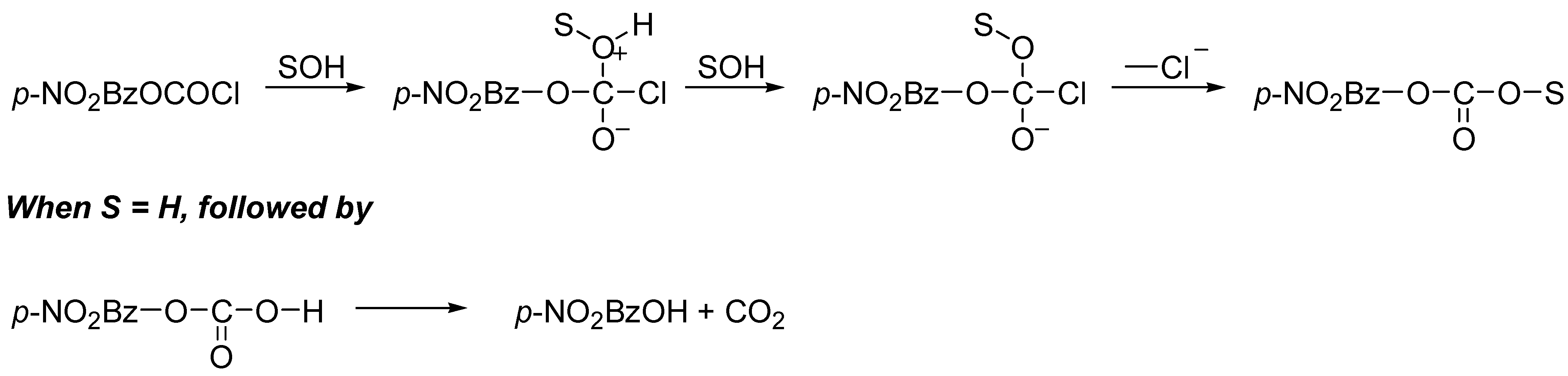1. Introduction
Chloroformate esters with primary alkyl groups solvolyze in most of the commonly studied solvents by an addition–elimination mechanism with the addition step being rate-determining. Only in solvents of very low nucleophilicity and very high ionizing power is an ionization mechanism observed. For methyl chloroformate solvolysis [
1] the ionization mechanism was observed only in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)–water mixtures with at least 90% HFIP content. The ionization range was larger for ethyl [
2] and
n-propyl [
3] chloroformates, and ionization was indicated with up to 50% water content in HFIP–H
2O and with up to 10% water content in 2,2,2-trifluoroethanol (TFE)–H
2O mixtures.
Incorporation of an aryl group into the methyl ester resulted in the addition–elimination pathway being followed for all commonly studied solvents for
p-nitrobenzyl chloroformate [
4] (
Scheme 1), but ionization being indicated in HFIP–H
2O and TFE–H
2O mixtures for the parent benzyl chloroformate [
4].
The ionization was accompanied by loss of carbon dioxide, such that the observed products (
Scheme 2) were benzyl chloride (decomposition) plus benzyl fluoroalkyl ether and benzyl alcohol (solvent capture of the cation).
For solvolyses of the secondary isopropyl [
5] and 2-adamantyl [
6] chloroformates, the ionization pathway was dominant and only in ethanol and methanol and their mixtures with 10% water was there an appreciable addition–elimination component. For the tertiary 1-adamantyl chloroformate, the ionization pathway was dominant in all solvents and only in 100% ethanol was a trace of the mixed carbonate, presumably from an addition–elimination reaction, detected [
7]. For phenyl chloroformate the addition–elimination pathway was observed over the full range of solvents [
8].
Studies have been made of the replacement of chlorine by fluorine for the
n-octyl [
9] and 1-adamantyl [
10] esters. For the
n-octyl fluoroformate, the addition–elimination pathway was observed over the full range of solvents and the
kF/
kCl specific rate ratios were only slightly below or above unity.
The present investigation involves a study of the solvolyses of benzyl fluoroformate. The corresponding chloroformate solvolyses divided, in the usually studied solvents, into two almost equally sized groups, with either ionization or addition–elimination as the rate-determining step [
4].
