Crystal Engineering Based on Polymeric Hydrogen-Bonded Supramolecules by Self-Assembling of 9, 10-Bis(3,5- dihydroxyphenyl)anthracene and 2,2′,4,4′- Tetrahydroxybenzophenone with Bipyridines
Abstract
:1. Introduction
2. Results and Discussion
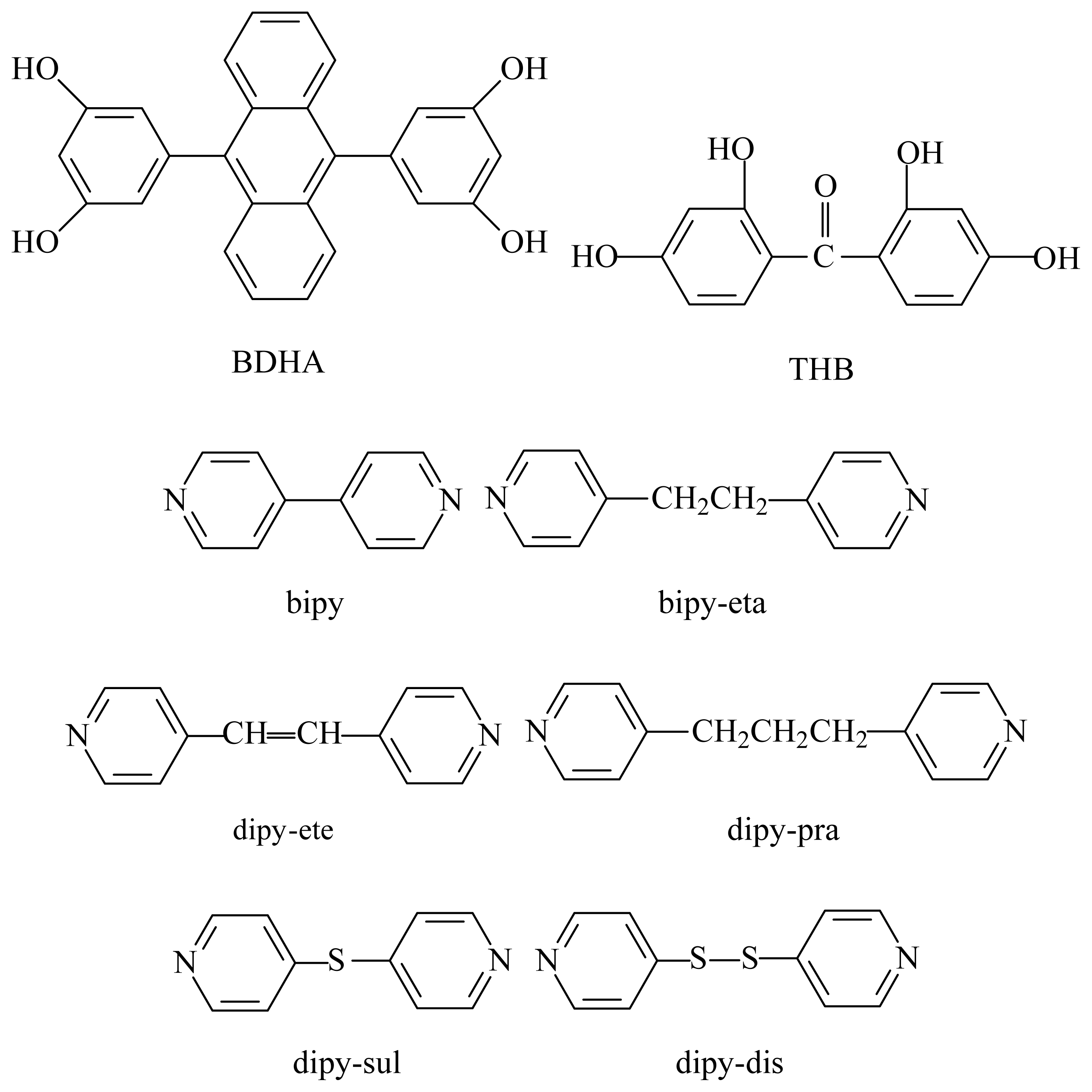
Synthesis
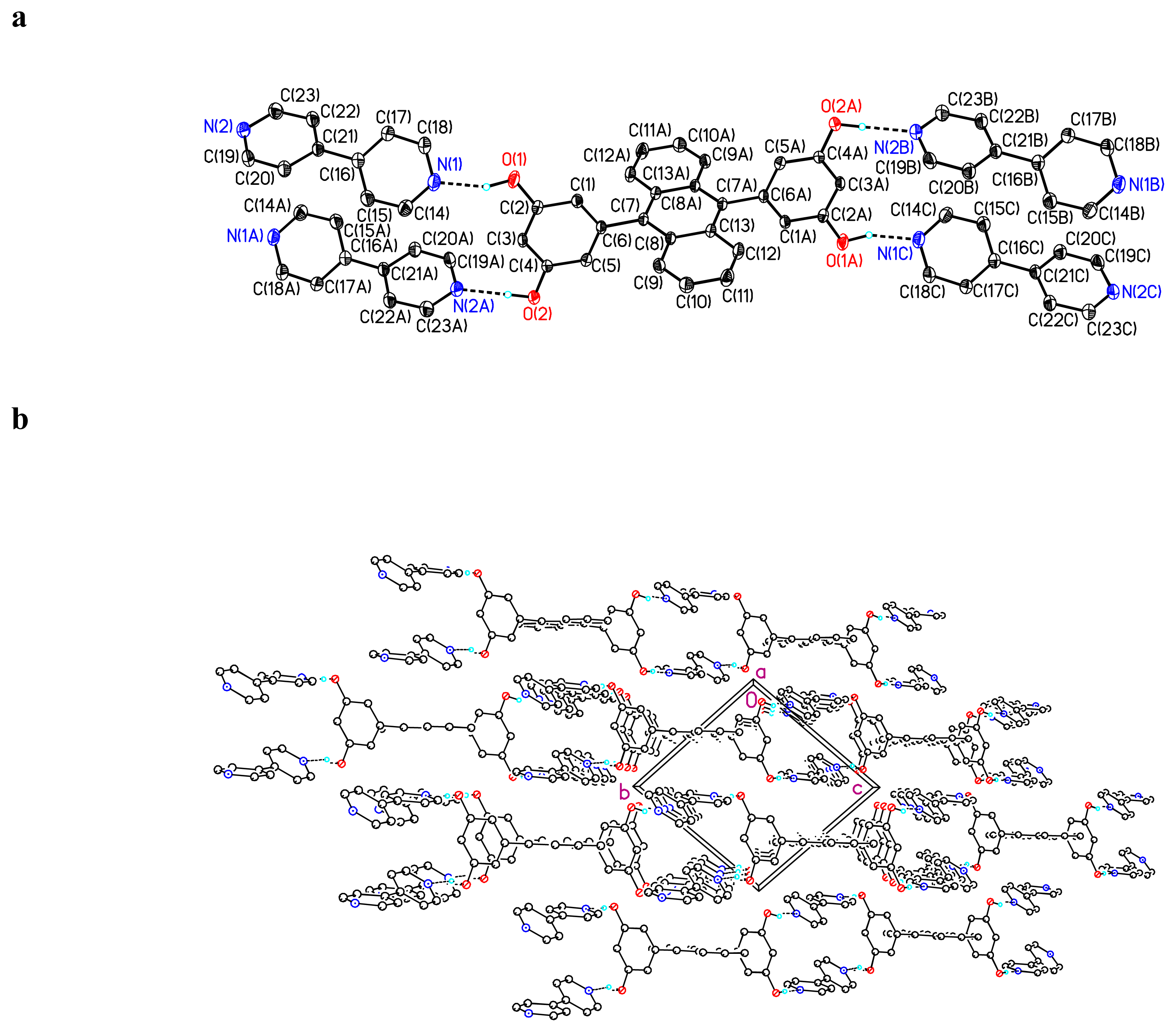
Crystal Structure of (BDHA) · (bipy)2 1
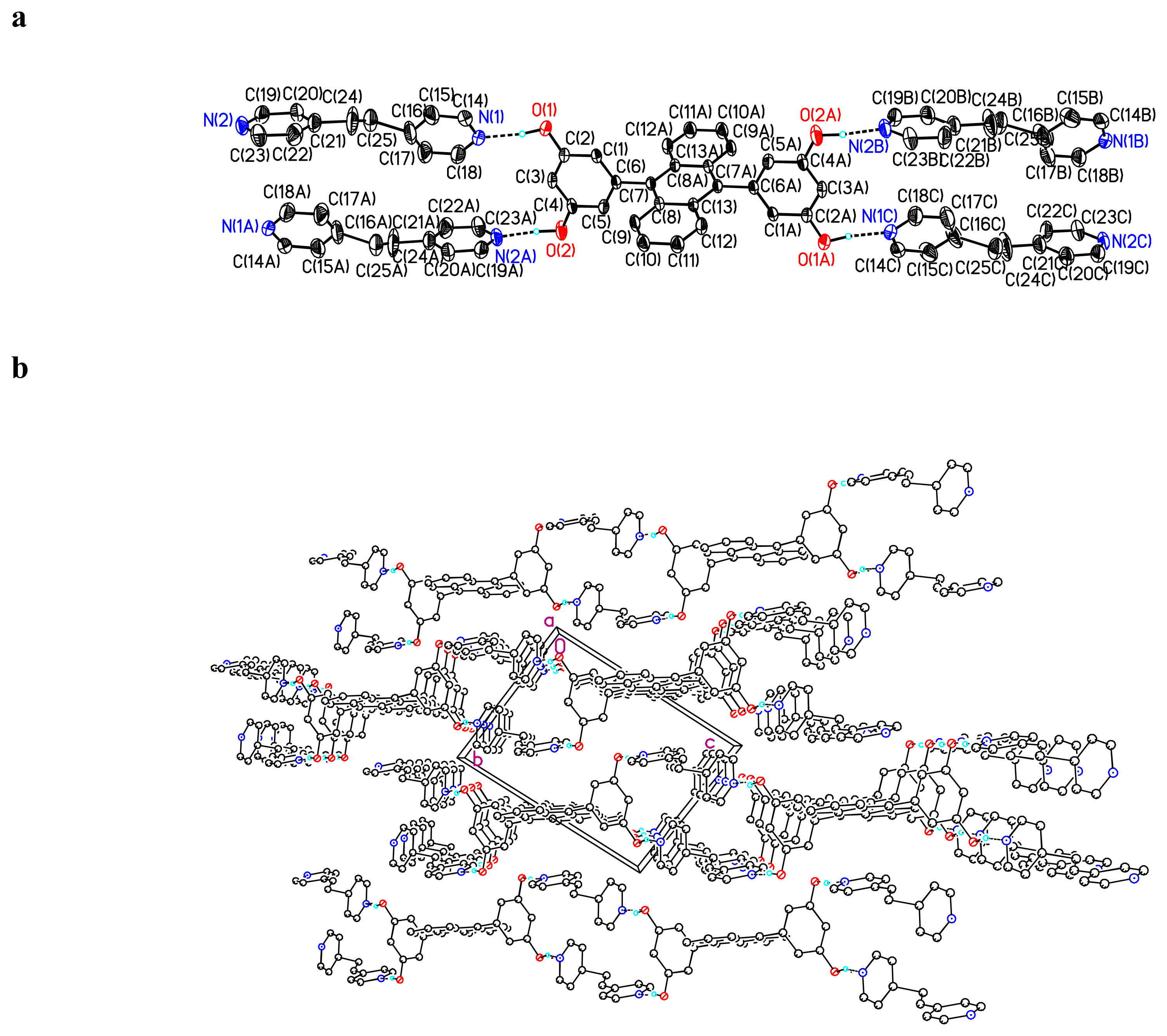
Crystal Structure of (BDHA) · (bipy-eta)2 2
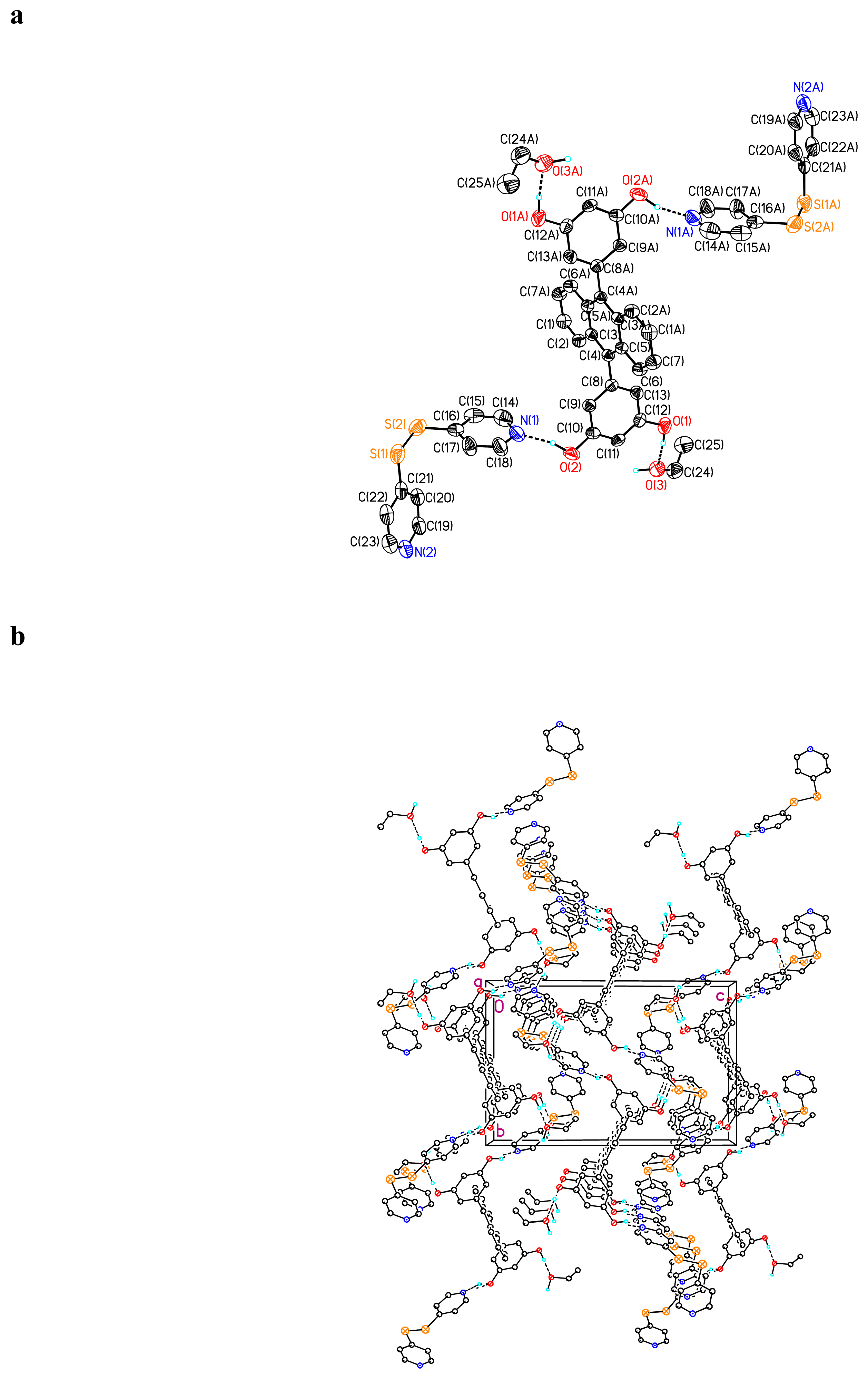
Crystal Structure of (BDHA)0.5· (dipy-pra) ·CH3CH2OH 3
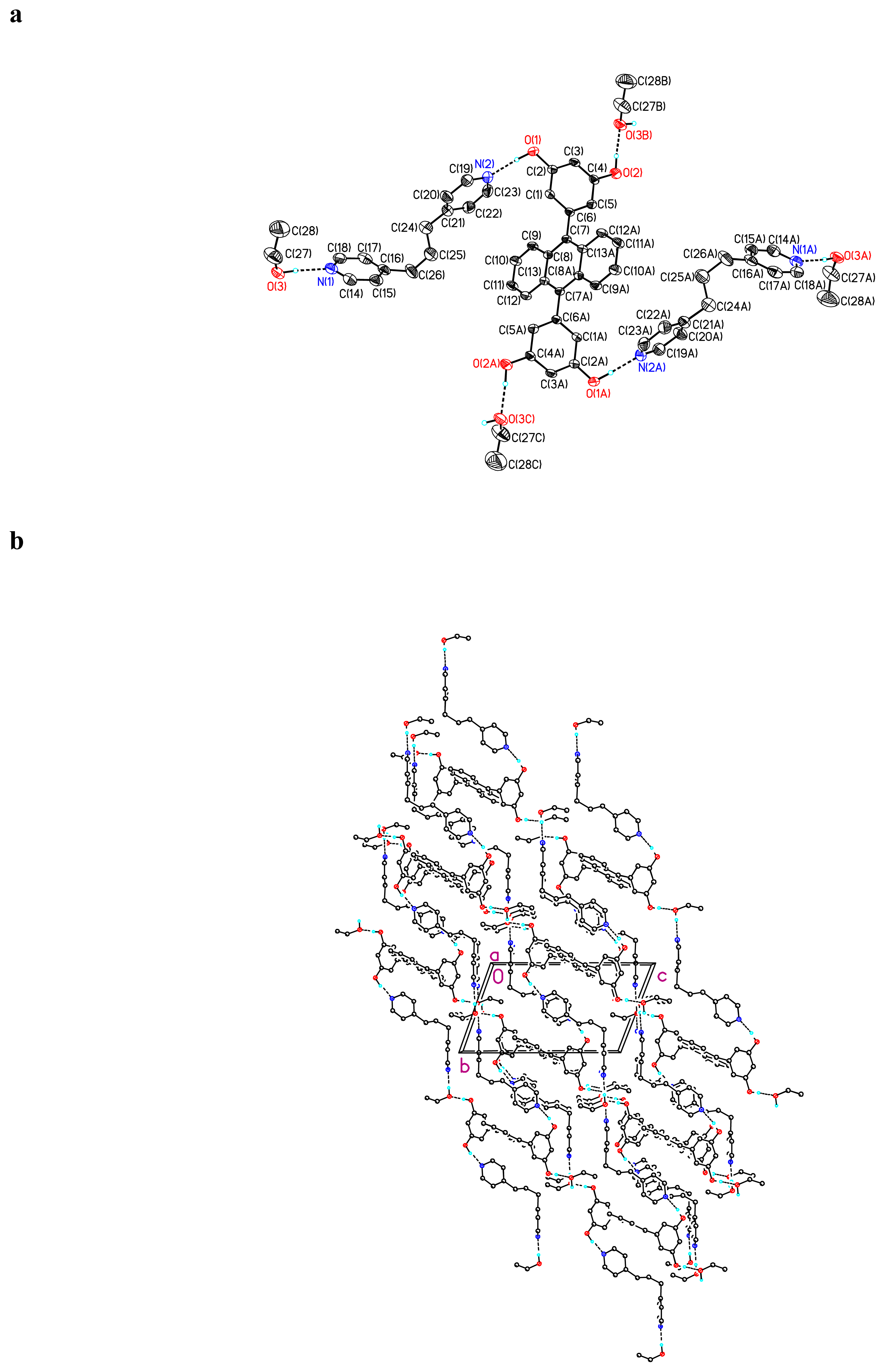
Crystal Structure of (BDHA)0.5· (dipy-sul) ·H2O 4
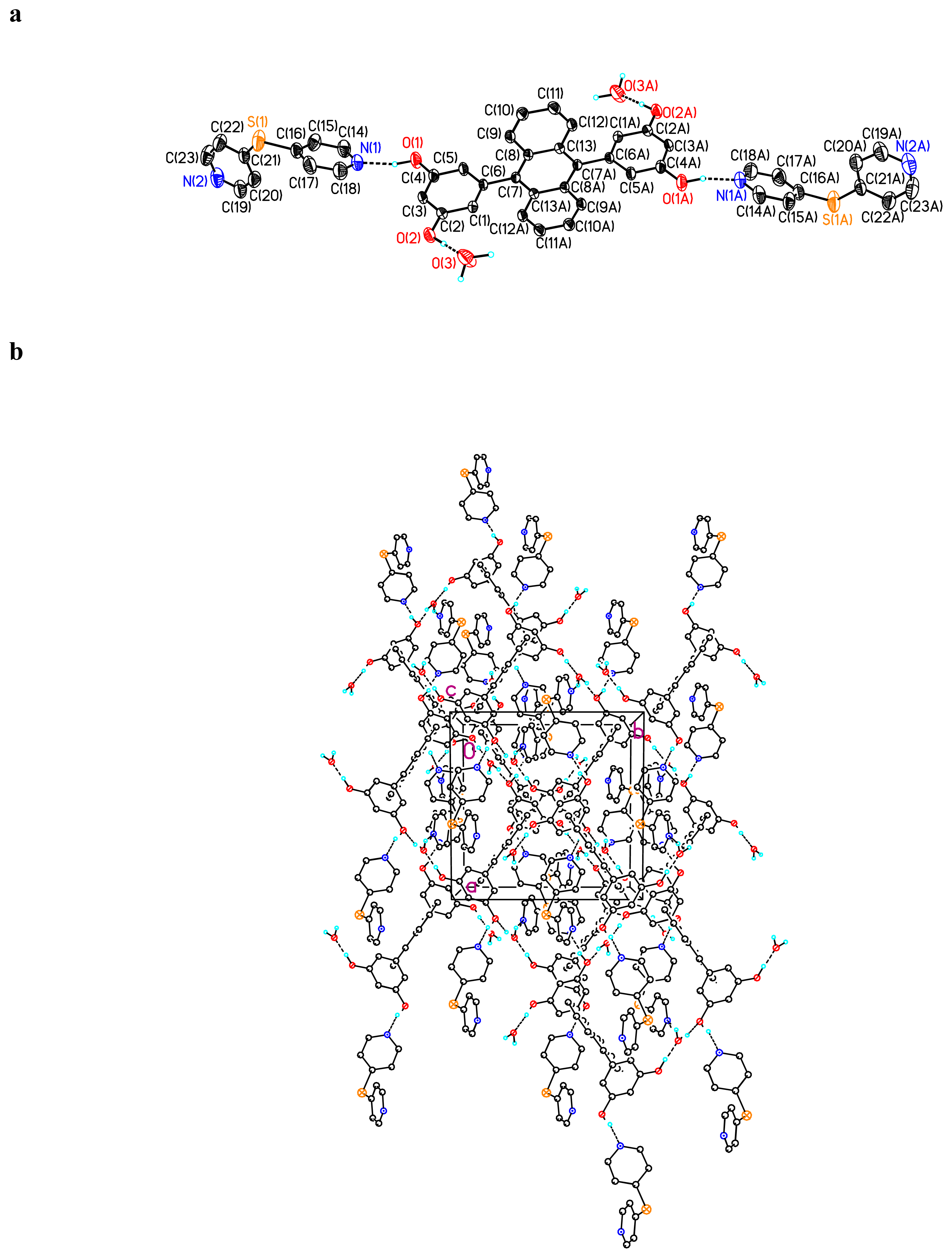
Crystal Structure of (BDHA)0.5· (dipy-dis) ·CH3CH2OH 5
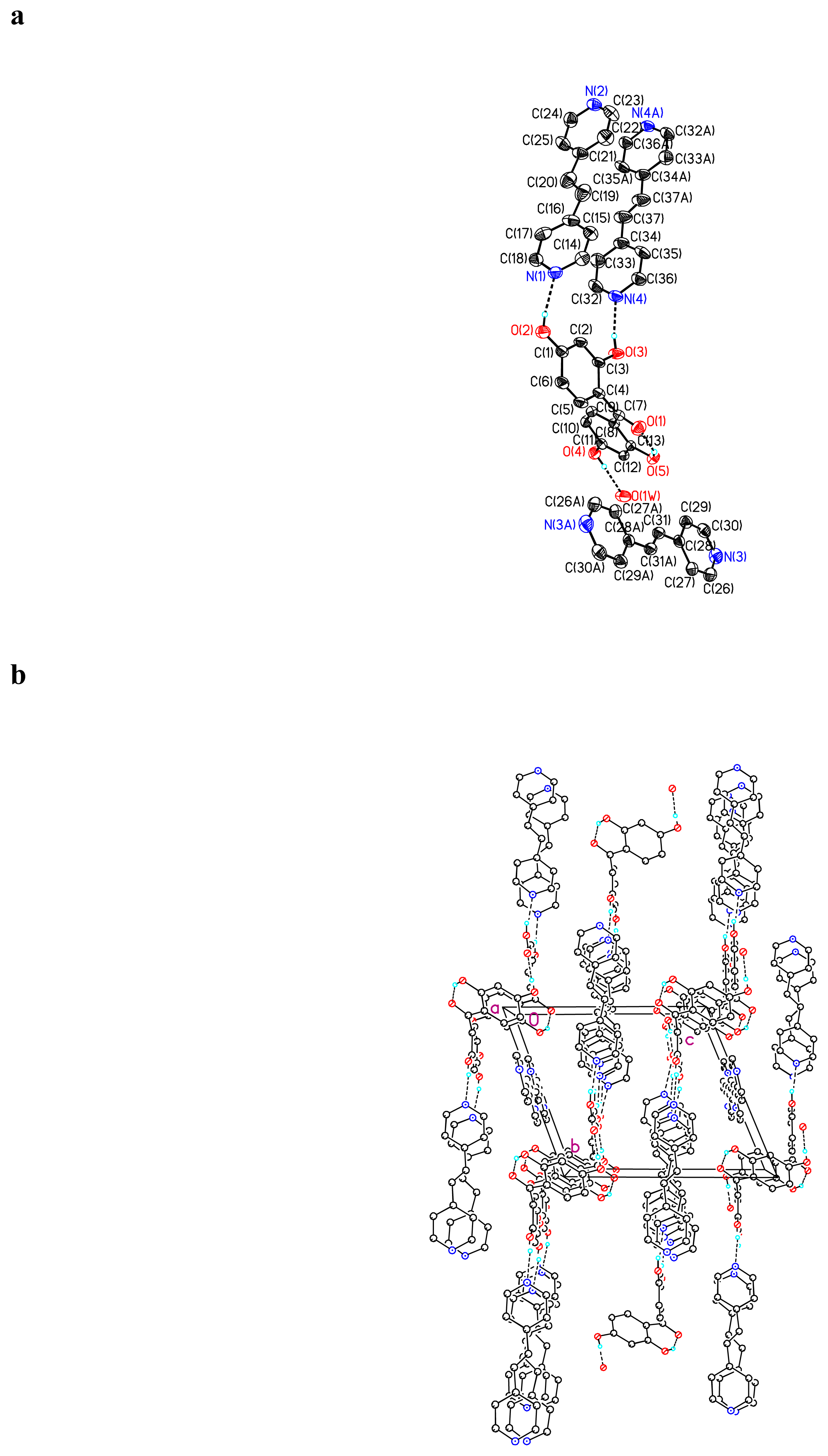
Crystal Structure of (THB) · (dipy-ete)2·H2O 6
3. Conclusions
4. Experimental Section
4.1. Syntheses of cocrystals 1–6
(BDHA) · (bipy)2 1
(BDHA) · (bipy-eta)2 2
(BDHA)0.5· (dipy-pra) ·CH3CH2OH 3
(BDHA)0.5· (dipy-sul) ·H2O 4
(BDHA)0.5· (dipy-dis) ·CH3CH2OH 5
(THB) · (dipy-ete)2·H2O 6
4.2. X-ray Crystallographic Analyses
| cocrystal | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| formula | C46H34N4O4 | C50H42N4O4 | C28H29N2O3 | C23H19N2O3S | C25H23N2O3S2 | C37H32N4O6 |
| Mr | 706.77 | 762.91 | 441.53 | 403.46 | 463.57 | 628.65 |
| Crystal size (mm3) | 0.35 × 0.15 × 0.10 | 0.70 × 0.40 × 0.10 | 0.50 × 0.35 × 0.15 | 0.30 × 0.25 × 0.10 | 0.60 × 0.40 × 0.35 | 0.79 × 0.70 × 0.70 |
| Crystal system | Triclinic | Triclinic | Triclinic | Monoclinic | Monoclinic | Triclinic |
| space group | P-1 | P-1 | P-1 | C2/c | P2(1)/c | P-1 |
| T (K) | 293(2) | 293(2) | 293(2) | 293(2) | 293(2) | 293(2) |
