Introduction
Substitution of any six-membered aromatic or heteroaromatic ring by a certain substituent causes changes of the chemical shifts of all carbon atoms in the ring. It is generally assumed that the substituent induced change in the
13C-NMR chemical shift is an additive property. The semiempirical methods still commonly used for prediction (precalculation) of the
13C-NMR chemical shifts for various kinds of compounds are based on this assumption [
1,
2,
3,
4,
5,
6]. The chemical shifts of carbon atoms in a compound containing a given substituent are calculated by addition of certain constant values, called additivity parameters, or SCS (for Substituent Induced Chemical Shifts), to the chemical shifts of carbon atoms in the parent unsubstituted compound or to the one containing a methyl group at this site. These parameters are characteristic for the substituent and its position with respect to the carbon atom in question. For example, chemical shifts of monosubstituted benzene derivatives are calculated from relation (1) [
3,
4]:
where 128.5 is the chemical shift of all carbon atoms in an unsubstituted benzene ring and Ai(R) are additivity parameters of substituents R in the
ipso-,
ortho-,
meta- or
para- positions with respect to the carbon atom C(k).
The same approach is applied for pyridine derivatives [
3,
4] and other heterocyclic compounds such as pyrrole [
7,
8], furan [
9,
10], thiophene [
9,
11]. This approach implies that for each type of heterocyclic ring and, moreover, for each site of substitution a separate set of parameters has to be used. For a given substituent the values of these parameters, even for analogous substitution sites (
e.g. for carbon atom bonded to substituent), are considerably different. Thus, for pyridine derivatives there are three sets of parameters: for α, β and γ substituted ones, and each set contains separate additivity parameters for each carbon atom [
4,
12,
13]. For other heterocycles other sets of parameters are used. Chemical shifts of carbon atoms in pyridine derivatives are calculated [
3,
4] from the relation (2):
where δC(k) is the chemical shift of the carbon atom
k in a pyridine ring containing substituents R
i; C
k is the chemical shift of the same atom in an unsubstituted pyridine; and Aik is a so called "additivity parameter", which tells us how much the chemical shift of carbon atom
k is changed as a result of substitution of carbon atom
i by the group R.
Additivity parameters for six-membered heteroaromatic rings considerably differ from those for the corresponding carbon atoms in benzene derivatives. The best example is provided by the C-2 atom in 2-substituted pyridine derivatives, where "additivity parameters" for pyridine are equal to about 0.5 of these for benzene. Similar variations of parameters are encountered for other aromatic and heteroaromatic compounds [
7,
8,
9,
10,
14,
15].
It is obvious that the changes of chemical shifts of carbon atoms in substituted compound with respect to unsubstituted ones depends mainly on the polarity of the substituent (which is constant), on the distance (in bonds) from the substituent to the carbon atom in question, and on the kind of the parent compound. Any substituent, however, has definite electron donating or electron withdrawing properties. Thus, it seemed reasonable to assume that different results of substitution by the same group in various compounds are due to differences in transmission of these effects through the bonds in the ring and to differences of susceptibility of a certain carbon atom in the ring of various types of aromatic and heteroaromatic compounds to polar effects of a substituent. As a consequence, chemical shifts of carbon atoms in all aromatic and heteroaromatic rings should correlate with just one common set of parameters, but these parameters will be multiplied by a certain factor depending on the nature of the ring and the position of substituent.
Some support for this assumption was provided by study on relations between basicity and substitution [
16,
17,
18,
19,
20] where it was shown that the polar effects of a substituent in a conjugated system can be considerably altered by changes of electron density in the system influencing transmission of these effects through the bonds.
In this work the 13C-NMR chemical shifts for monosubstituted derivatives of pyridine (1-3), pyrazine (4) and pyrimidine (5) have been collected from literature (total 77 cpds.) and to check aforesaid assumption were correlated with one set of corresponding parameters common for all studied compounds.