The dependence of the rates of solvolysis on solvent nucleophilicity (
NT) and solvent ionizing power (
YCl) is intimately associated with the details of the reaction mechanism. In particular, for attack at an acyl carbon, very different values for the parameters are observed dependent upon whether the solvolyses follow the addition–elimination or the ionization mechanism. The extended Grunwald–Winstein equation used in these analyses can be expressed as in equation 1:
In equation 1,
l represents the sensitivity towards changes in the
NT value;
m represents the sensitivity towards changes in the
YCl value;
c is a constant (residual) term; and
k and
k0 represent the specific rates of solvolysis in the solvent under consideration and in the arbitrarily chosen standard solvent [
11] (80% ethanol), respectively.
2. Results
The specific rates of solvolysis of benzyl fluoroformate have been determined, at 25.0 ºC, in methanol, ethanol, and TFE, and in binary aqueous solvents with the other component being methanol, ethanol, acetone, TFE, or HFIP. Determinations were also made in TFE–ethanol mixtures. In
Table 1 are presented the 16 data points used in extended Grunwald-Winstein analyses, together with the appropriate
NT [
12] and
YCl [
13] values. A determination was also made in methanol–d (MeOD). For ethanol and 80% ethanol, specific rates were determined at two additional temperatures, and activation parameters were calculated.
The product ratio for several of the solvolyses was determined by response-calibrated gas chromatography. Determination was at 10 half-lives. For 90% EtOH, 50% EtOH, 70% TFE, and 50% TFE, it was shown that the products were formed in amounts which remained essentially unchanged if left for longer periods of time (20 to 40 half-lives) prior to the gas chromatography analysis.
For the aqueous–alcohol solvents, selectivity values (
S) were calculated according to equation 2,
and for the reaction in TFE–ethanol, equation 3 was used. In both equations, the product
concentrations are divided by the concentration of the component of the solvent producing them. The selectivity values are presented in
Table 2. For comparison,
SCl values previously reported [
4] for the solvolyses of benzyl chloroformate are also tabulated.
Table 1.
First-order rate coefficients (kF) for the solvolysis of benzyl fluoroformate in pure and binary solvents at 25.0 °C together with the appropriate solvent nucleophilicity (NT) and solvent ionizing power (YCl) values and kF/kCl values.
Table 1.
First-order rate coefficients (kF) for the solvolysis of benzyl fluoroformate in pure and binary solvents at 25.0 °C together with the appropriate solvent nucleophilicity (NT) and solvent ionizing power (YCl) values and kF/kCl values.
| Solvent (%)a | 104 kF (s–1) | kF/kClb | NTc | YCld |
|---|
| 100% EtOH | 0.612 ± 0.003e | 1.19 | 0.37 | –2.52 |
| 90% EtOH | 7.66 ± 0.21 | 5.94 | 0.16 | –0.94 |
| 80% EtOH | 20.4 ± 0.3f | 11.5 | 0.00 | 0.00 |
| 70% EtOH | 31.4 ± 0.3 | 14.6 | –0.20 | 0.78 |
| 100% MeOH | 3.36 ± 0.02g | 1.78 | 0.17 | –1.17 |
| 90% MeOH | 27.6 ± 0.2 | 7.18 | –0.01 | –0.18 |
| 90% Acetone | 0.175 ± 0.003 | | –0.35 | –2.22 |
| 80% Acetone | 1.26 ± 0.02 | 5.89 | –0.37 | –0.83 |
| 70% Acetone | 3.87 ± 0.02 | 9.16 | –0.42 | 0.17 |
| 60% Acetone | 8.88 ± 0.02 | 11.6 | –0.52 | 0.95 |
| 50% Acetone | 19.3 ± 0.2 | | –0.70 | 1.73 |
| 70% TFE | 3.07 ± 0.03 | 6.36 | –1.98 | 2.96 |
| 50% TFE | 11.6 ± 0.2 | 12.3 | –1.73 | 3.16 |
| 60T–40Eh | 0.420 ± 0.020 | 4.23 | –0.94 | 0.63 |
| 40T–60Eh | 0.758 ± 0.024 | 3.46 | –0.34 | –0.48 |
| 20T–80Eh | 0.965 ± 0.026 | 2.47 | 0.08 | –1.42 |
Table 2.