| a (Å) | 9.4497(19) | 9.1440(18) | 9.1264(18) | 12.801(3) | 9.776(2) | 12.233(2) |
| b (Å) | 10.065(2) | 9.5925(19) | 10.107(2) | 12.968(3) | 12.530(3) | 12.323(3) |
| c (Å) | 10.151(2) | 12.159(2) | 15.870(3) | 26.107(5) | 19.178(4) | 12.721(3) |
| α (º) | 93.60(3) | 92.53(3) | 107.16(3) | 90 | 90 | 68.39(3) |
| β (º) | 103.94(3) | 98.09(3) | 93.50(3) | 100.68(3) | 92.57(3) | 84.53(3) |
| γ (º) | 108.30(3) | 109.22(3) | 113.27(3) | 90 | 90 | 63.89(3) |
| Z | 1 | 1 | 2 | 8 | 4 | 2 |
| Volume (Å3) | 879.6(3) | 992.4(3) | 1258.3(4) | 4258.7(15) | 2346.8(8) | 1595.9(5) |
| Dcalc (g cm−3) | 1.334 | 1.280 | 1.165 | 1.259 | 1.312 | 1.304 |
| μ (mm−1) | 0.086 | 0.082 | 0.076 | 0.178 | 0.256 | 0.090 |
| 2θ scan range (º) | 4.72 – 54.96 | 4.52 – 54.96 | 5.14 – 54.6 | 5.98 – 54.96 | 5.28 – 50.06 | 3.46 – 54.94 |
| range h | −12 to 11 | −11 to 11 | −11 to 10 | −16 to 16 | −11 to 11 | 0 to 15 |
| range k | −13 to 13 | −12 to 12 | −12 to 12 | −16 to 16 | −14 to 14 | −13 to 15 |
| range l | −13 to 13 | −15 to 15 | −20 to 19 | −33 to 33 | −22 to 22 | −16 to 16 |
| reflns collected | 8015 | 7757 | 10521 | 15434 | 15080 | 11133 |
| unique reflns | 3932 | 4232 | 5407 | 4819 | 4127 | 6601 |
| observed reflns | 2061 | 2060 | 2492 | 1619 | 2269 | 3722 |
| Goodness-of-fit | 0.821 | 1.183 | 1.120 | 0.897 | 0.997 | 1.043 |
| R1, wR2 | 0.0406,0.083 | 0.0545,0.131 | 0.0551,0.116 | 0.0475,0.065 | 0.0458,0.108 | 0.0808,0.237 |
| [I>2σ(I)] | 9 | 2 | 7 | 5 | 5 | 6 |
| cocrystal | D-H· · ·A | d(D-H)/Å | d(H· · ·A)/Å | d(D· · ·A)/Å | <(DHA)/ | Symmetry |
|---|---|---|---|---|---|---|
| 1 | O(1)-H· · ·N(1) | 0.98(2) | 1.80(2) | 2.7445(18) | 159.4(18) | |