![Ijms 06 00011 i001]()
Results and Discussion
As the most convenient common parameters for the purpose I have chosen so called "additivity parameters" derived from monosubstituted benzene derivatives, because they are are the most thoroughly studied ones, and their
13C-NMR spectra recorded in various solvents provide a rich collection of the data [
21,
22,
23,
24]. The correlations are in the form of the equation (3):
where δ
oC(k) is the chemical shift of the carbon atom
k in an unsubstituted six-membered ring, A
g(R) is a so called "additivity parameter" of a substituent R at the site s, derived from chemical shift in corresponding monosubstituted phenyl derivative;
is the coefficient specific for the substitution site
s and its geometrical position
g,
i.e. i (
ipso), o (
ortho), m (
meta) or p (
para) with respect to the substituent R in a certain heterocyclic ring. On the assumption that the polar effects exerted by a substituent in any six-membered heteroaromatic ring on chemical shift are the same as in benzene, the chemical shifts of carbon atom bonded to a substituent, according to equation (3), can be correlated with A
i parameters for benzene derivatives. Those of carbon atoms two bonds away from a substituent,
i.e. in a position corresponding to
ortho- in benzene, with A
o parameters, those of carbon atoms three bonds away with A
m, whereas for carbon atoms four bonds away,
i.e. in position corresponding to
para- in benzene, A
p parameters should be appropriate.
The
13C-NMR spectra of studied compounds accessible in the literature were recorded in various solvents such as CDCl
3, acetone, DMSO, or CS
2. Therefore calculations for a given group of compounds in each solvent were made separately to obtain reliable results. The results indicate that the chemical shifts in all the above mentioned solvents can be correlated with parameters derived for benzene derivatives. For some carbon atoms, however, the slopes of correlation lines obtained for spectra recorded in acetone, DMSO or CS
2 are significantly different from those obtained for spectra recorded in CDCl