Percentages of the products present after the solvolysis of benzyl fluoroformate (BzOCOF) in a binary hydroxylic solvent at 25.0 °C and the calculated selectivity values.
Table 2.
Percentages of the products present after the solvolysis of benzyl fluoroformate (BzOCOF) in a binary hydroxylic solvent at 25.0 °C and the calculated selectivity values.
| Solventa | %BzOCO2CH2CF3 | %BzOH | %BzOCO2Et | Sb | ScCl |
|---|
| 100% EtOH | | 4.1 | 95.9 | | |
| 90% EtOH | | 17.8 | 82.2 | 2.2 | 2.0 |
| 80% EtOH | | 25.7 | 74.3 | 2.8 | 2.7 |
| 70% EtOH | | 34.0 | 66.0 | 3.3 | 3.3 |
| 60% EtOH | | 41.2 | 58.8 | 3.4 | 3.7 |
| 50% EtOH | | 49.7 | 50.3 | 3.6 | 4.1 |
| 70% TFE | 4.7 | 95.3 | | 0.12 | 1.04d |
| 50% TFE | 3.2 | 96.8 | | 0.19 | 1.20d |
| 80T–20Ee | 11.5 | 3.2 | 85.3 | 0.043f | |
| 60T–40Ee | 4.0 | 3.8 | 92.2 | 0.036f,g | |
3. Discussion
With the tertiary 1-adamantyl group incorporated into the haloformate esters, the chloroformate reacted across the full range of solvents by an ionization mechanism incorporating loss of CO
2 and formation of products by capture of the 1-adamantyl cation [
7]. The tendency towards ionization was considerably reduced for the solvolyses of the fluoroformate [
10] and a change in mechanism to the addition–elimination pathway occurred as one moved to less ionizing and/or more nucleophilic solvents. For example, in the standard solvent (80% ethanol), more than 90% of the reaction proceeded by the rate-determining addition to the acyl carbon. In this region, the solvolyses parallel closely those of
n-octyl fluoroformate, incorporating a primary alkyl group [
9].
In the present study, we have extended the previous study [
4] of the solvolyses of benzyl chloroformate to the fluoroformate. The chloroformate showed a change in mechanism similar to that observed for 1-adamantyl fluoroformate as one moved across the usual range of solvents. Accordingly, we would not be surprised if the expected move away from ionization and toward addition–elimination led to the solvolyses of benzyl fluoroformate over an extensive range of solvents involving rate-determining addition to the acyl carbon, followed by a relatively rapid expulsion of fluoride ion (as in
Scheme 1).
The specific rates of solvolysis in 16 representative solvents, at 25.0 °C, are reported in
Table 1 and, in conjunction with the appropriate
NT and
YCl values, a correlation analysis has been carried out using equation 1. The sensitivity values (
l and
m) and several goodness-of-fit parameters are reported in
Table 3.
Table 3.
Correlationsa of the specific rates of the solvolyses by the addition–elimination mechanism for benzyl fluoroformate, several other chloroformate and fluoroformate esters, and benzoyl fluoride.
Table 3.
Correlationsa of the specific rates of the solvolyses by the addition–elimination mechanism for benzyl fluoroformate, several other chloroformate and fluoroformate esters, and benzoyl fluoride.