| O(2)-H· · ·N(2)#2 | 0.946(18) | 1.872(19) | 2.8165(18) | 175.7(16) | −x + 2, −y + 2, −z + 1 | |
| 2 | O(1)-H· · ·N(1) | 0.99(3) | 1.77(3) | 2.742(2) | 164(2) | |
| O(2)-H· · ·N(2)#2 | 1.04(3) | 1.68(3) | 2.718(2) | 173(2) | −x + 2, −y −1, −z + 2 | |
| 3 | O(1)-H· · ·N(2) | 0.99(3) | 1.79(3) | 2.764(3) | 167(2) | |
| O(3)-H· · ·N(1) | 0.93(3) | 1.90(3) | 2.818(3) | 174(3) | ||
| O(2)-H· · ·O(3)#2 | 0.96(4) | 1.76(4) | 2.713(2) | 175(3) | x − 1, y − 2, z − 1 | |
| 4 | O(1)-H· · ·N(1) | 1.02(3) | 1.73(3) | 2.727(3) | 166(2) | |
| O(2)-H· · ·O(3) | 0.96(3) | 1.67(3) | 2.628(2) | 176(2) | ||
| O(3)-H· · ·O(1)#2 | 0.9526(16) | 1.8539(17) | 2.790(2) | 166.95(16) | x + 1/2, y −1/2, z | |
| O(3)-H· · ·N(2)#3 | 1.1004(17) | 1.765(2) | 2.810(3) | 156.71(13) | x + 1, −y, z + 1/2 | |
| 5 | O(1)-H· · ·O(3) | 1.00(2) | 1.69(3) | 2.683(2) | 171.8(2) | |
| O(2)-H· · ·N(1) | 1.09(3) | 1.71(3) | 2.759(3) | 159.4(3) | ||
| O(3)-H· · ·N(2)#2 | 1.12(3) | 1.69(3) | 2.801(2) | 170.2(2) | x, −y + 1/2, z + 1/2 | |
| 6 | O(2)-H· · ·N(1)#3 | 0.82(3) | 1.95(3) | 2.765(3) | 170.1(2) | x + 1, y − 1, z |
| O(3)-H· · ·N(4) | 0.82(3) | 1.85(3) | 2.664(2) | 170.3(2) | ||
| O(4)-H· · ·O(1w) | 0.82(3) | 1.82(2) | 2.638(2) | 175.3(3) | ||
| O(5)-H· · ·O(1) | 0.82(3) | 1.80(2) | 2.528(3) | 147.7(3) |
Acknowledgements
References and Notes
- Kondo, M.; Yoshitomi, T.; Seki, K.; Matsuzaka, H.; Kitagawa, S. Three-dimensional framework with channeling cavities for small molecules: {[M-2(4,4′-bpy)(3)(NO3)(4)]center dot xH(2)O}(n) (M = Co, Ni, Zn). Angew. Chem. Int. Ed. Engl 1997, 36, 1725–1727. [Google Scholar]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Fujita, M.; Kwon, Y. J.; Washizu, S.; Ogura, K. Preparation, clathration ability, and catalysis of a 2-dimensional square network material composed of cadmium(II) and 4,4′-bipyridine. J. Am. Chem. Soc 1994, 116, 1151–1152. [Google Scholar]
