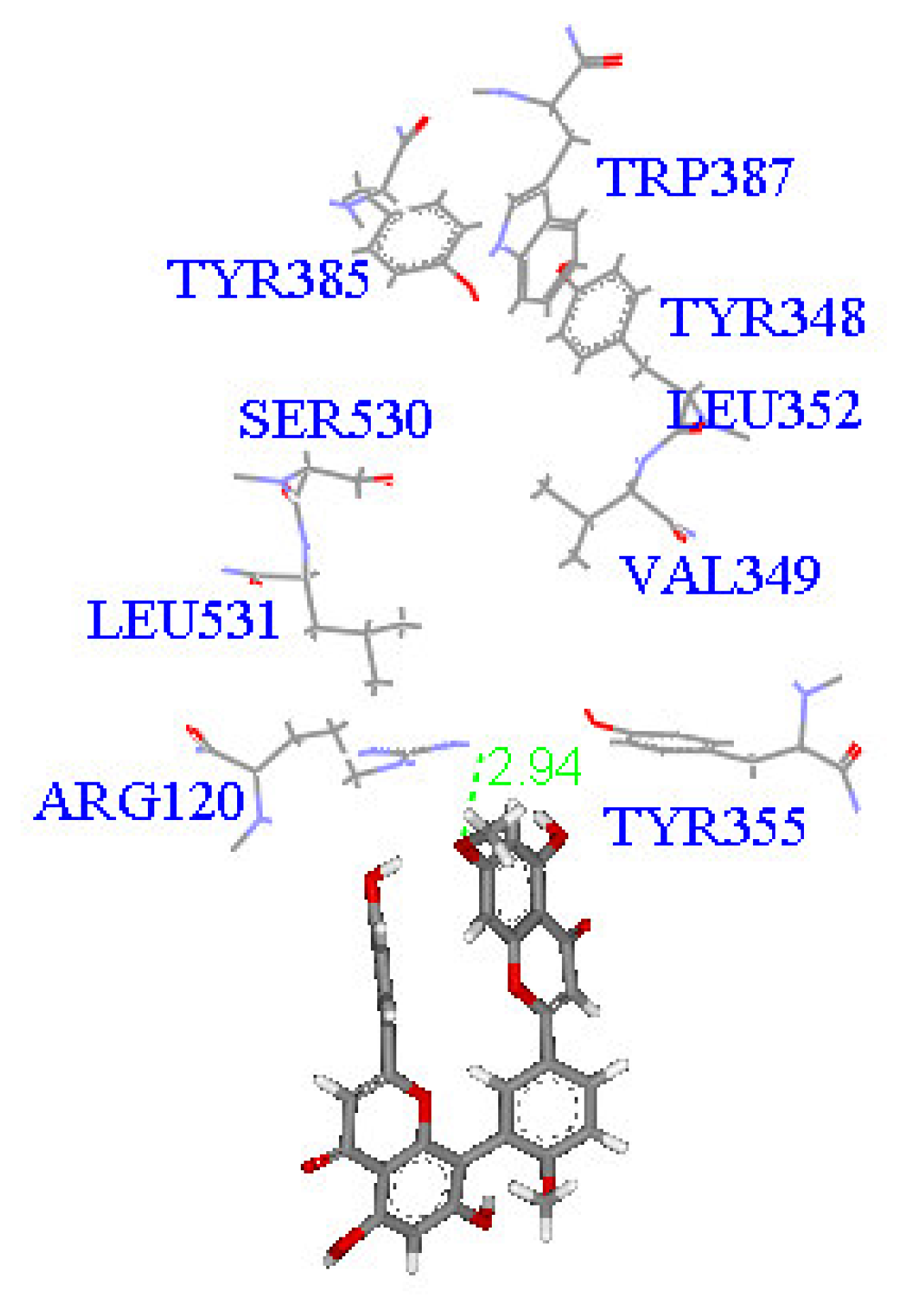
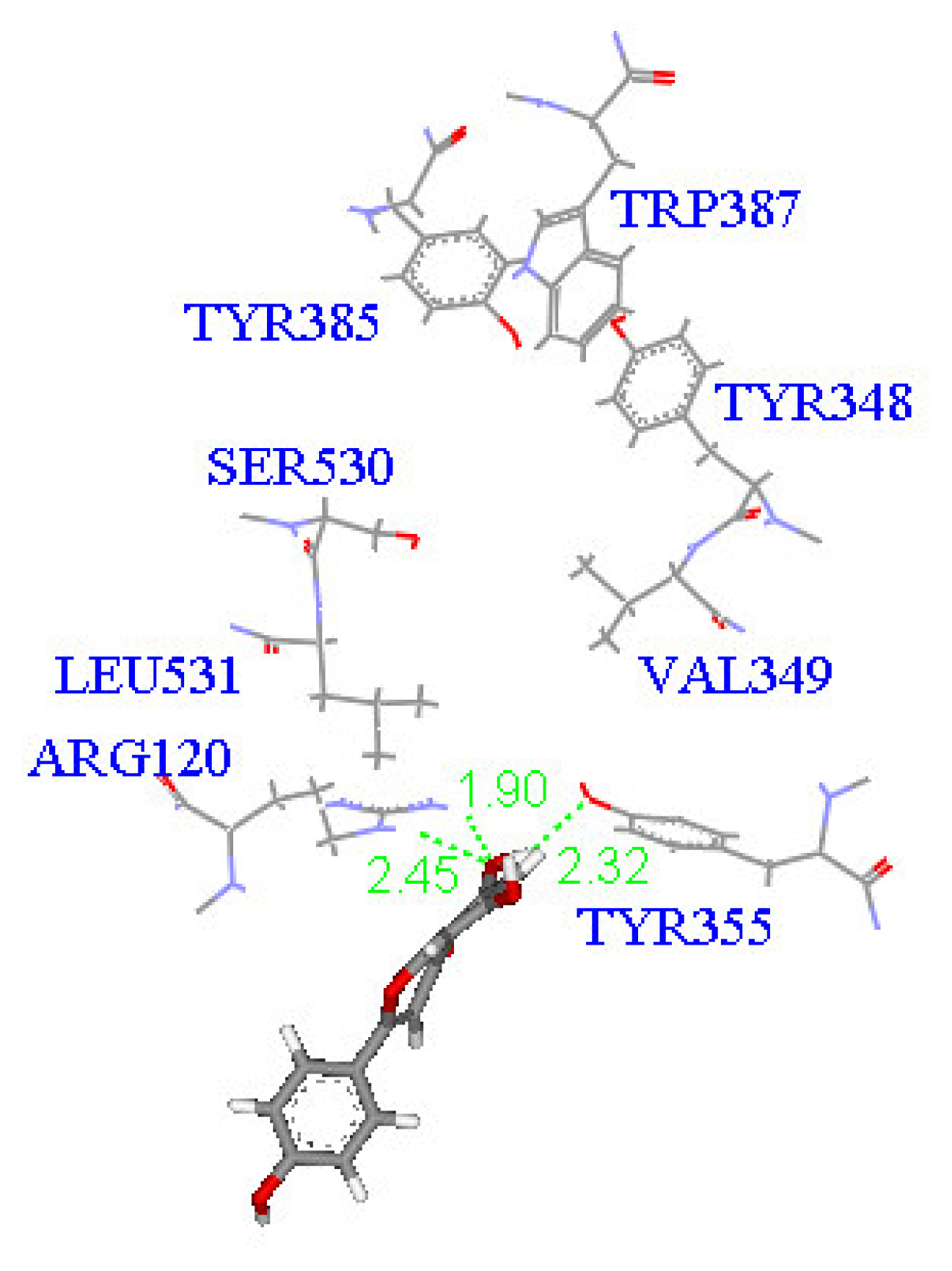
2. Results and Discussion
Conclusions
3. Experimental Section
General
Extraction and Isolation
Platelet aggregation
COX-1 activity
Molecular docking study
Data analysis
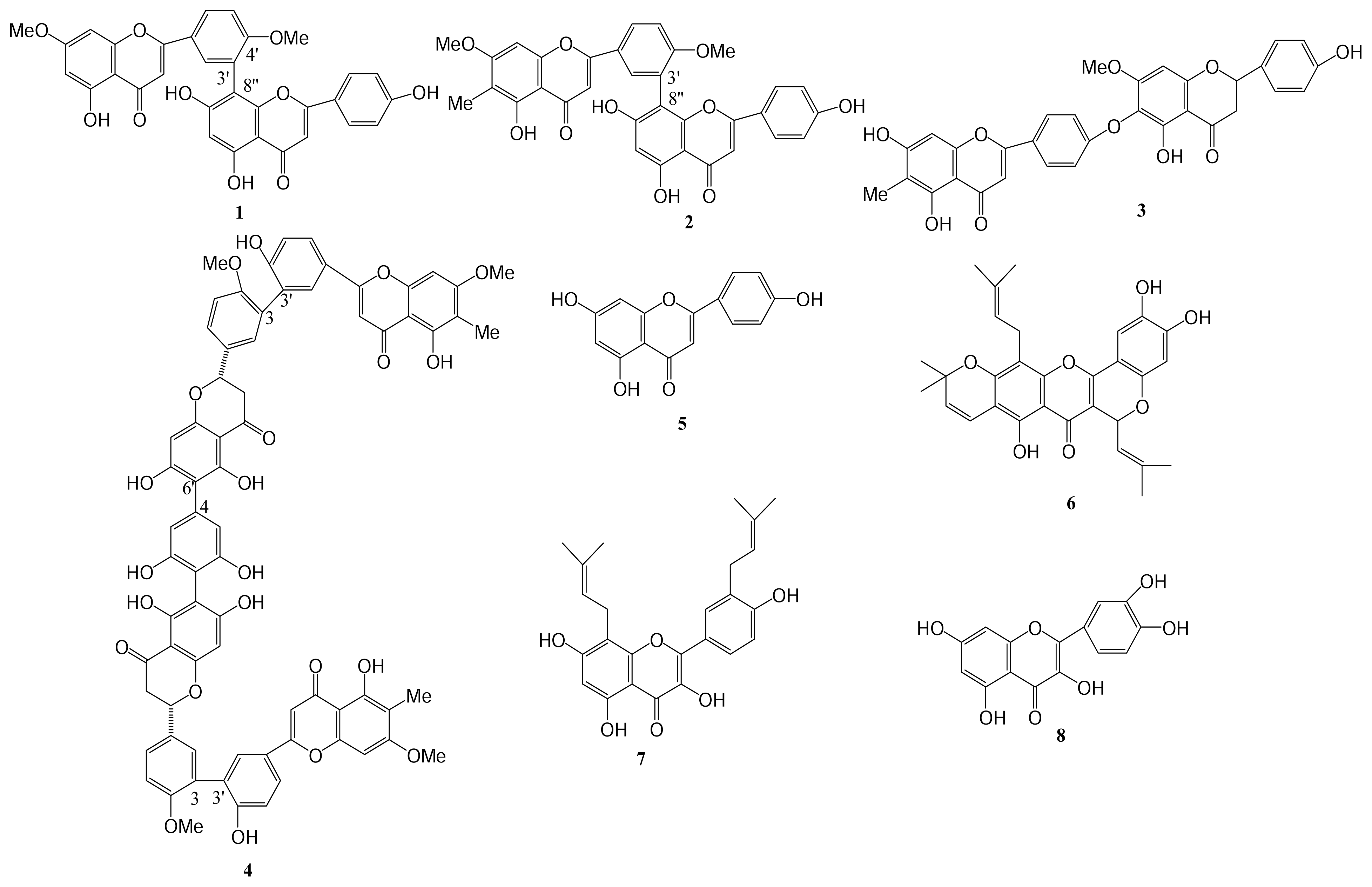
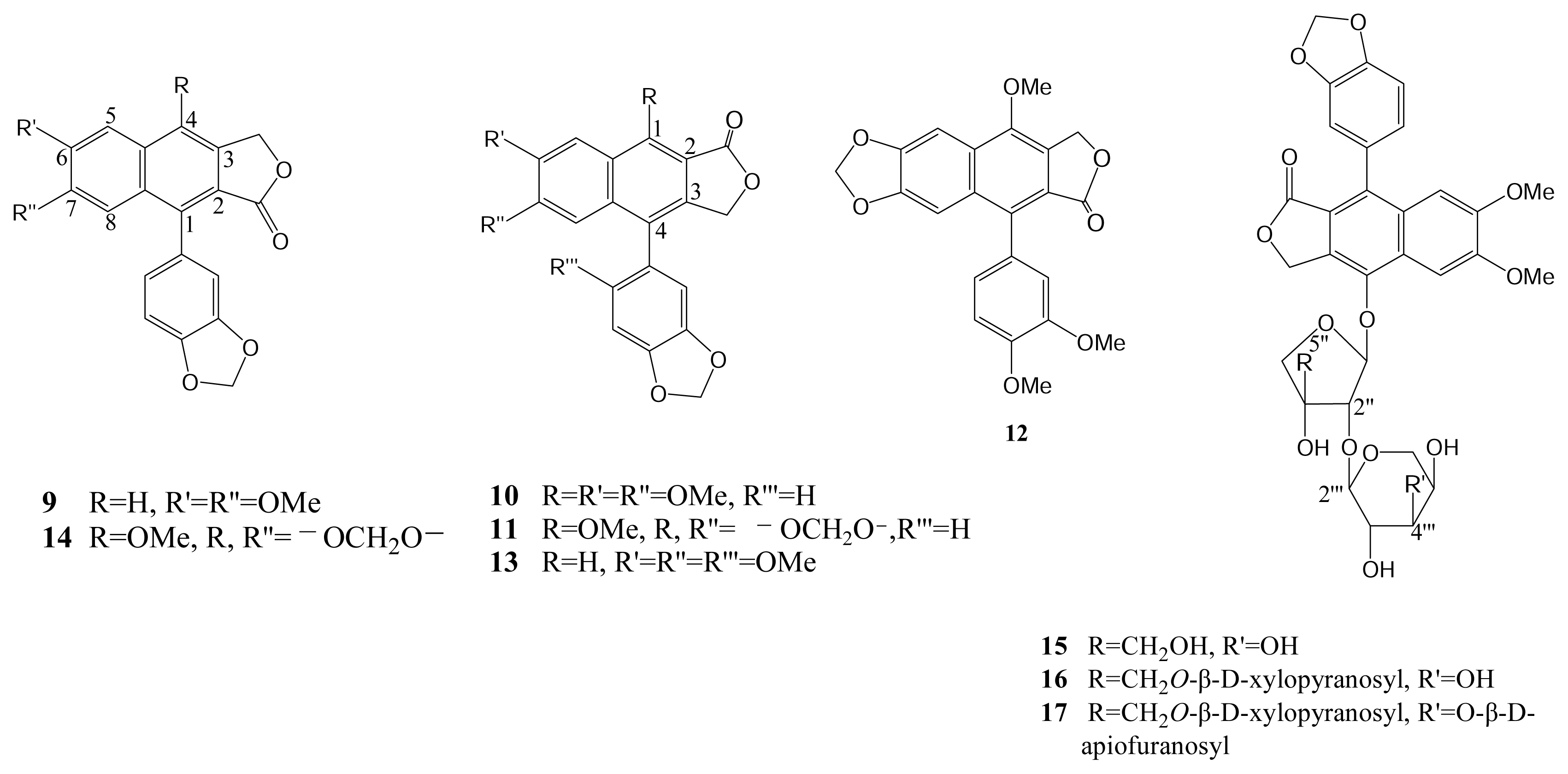
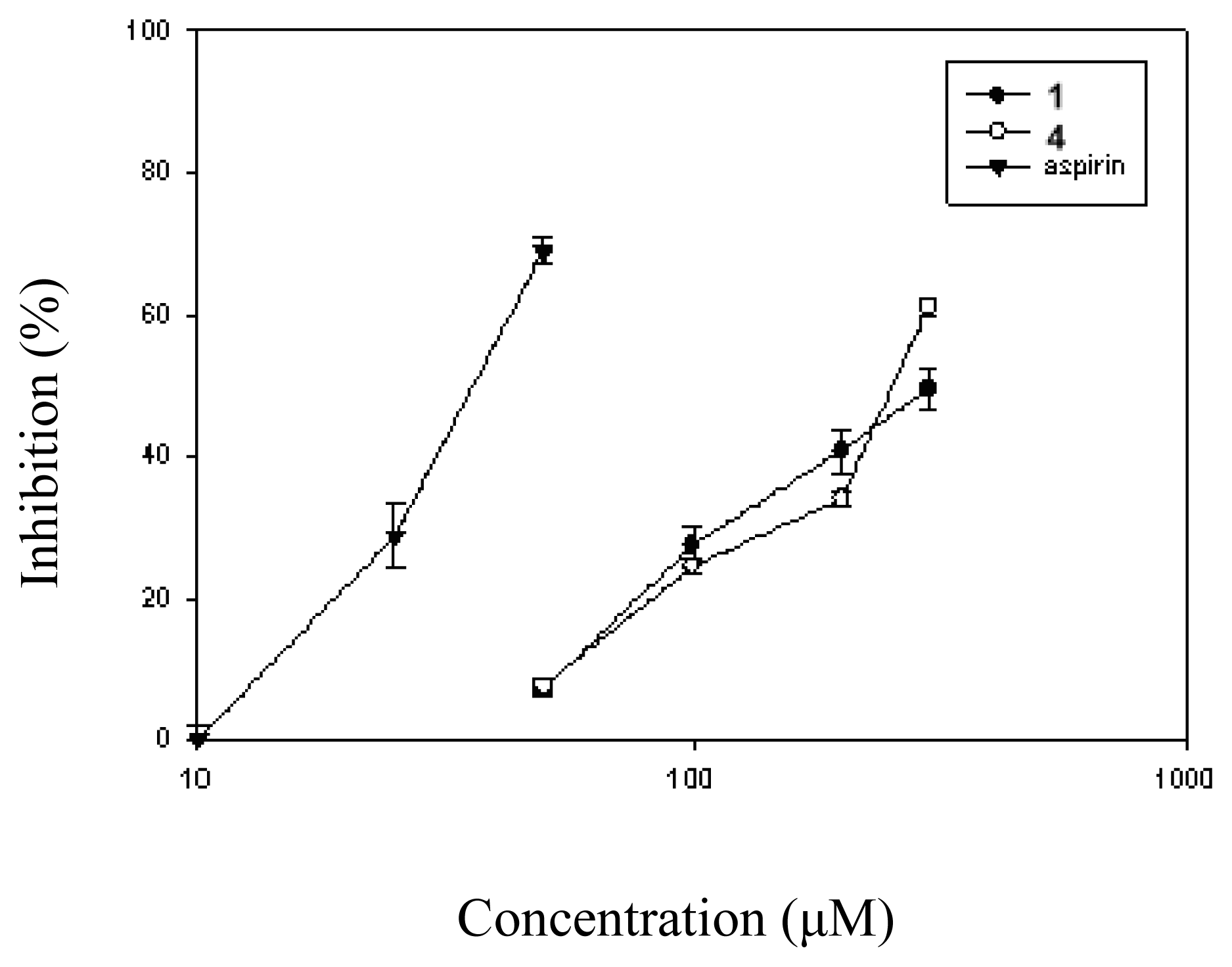
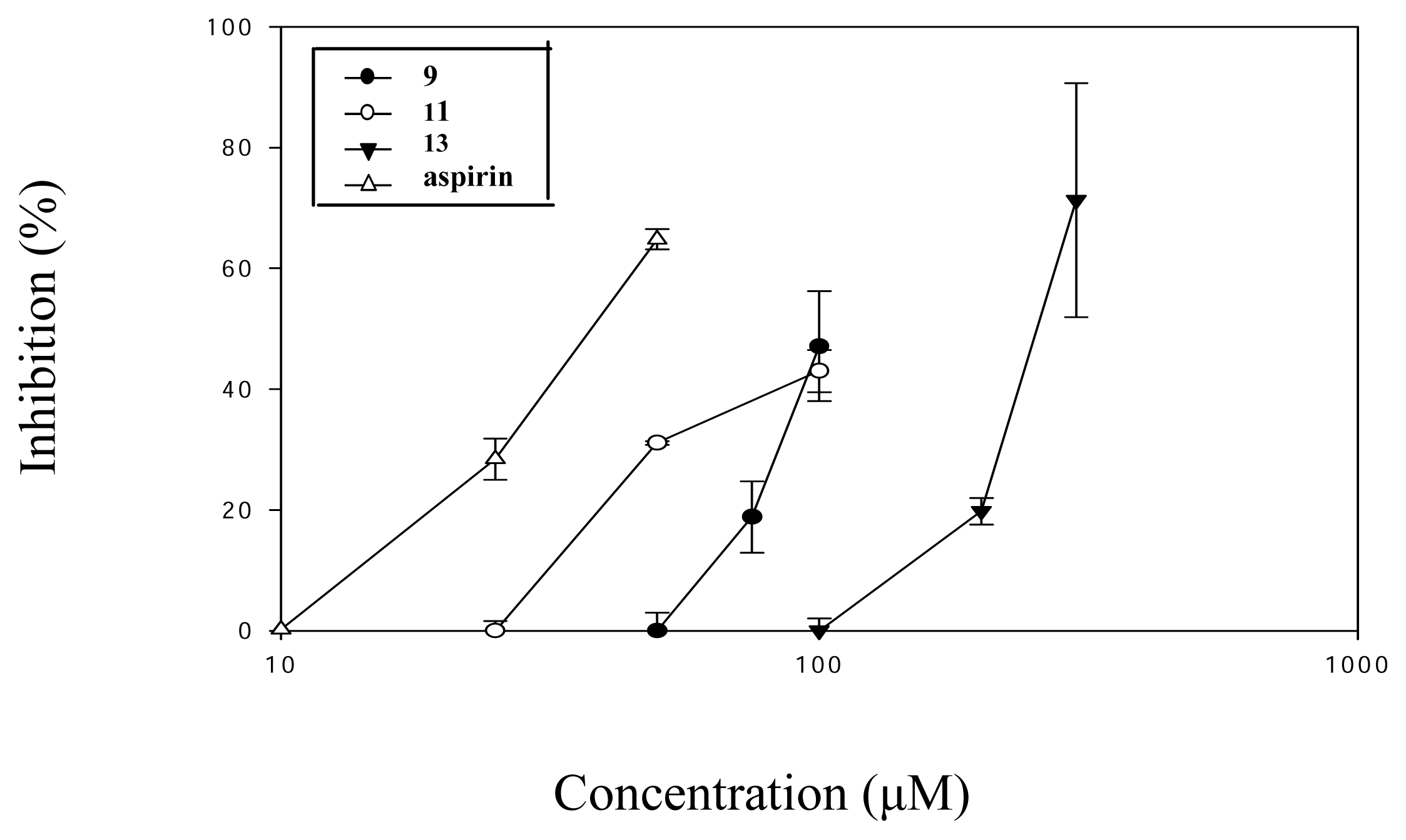
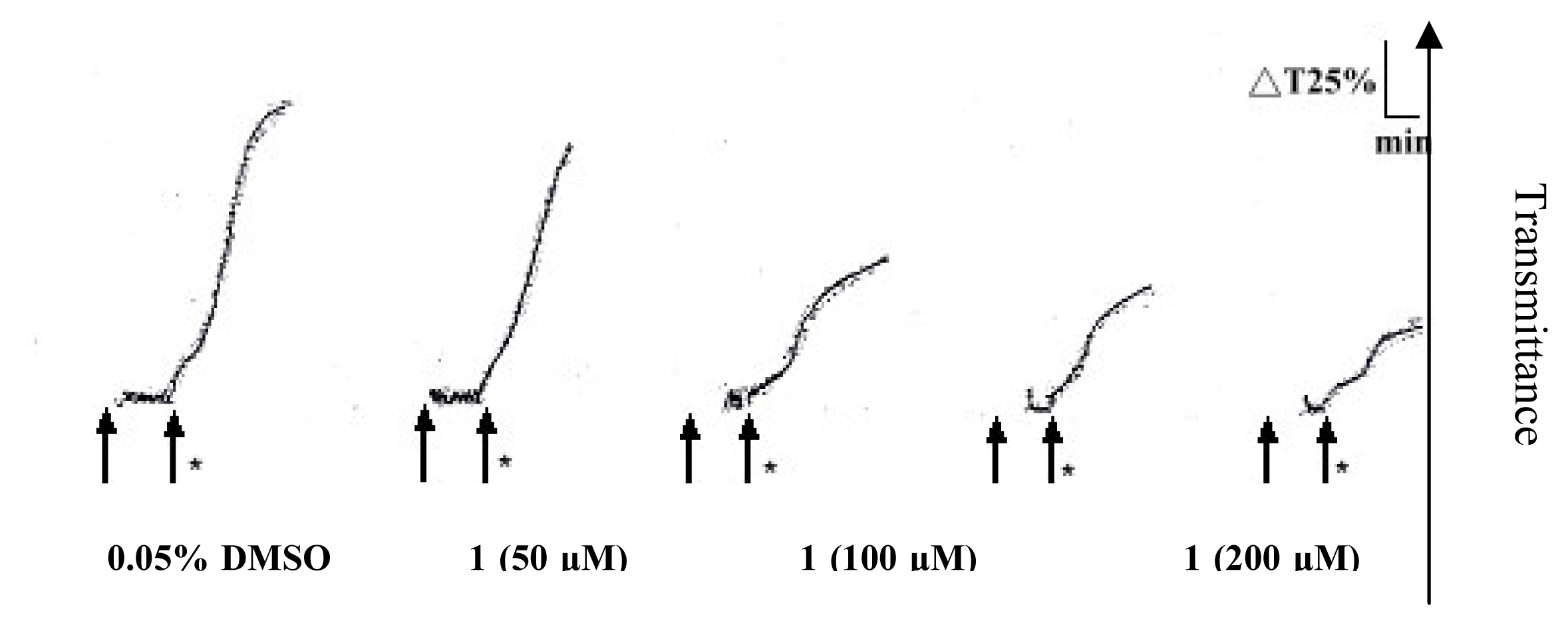
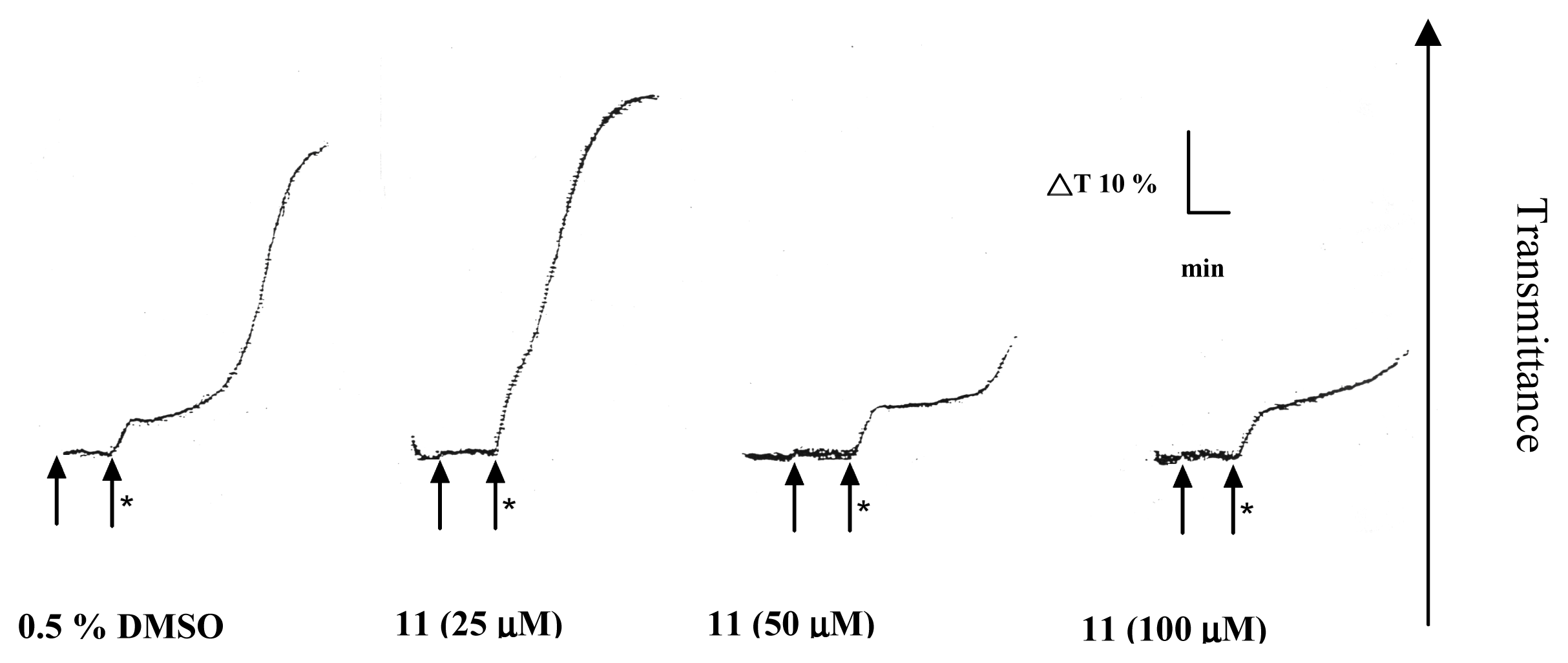
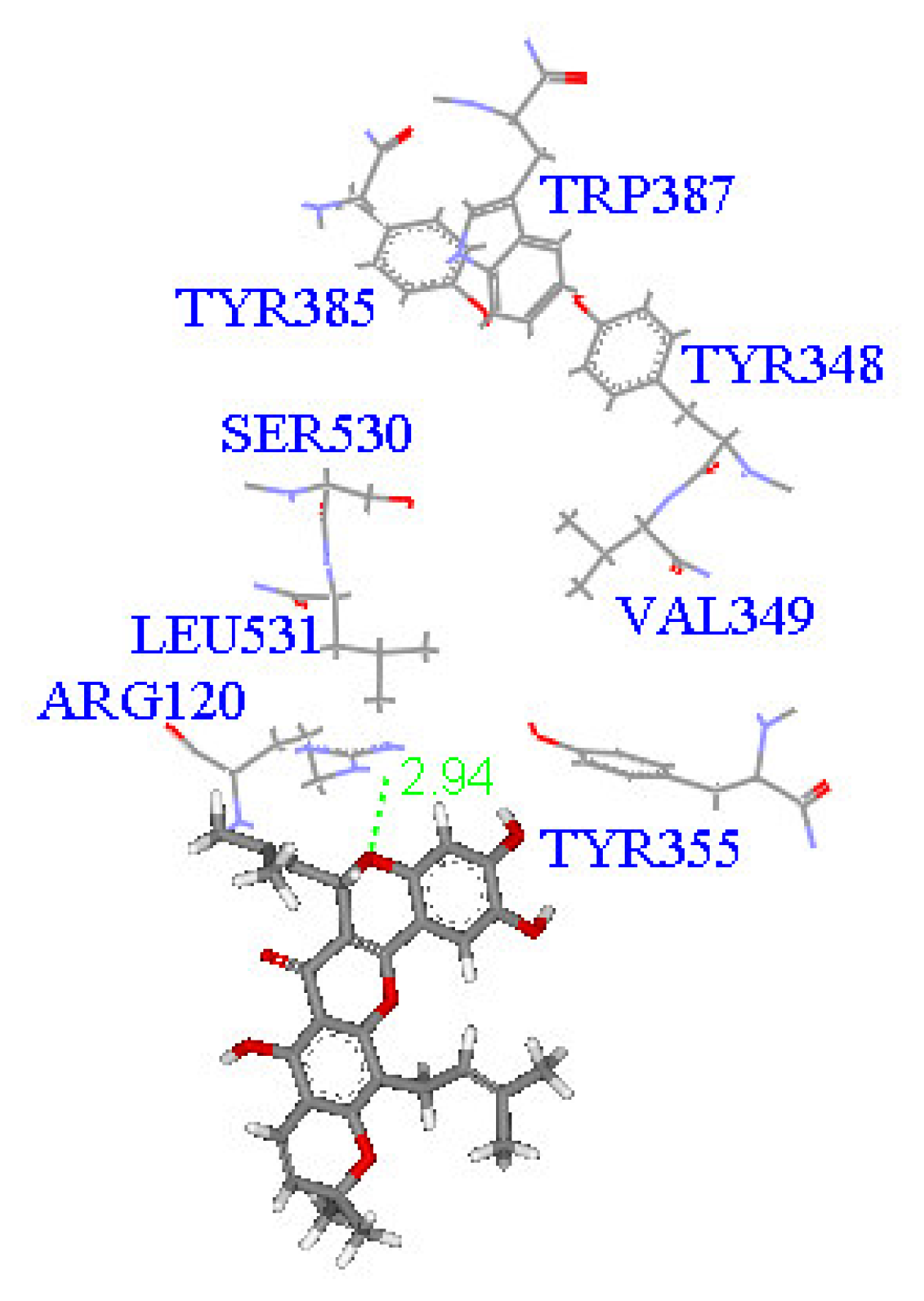
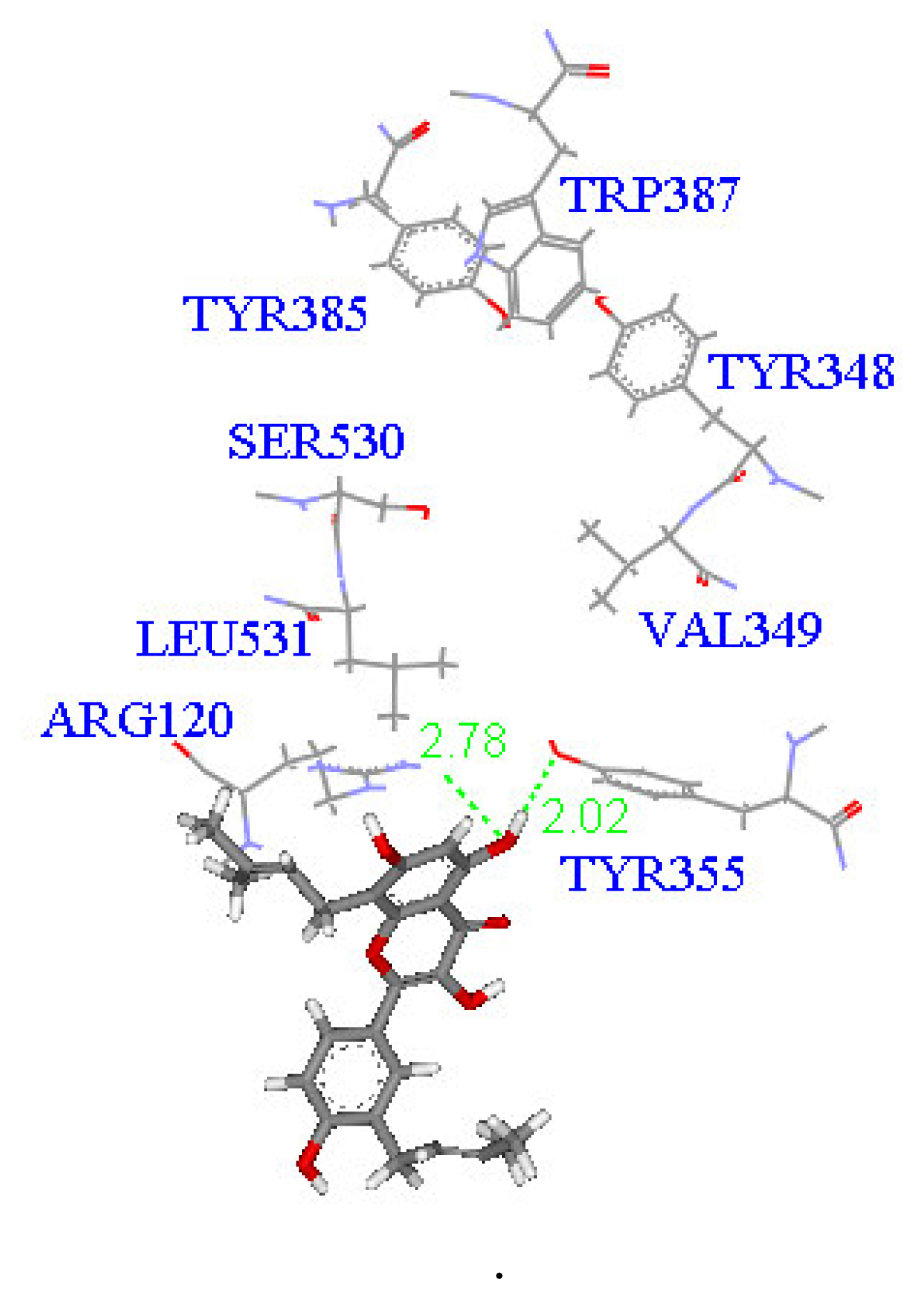
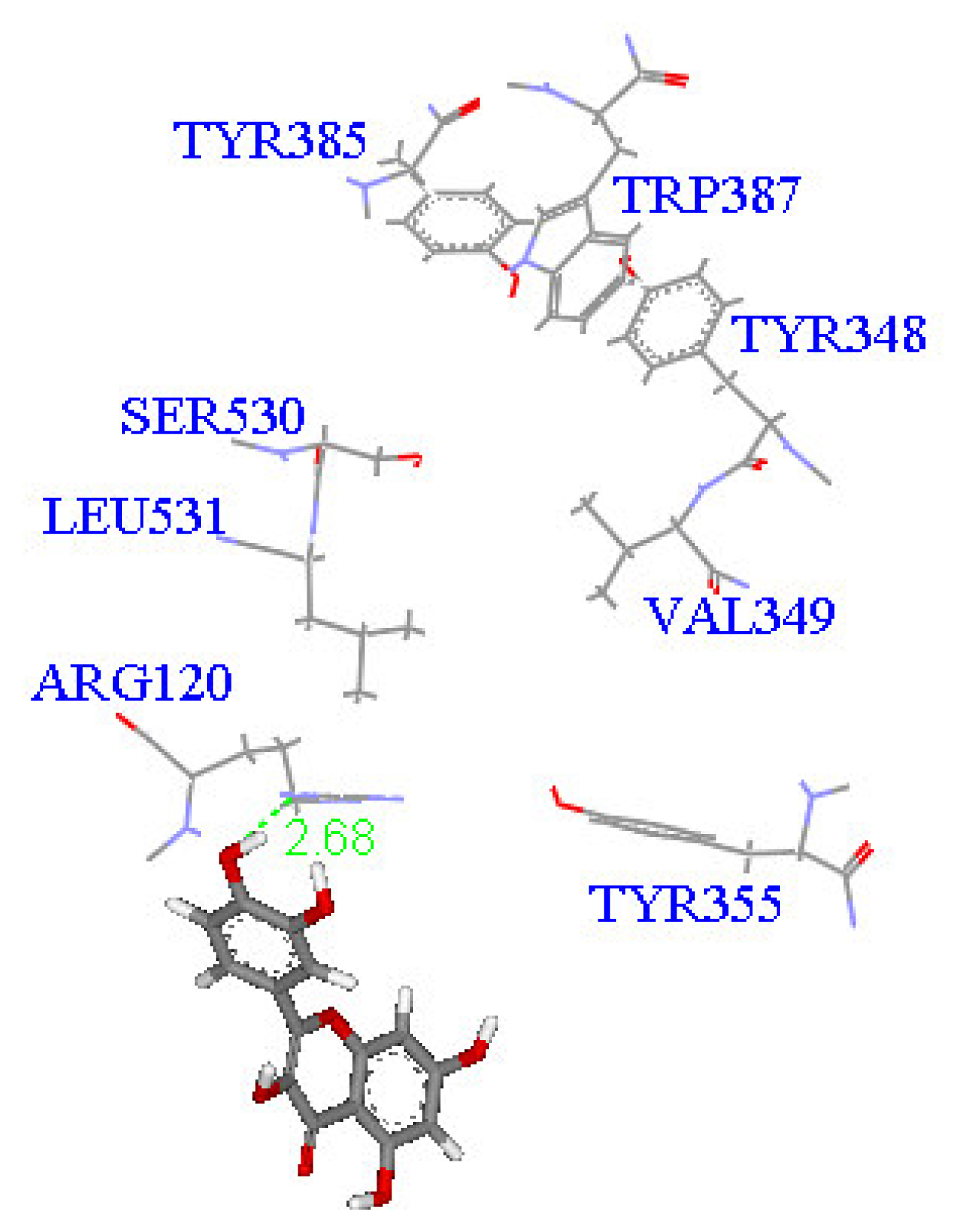
| Compound (μM) | Aggregation (%) |
|---|---|
| Control | 98.7 ± 0.3 |
| 1 (300) | 49.9 ± 3.0* |
| 2 (300) | 97.4 ± 0.8 |
| 3 (300) | 98.8 ± 1.6 |
| 4 (300) | 38.5 ± 1.2* |