| | n | lb | mb | cb | Rc | l/m |
|---|
| PhOCOCld | 21 | 1.68 ± 0.10 | 0.57 ± 0.06 | 0.12 ± 0.41 | 0.973 | 2.95 |
| MeOCOCle | 19 | 1.59 ± 0.09 | 0.58 ± 0.05 | 0.16 ± 0.17 | 0.977 | 2.74 |
| EtOCOClf | 28 | 1.56 ± 0.09 | 0.55 ± 0.03 | 0.19 ± 0.24 | 0.967 | 2.84 |
| PrOCOClg | 22 | 1.57 ± 0.12 | 0.56 ± 0.06 | 0.15 ± 0.08 | 0.947 | 2.79 |
| p-NO2BzOCOClh | 19 | 1.61 ± 0.09 | 0.46 ± 0.04 | 0.04 ± 0.22 | 0.975 | 3.50 |
| BzOCOClh | 15 | 1.95 ± 0.16 | 0.57 ± 0.05 | 0.16 ± 0.15 | 0.966 | 3.42 |
| OctOCOFi | 19 | 1.67 ± 0.07 | 0.76 ± 0.03 | –0.08 ± 0.18 | 0.988 | 2.20 |
| BzOCOF | 16 | 1.57 ± 0.20 | 0.76 ± 0.08 | –0.13 ± 0.27 | 0.933 | 2.07 |
| BzOCOF | 13j | 1.43 ± 0.13 | 0.70 ± 0.05 | –0.09 ± 0.17 | 0.974 | 2.04 |
| C6H5COFk | 41 | 1.58 ± 0.09 | 0.82 ± 0.05 | –0.09 ± 0.10 | 0.953 | 1.93 |
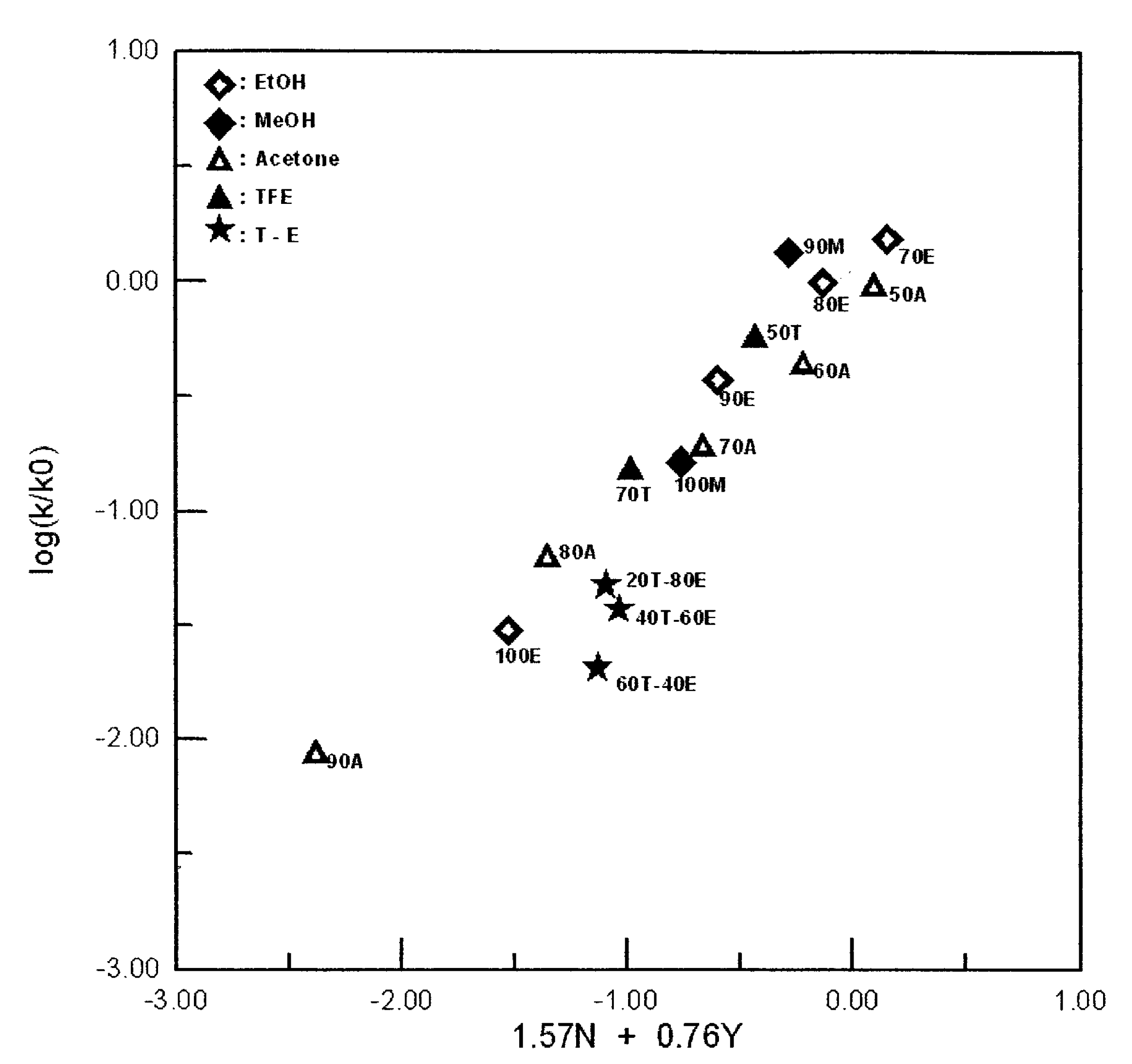
With incorporation of all 16 of the standard systems, the correlation coefficient of 0.933 (
F-test value of 44) is rather low and an inspection of the plot shows that, as is frequently the case [
14], the TFE–ethanol points lie somewhat below the plot (
Figure 1). Omission of these three points does not appreciably change the
l and
m values (
Figure 2), but it does lead to a considerable improvement in the correlation coefficient, to 0.974 (
F-test value of 90). The entries presented in
Table 3 for comparison show that the
l and
m values are very similar to those previously observed for
n-octyl fluoroformate [
9] and benzoyl fluoride [
15]. These three solvolyses have an
l/
m ratio which is very close to two in value. A direct logarithmic comparison of the specific rates of solvolysis of benzyl fluoroformate with those for
n-octyl fluoroformate in 14 solvents (no values for the
n-octyl ester in 70% and 50% acetone) gave an acceptable correlation, with a slope of 0.95 ± 0.05, intercept of 0.10 ± 0.06, correlation coefficient of 0.986, and an
F-test value of 415.
Figure 1.
The plot of log(k/k0) vs. (1.57NT + 0.76YCl) for the solvolyses of benzyl fluoroformate in pure and binary solvents at 25.0 °C. In the key to symbols, only components other than water are indicated.
Figure 1.