- Kosal, M. E.; Chou, J.–H.; Wilson, S. R.; Suslick, K. S. A functional zeolite analogue assembled from metalloporphyrins. Nat. Mater 2002, 1, 118–121. [Google Scholar]
- Albrecht, M.; Lutz, M.; Spek, A. L.; van Koten, G. Organoplatinum crystals for gas-triggered switches. Nature 2000, 406, 970–974. [Google Scholar]
- Yuen, T.; Lin, C. L.; Mihalisin, T. W.; Lawandy, M. A.; Li, J. Magnetic ordering in M(ox)(bpy) system (M=Fe, Co, Ni; ox=C2O42-; bpy=4,4′-bipyridine). J. Appl. Phys 2000, 87, 6001–6003. [Google Scholar]
- Lin, W.; Wang, Z.; Ma, L. A novel octupolar metal-organic NLO material based on a chiral 2D coordination network. J. Am. Chem. Soc 1999, 121, 11249–11250. [Google Scholar]
- Pedireddi, V. R. Design and synthesis of novel aza-aromatic host-guest complexes: Crystal structures of 1,7-phenanthroline with thiodipropionic and thiodiglycolic acids. Cryst. Growth Des 2001, 1, 383–385. [Google Scholar]
- Broder, C. K.; Davidson, M. G.; Forsyth, V. T.; Howard, J. A. K.; Lamb, S.; Mason, S. A. On the reliability of C-H center dot center dot center dot O interactions in crystal engineering: Synthesis and structure of two hydrogen bonded phosphonium bis(aryloxide) salts. Cryst. Growth Des 2002, 2, 163–169. [Google Scholar]
- Bhogala, B. R.; Nangia, A. Cocrystals of 1,3,5-cyclohexanetricarboxylic acid with 4,4′-bipyridine homologues: Acid center dot center dot center dot pyridine hydrogen bonding in neutral and ionic complexes. Cryst. Growth Des 2003, 3, 547–554. [Google Scholar]