| 9 (100) | 50.9 ± 5.3*** |
| 10 (100) | 74.7 ± 2.2** |
| 11 (100) | 54.9 ± 2.0*** |
| 13 (300) | 27.7 ± 11.0** |
| Aspirin (50) | 29.5 ± 1.0* |
| Compounds (μM) | COX-1 activity nmol/min (% inhibition) | IC50 (μM) |
|---|---|---|
| Control | 10.9 ± 1.1 | |
| 1 (10) | 8.5 ± 1.4 (25.1 ± 6.9) | |
| (30) | 7.6 ± 1.7 (33.5 ± 7.5)* | > 100 |
| (100) | 6.6 ± 1.3 (42.0 ± 6.4)* | |
| 5a | > 100 | |
| 6b | 52.0 | |
| 7b | 34.6 | |
| 8a | 8.0 | |
| 14 (10) | 9.9 ± 1.6 (12.9 ± 8.1) | > 100 |
| (100) | 10.2 ± 1.0 (9.9 ± 5.3) | |
| Indomethacin (0.3) | 9.1 ± 1.1 (20.6 ± 6.2) | |
| (1) | 5.6 ± 1.0 (49.1 ± 6.4)** | 14 ± 0.2 |
| (3) | 3.8 ± 0.7 (61.9 ± 5.2)** |
| Compound | DockScore | Van der Waals force (kcal/mol) | Interaction with following amino acids near the gate of active site of COX-1 |
|---|---|---|---|