The plot of log(k/k0) vs. (1.57NT + 0.76YCl) for the solvolyses of benzyl fluoroformate in pure and binary solvents at 25.0 °C. In the key to symbols, only components other than water are indicated.
Figure 2.
The plot of log(k/k0) vs. (1.43NT + 0.70YCl) for the solvolyses of benzyl fluoroformate in pure and binary solvents at 25.0 °C. The three TFE–ethanol systems are omitted from the correlation. In the key to symbols, only components other than water are indicated.
Figure 2.
The plot of log(k/k0) vs. (1.43NT + 0.70YCl) for the solvolyses of benzyl fluoroformate in pure and binary solvents at 25.0 °C. The three TFE–ethanol systems are omitted from the correlation. In the key to symbols, only components other than water are indicated.
Data from the correlations of several chloroformate esters, in the region where they solvolyze by the addition–elimination mechanism, are also included in
Table 3. For the fluoroformates, the
m values are appreciably higher, by some 30%–40%. Accordingly, the
l/
m ratio is higher for the chloroformates, with values ranging from 2.7–3.5.
The higher
m values for the solvolyses of fluoroformates, relative to chloroformates, may reflect the influence of the high electronegativity of fluorine. This can lead to the need for increased interactions with the solvent, in the process moving the Π electrons of the carbonyl group away from the fluorine and onto the oxygen (
Scheme 1). Such interactions can involve increased solvation of the developing anionic center (higher
m values). Another way of describing this situation is in terms of a later transition state for the rate-determining addition step.
For methanolysis, the solvent deuterium isotope effect (
kMeOH/
kMeOD) was determined (
Table 1). The value of 3.19 ± 0.14 at 25.0 °C is higher than for the methanolysis of
p-nitrobenzyl chloroformate (2.42 ± 0.03) [
4] or for the ethanolysis of a series of
para-substituted phenyl chloroformates, where values in the range of 2.1–2.4 were obtained [
16]. The higher value gives further support for the proposal that bond formation is more advanced at the transition state for addition to fluoroformates than for chloroformates.