- Zaman, M. B.; Udachin, K. A.; Ripmeester, J. A. Supramolecular grid and layer architectures. hydrogen bonds and halogen-halogen interactions influenced by bromo-, chloro-, and cyano-substituted anilic acids. Cryst. Growth Des 2004, 4, 585–589. [Google Scholar]
- MacDonald, J. C.; Whitesides, G. M. Solid-state structures of hydrogen-bonded tapes based on cyclic secondary diamides. Chem. Rev 1994, 94, 2383–2420. [Google Scholar]
- Lawrence, D. S.; Jianf, T.; Levett, M. Self-assembling supramolecular complexes. Chem. Rev 1995, 95, 2229–2260. [Google Scholar]
- Aakeroy, C. B.; Seddon, K. R. The hydrogen-bond and crystal engineering. Chem. Rev. Soc 1993, 22, 397–407. [Google Scholar]
- Desiraju, G. R. Crystal Engineering: The Design of Organic Solids; Elsevier: New York, 1989. [Google Scholar]
- Steed, J. W.; Atwood, J. L. Supramolecular Chemistry; Wiley: Chichester, 2000. [Google Scholar]
- Jeffrey, G. A.; Saenger, W. Hydrogen bonding in Biological Structures; Springer-Verlag: Berlin, 1991. [Google Scholar]
- MacGillivray, L. R.; Atwood, J. L. Cavity-containing materials based upon resorcin[4]arenes by discovery and design. J. Solid State Chem 2000, 152, 199–210. [Google Scholar]
- MacGillivray, L. R.; Reid, J. L.; Ripmeester, J. A. Conformational isomerism leads to supramolecular isomerism and nanoscale inclusion in 2D extended framework solids based on C-methylcalix[ 4]resorcinarene. Chem. Commun. 2001, 1034–1035. [Google Scholar]
- MacGillivray, L. R.; Spinney, H. A.; Reid, J. L.; Ripmeester, J. A. Entrapment of ferrocenes within supramolecular, deep-cavity resorcin[4]arenas. Chem. Commun. 2000, 517–518. [Google Scholar]