3 solutions (cf.
Figure 1 and
Table 1,
Table 2 and
Table 3), thus indicating that the changes of chemical shifts following the change of a solvent are due not only to a magnetic susceptibility of a solvent, but also to a considerable extent to solvation effects. First attempts at correlations revealed that experimental points for all hydroxy derivatives deviate considerably from the obtained correlations. This can be explained by hydrogen bond formation between the hydroxy group and the nitrogen atom in the ring. Nitrogen heterocycles, as bases, may form hydrogen bonds with proton donating groups such as OH, which involves a change in the chemical shifts of all carbon atoms in a ring, analogous to those observed in the case of protonation of pyridine [
25]. For this reason experimental points for hydroxy derivatives may deviate considerably from obtained correlations, and thus were not taken into account in calculations of regression parameters. The parameters of equation 3 found by the least squares method for carbon atoms in studied compounds are summarized in
Table 1,
Table 2 and
Table 3.
Carbon atoms bonded to a substituent
The very large range of both chemical shifts of carbon atoms in studied compounds (80 ppm) as well as A
ipso(R) parameters (~70 ppm) makes the correlations the most reliable. Close inspection of experimental points pattern for C-2 in 2-substuituted pyridines indicated, however, that there are two separate correlation lines: one for bulky substituents such as Br or substituents of the general formula CH2R, and a second line for other substituents (cf.
Figure 2). Therefore they were calculated separately.
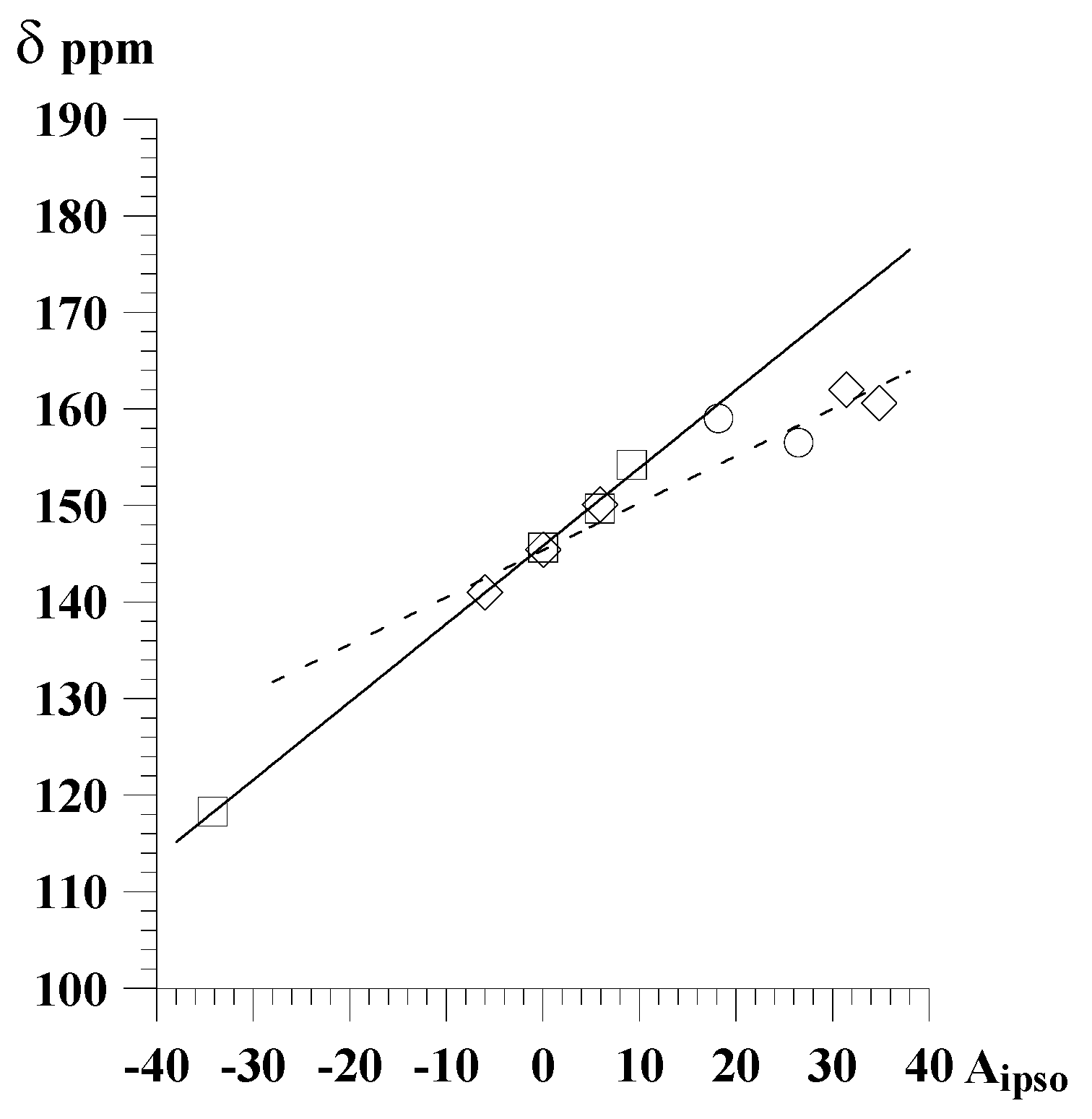
Figure 1.
Correlation of chemical shifts of carbon atoms bonded to a substituent in 2-substituted pyrazine derivatives with the A
i parameters
- □ -
in CDCl3 solutions,
- ○ -
in DMSO solutions
Figure 1.
Correlation of chemical shifts of carbon atoms bonded to a substituent in 2-substituted pyrazine derivatives with the A
i parameters
- □ -
in CDCl3 solutions,
- ○ -
in DMSO solutions
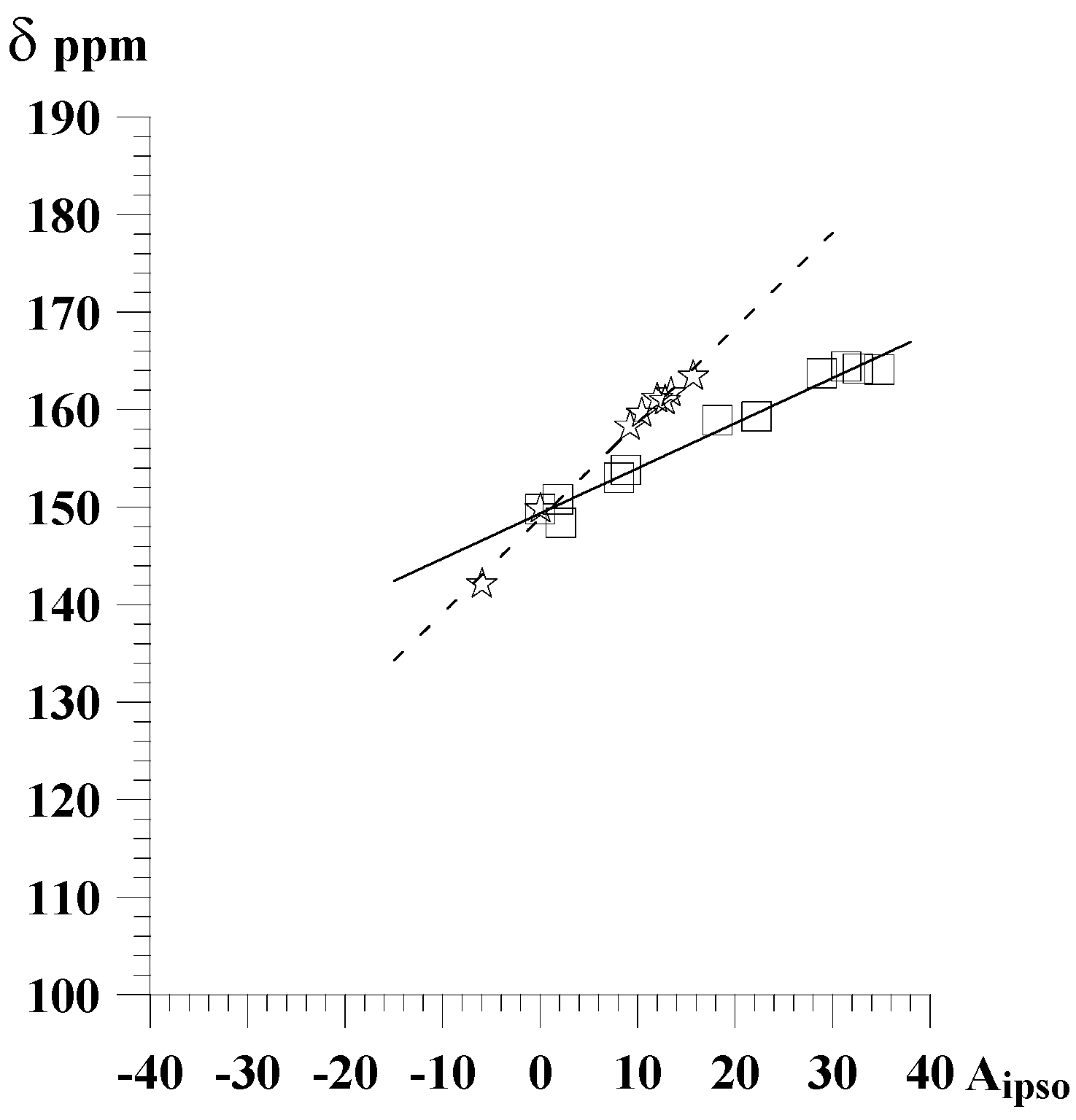