| 1 | 59.13 | −32.91 | Arg120 |
| 5 | 50.65 | −16.67 | Arg120a, Tyr355 |
| 6 | 53.93 | −33.85 | Arg120 |
| 7 | 63.17 | −34.14 | Arg120, Tyr355 |
| 8 | 60.24 | −2.01 | Arg120 |
Acknowledgements
References and Notes
- Wang, L. W.; Su, H. J.; Yang, S. Z.; Won, S. J.; Lin, C. N. New alkaloids and a tetraflavonoid from Cephalotaxus wilsoniana. J. Nat. Prod 2004, 67, 1182–1185. [Google Scholar]
- Day, S. H.; Chiu, N. Y.; Won, S. J.; Lin, C. N. Cytotoxic lignans of Justicia ciliata. J. Nat. Prod 1999, 62, 1033–1035. [Google Scholar]
- Day, S. H.; Chiu, N. Y.; Tsao, L. T.; Wang, J. P.; Lin, C. N. New lignan glycosides with potent anti-inflammatory effect, isolated from Justicia ciliata. J. Nat. Prod 2000, 63, 1560–1562. [Google Scholar]
- Day, S. H.; Lin, Y. C.; Tsao, M. L.; Tsao, L. T.; Ko, H. H.; Chung, M. I.; Lee, J. C.; Wang, J. P.; Won, S. J.; Lin, C. N. Potent cytotoxic lignans from Justicia procumbens and their effects on nitric oxide and tumor necrosis factor-α production in mouse macrophages. J. Nat. Prod 2002, 65, 379–381. [Google Scholar]
- Weng, J. R.; Ko, H. H.; Yeh, T. L.; Lin, C. H.; Lin, C. N. Two new arylnaphthalide lignans and antiplatelet constituents from Justicia procumbens. Arch. Pharm. Pharm. Med. Chem 2004, 337, 207–212. [Google Scholar]
- Chen, C. C.; Hsin, W. C.; Ko, F. N.; Huang, Y. L.; Ou, J. C.; Teng, C. M. Antiplatelet arylnaphthalide lignans from Justicia procumbens. J. Nat. Prod 1996, 59, 1149–1150. [Google Scholar]
- Ko, H. H.; Hsieh, H. K.; Liu, C. T.; Lin, C. H.; Teng, C. M.; Lin, C. N. Structure-activity relationship studies on chalcone derivatives: The potent inhibition of platelet aggregation. J. Pharm. Pharmacol 2004, 56, 1333–1337. [Google Scholar]