- MacGillivray, L. R.; Diamente, P. R.; Reid, J. L.; Ripmeester, J. A. Encapsulation of two aromatics by a carcerand-like capsule of nanometre-scale dimensions. Chem. Commun. 2000, 359–360. [Google Scholar]
- MacGillivray, L. R.; Atwood, J. L. Rational design of multicomponent calix[4]arenes and control of their alignment in the solid state. J. Am. Chem. Soc 1997, 119, 6931–6932. [Google Scholar]
- MacGillivray, L. R.; Papaefstathiou, G. S.; Reid, J. L.; Ripmeester, J. A. A rod-shaped guest leads to architectural isomerism in a multicomponent crystalline framework based on a resorcin[4]arene. Cryst. Growth Des. 2001, 5, 373–375. [Google Scholar]
- Du, M.; Zhang, Z.-H.; Zhao, X.-J. Cocrystallization of bent dipyridyl type compounds with aromatic dicarboxylic acids: Effect of the geometries of building blocks on hydrogen-bonding supramolecular patterns. Cryst. Growth Des 2005, 5, 1199–1208. [Google Scholar]
- Du, M.; Zhang, Z.-H.; Zhao, X.-J. Cocrystallization of trimesic acid and pyromellitic acid with bent dipyridines. Cryst. Growth Des 2005, 5, 1247–1254. [Google Scholar]
- Du, M.; Zhang, Z.-H.; Zhao, X.-J. A search for predictable hydrogen-bonding synthons in cocrystallization of unusual organic acids with a bent dipyridine. Cryst. Growth Des 2006, 6, 390–396. [Google Scholar]
- Du, M.; Zhang, Z.-H.; Zhao, X.-J.; Cai, H. Synthons competition/prediction in cocrystallization of flexible dicarboxylic acids with bent dipyridines. Cryst. Growth Des 2006, 6, 114–121. [Google Scholar]
- Du, M.; Zhang, Z.-H.; Wang, X.-G.; Wu, H.-F.; Wang, Q. Flexible building blocks of N,N′-bis(picolinoyl)hydrazine for hydrogen-bonding directed cocrystallization: Structural diversity, concomitant polymorphs, and synthon prediction. Cryst. Growth Des 2006, 6, 1867–1875. [Google Scholar]