For solvolyses in ethanol and 80% ethanol, activation parameters were determined (
Table 1). The appreciably negative entropy of activation values of –39.4 and –30.3 cal mol
–1 K
–1, respectively, are consistent with the bimolecular character of the proposed rate-determining addition.
Product studies were based on determinations by gas chromatography at 10 half lives, estimated from the specific rates of
Table 1. The values remained unchanged for up to at least 20 half lives. The percentage compositions and the selectivity values calculated using equation 2 or 3 (as appropriate) are reported in
Table 2. The 4.1% of alcohol after solvolysis in 100% ethanol is similar to the 2.7% found after ethanolysis of 1-adamantyl chloroformate [
7] and the 3.3% found after ethanolysis of 1-adamantyl fluoroformate [
10]. As previously proposed [
7,
10], this probably results from reaction of the substrate with moisture during manipulation. In the calculations of selectivities using equation 2, this amount is deducted from the percentages of benzyl alcohol entered into the equation, with percentage-composition ratios being used to represent the molar ratio of products.
For solvolyses in aqueous ethanol, the selectivity values increase steadily from a value of 2.2 in 90% ethanol to 3.6 in 50% ethanol. These values are remarkably similar to those observed in the corresponding solvolyses of benzyl chloroformate (2.0–4.1). Since the benzyl esters of both chloroformic and fluoroformic acids give these similar ratios, it can be taken as a strong indication that they both solvolyze by the same mechanism, which we believe to be addition–elimination, with the addition step being rate determining.
In contrast, for solvolyses in 70% and 50% TFE, the ratios of 0.12 and 0.19 for the fluoroformate are considerably lower than the solvolysis–decomposition values of 1.04 and 1.20 for the chloroformate, consistent with the addition–elimination pathway remaining dominant for the fluoroformate but, as previously proposed [
4], the chloroformate having switched over to the ionization pathway (
Scheme 2).
The solvolyses in TFE–ethanol mixtures very much favor product formation involving attack by the considerably more nucleophilic ethanol, with
S values (equation 3) of 0.043 in 80TFE–20E and 0.036 in 60TFE–40E. Since, for 1-adamantyl fluoroformate, the 60TFE–40E solvolysis is primarily within the addition–elimination region, it was possible [
10] to apply equation 3. The
S value of 0.039 reported is essentially identical to that of the present study.
The consideration of fluoride/chloride ratios (
kF/
kCl) in nucleophilic substitution reactions has long been recognized as a useful tool in studying the reaction mechanism [
17]. This is especially so when the attack is at an acyl carbon. For S
N1 reaction, a value as low as 10
–7 was observed in 4-(
N,N-dimethylamino)benzoyl halide solvolyses [
18] and a low value of 1.3∙10
–4 was also observed for acetyl halide solvolyses in 75% acetone [
17]. These values reflect an appreciable ground-state stabilization for the fluoride [
19] and the need to break a strong carbon–fluorine bond in the rate determining step. In contrast, if the addition step is rate-determining, values of close to unity (and frequently above it), reflecting a large electron deficiency at the carbonyl carbon of a haloformate incorporating fluorine [
15], are frequently observed. This situation has recently been discussed in a consideration of
n-octyl haloformate solvolyses [
9], where
kF/
kCl specific rate ratios of 0.6 to 15 were observed.
Due to the previous study of benzyl chloroformate [
4] involving 14 of the 16 solvent compositions of the present study, a wide range of
kF/
kCl values are available. These vary from a low of 1.1 in 100% ethanol (similar to the 0.6 for
n-octyl haloformates) to a high of 14.6 in 70% ethanol (similar to the 15 for
n-octyl haloformates in 60% ethanol). The values for 70% TFE and 50% TFE (6.36 and 12.3) are quite high despite there being an appreciable ionization component for the chloride [
4], which will lead to the experimental
kCl value being higher than the value required for a consideration of the
kF/
kCl ratio for the addition–elimination pathway and a low value for this ratio. One can roughly extrapolate to a value of about 4 for the
kF/
kCl ratio in 80% TFE, a value somewhat less than the value of 10.2 obtained [
9] for the solvolyses of
n-octyl haloformates, giving some support to this prediction.