- Mitchell, J. R. A.; Sharp, A. A. Platelet clumping in vitro. Br. J. Haematol 1964, 10, 78–93. [Google Scholar]
- Mustard, J. F.; Perry, D. W.; Kinlough-Rathbone, R. L.; Pockham, M. A. Factors responsible for ADP-induced release reaction of human platelets. Am. J. Physiol 1975, 228, 1757–1765. [Google Scholar]
- Lin, C. N.; Lu, C. M.; Lin, H. C.; Fang, S. C.; Shieh, B. J.; Hsu, M. F.; Wang, J. P.; Ko, F. N.; Teng, C. M. Novel antiplatelet constituents from Formosan Moraceous plants. J. Nat. Prod 1996, 59, 834–838. [Google Scholar]
- Lin, C. N.; Shieh, W. L.; Ko, F. N.; Teng, C. M. Antiplatelet effect of prenylflavonoids. Biochem. Pharmacol 1993, 45, 509–512. [Google Scholar]
- Kalgutkar, A. S.; Crews, B. C.; Rowlison, C. G.; Seibest, K.; Marnett, L. J. Aspirin-like molecules that covalently inactive cyclooxygenase-2. Science 1998, 280, 1268–1270. [Google Scholar]
- Chi, Y. S.; Jong, H. G.; Son, K. H.; Chang, H. W.; Kang, S. S.; Kim, H. P. Effect of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid : cyclooxygenases and lipoxygenases. Biochem. Pharmacol 2001, 62, 1185–1191. [Google Scholar]
- DS Modeling 1.2-SBD; Accelrys Inc: 9685 Scranton Rd. San Diego, CA.
- Ohta, K.; Munaka, K. Justicidin C and D, the 1-methoxy-2,3-naphthalide lignans, isolated from Justicia procumbens L. Tetrahedron Lett 1970, 12, 923–925. [Google Scholar]
- Okigawa, M.; Maeda, T.; Kawano, N. The isolation and structure of three new lignans from Justicia procumbens Linn. var. leucantha Honda. Tetrahedron 1970, 26, 4301–4305. [Google Scholar]