- Zeng, Q. D.; Wu, D. X.; Liu, C. M.; Ma, H. W.; Lu, J.; Xu, S. D.; Li, Y.; Wang, C.; Bai, C. L. Crystal engineering based on polymeric hydrogen-bonded supramolecules by self-assembling of 4,4′-(9-fluorenylidene)diphenol and 4,4′-cyclohexylidenebisphenol with bipyridines. Cryst. Growth Des 2005, 5, 1041–1047. [Google Scholar]
- Zeng, Q. D.; Wu, D. X.; Wang, C.; Ma, H. W.; Lu, J.; Liu, C. M.; Xu, S. D.; Li, Y.; Bai, C. L. Supramolecular structures based on bis(2-hydroxy-5-chlorophenyl) sulfide and spirobicromane with bipyridines. Cryst. Growth Des 2005, 5, 1889–1896. [Google Scholar]
- Zeng, Q. D.; Wu, D. X.; Ma, H. W.; Shu, C. Y.; Li, Y.; Wang, C. Polymeric hydrogen-bonded supramolecules by selfassembling of adamantane derivatives with bipyridines. CrystEngComm 2006, 8, 189–201. [Google Scholar]
- Endo, K.; Ezuhara, T.; Koyanagi, M.; Masuda, H.; Aoyama, Y. Functional self-assembly of hydrogen-bonded networks. Construction of aromatic stacks and columns and cavity-size control via flexible intercalation of 1D chains having orthogonal aromatic substituents. J. Am. Chem. Soc 1997, 119, 499–505. [Google Scholar]
- Dewa, T.; Endo, K.; Aoyama, Y. Dynamic aspects of lattice inclusion complexation involving a phase change. Equilibrium, kinetics, and energetics of guest-binding to a hydrogen-bonded flexible organic network. J. Am. Chem. Soc 1998, 120, 8933–8940. [Google Scholar]
- Tanaka, T.; Endo, K.; Aoyama, Y. A hydrogen-bonded molecular ladder. The crystal structure and guest-binding properties of a bishydroquinone derivative of anthracene. Chem. Lett. 2000, 887–888. [Google Scholar]
- Schlemper, E. O.; Hussain, M. S. X-ray and neutron diffraction studies of the hydrongen bonding in hydroxybenzophenone. Acta Cryst. A 1981, 37, C96–C96. [Google Scholar]
- Schlemper, E. O. Intramolecular hydrogen bonding in 2,2′,4,4′-tetrahydroxybenzophenone. Acta Cryst. B 1982, 38, 554–559. [Google Scholar]
- Sheldrick, G. M. SHELXS 97, Program for the Solution of Crystal Structure; University of Göttingen, 1997. [Google Scholar]
- Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure; University of Göttingen, 1997. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Li, X.; Zhen, L.; Fan, Y.; Fan, X.; Zeng, Q. Crystal Engineering Based on Polymeric Hydrogen-Bonded Supramolecules by Self-Assembling of 9, 10-Bis(3,5- dihydroxyphenyl)anthracene and 2,2′,4,4′- Tetrahydroxybenzophenone with Bipyridines. Int. J. Mol. Sci. 2007, 8, 241-258. https://doi.org/10.3390/i8030241
Li X, Zhen L, Fan Y, Fan X, Zeng Q. Crystal Engineering Based on Polymeric Hydrogen-Bonded Supramolecules by Self-Assembling of 9, 10-Bis(3,5- dihydroxyphenyl)anthracene and 2,2′,4,4′- Tetrahydroxybenzophenone with Bipyridines. International Journal of Molecular Sciences. 2007; 8(3):241-258. https://doi.org/10.3390/i8030241
Chicago/Turabian StyleLi, Xiaokang, Lvyin Zhen, Yulan Fan, Xiaolin Fan, and Qingdao Zeng. 2007. "Crystal Engineering Based on Polymeric Hydrogen-Bonded Supramolecules by Self-Assembling of 9, 10-Bis(3,5- dihydroxyphenyl)anthracene and 2,2′,4,4′- Tetrahydroxybenzophenone with Bipyridines" International Journal of Molecular Sciences 8, no. 3: 241-258. https://doi.org/10.3390/i8030241