- Hsu, M. F.; Lu, C. M.; Tsao, L. T.; Kuan, Y. H.; Chen, C. C.; Wang, J. P. Mechanisms of the influence of magnolol on eicosanoid metabolism in neutrophils. Biochem. Pharmacol 2004, 67, 831–840. [Google Scholar]
- Wiglenda, T.; Ott, I.; Kircher, B.; Schumacher, P.; Schuster, D.; Langer, T.; Gust, R. Synthesis and pharmacological evaluation of 1H-imidazoles as ligands for the estrogen receptor and cytotoxic inhibitors of cyclooxygenase. J. Med. Chem 2005, 48, 6516–6521. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Wu, C.-M.; Wu, S.-C.; Chung, W.-J.; Lin, H.-C.; Chen, K.-T.; Chen, Y.-C.; Hsu, M.-F.; Yang, J.-M.; Wang, J.-P.; Lin, C.-N. Antiplatelet Effect and Selective Binding to Cyclooxygenase (COX) by Molecular Docking Analysis of Flavonoids and Lignans. Int. J. Mol. Sci. 2007, 8, 830-841. https://doi.org/10.3390/i8080830
Wu C-M, Wu S-C, Chung W-J, Lin H-C, Chen K-T, Chen Y-C, Hsu M-F, Yang J-M, Wang J-P, Lin C-N. Antiplatelet Effect and Selective Binding to Cyclooxygenase (COX) by Molecular Docking Analysis of Flavonoids and Lignans. International Journal of Molecular Sciences. 2007; 8(8):830-841. https://doi.org/10.3390/i8080830
Chicago/Turabian StyleWu, Chien-Ming, Shu-Chun Wu, Wan-Jung Chung, Hsien-Cheng Lin, Kun-Tze Chen, Yu-Chian Chen, Mei-Feng Hsu, Jwu-Maw Yang, Jih-Pyang Wang, and Chun-Nan Lin. 2007. "Antiplatelet Effect and Selective Binding to Cyclooxygenase (COX) by Molecular Docking Analysis of Flavonoids and Lignans" International Journal of Molecular Sciences 8, no. 8: 830-841. https://doi.org/10.3390/i8080830