Figure 2.
Correlation of chemical shifts of carbon atoms bonded to a substituent in 2-substituted pyridine derivatives with the A
i parameters
- ✰ -
bulky substituents
- □ -
other substituents
Figure 2.
Correlation of chemical shifts of carbon atoms bonded to a substituent in 2-substituted pyridine derivatives with the A
i parameters
- ✰ -
bulky substituents
- □ -
other substituents
Considerable differences between the slopes of correlation lines are the most probaly due to the change in geometry of a molecule caused by steric repulsion between bulky substituent and lone electron pair of pyridine nitrogen atom, causing changes in transmission of polar effects.
Thus obtained correlations of chemical shifts of carbon atoms bonded to a substituent with Ai parameters are for most of studied series of at least good quality, as indicated by the correlation coefficients r (over 0.95). In 2- 3- or 4-substituted pyridines for carbon atoms bonded to substituent the correlations with the Ai parameters are of excellent quality, as indicated by the correlation coefficients r (over 0.99). The parameter
for 3- and 4-substituted pyridines, as well as for 2-substituted ones with bulky substituents is close to unity, indicating that in this case influence of a substituent on chemical shift is similar to that of benzene. However, for 2-substituted pyrazines, pyrimidines, and pyridines containing substituents without steric hindrance theyare are considerably lower (0.5 – 0.7).
As a consequence, the differences between chemical shifts of the C-2 atoms in substituted and unsubstituted compound (additivity parameters) are equal to approximately one half of that for benzene. This provides satisfactory explanation for variety of so called “additivity parameters” for pyridine derivatives reported in the literature [
1,
3,
4].
Table 1.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms bonded to substituent with Ai parameters.
Table 1.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms bonded to substituent with Ai parameters.
| Series | Atom | Solvent | na | doC(k) | | r |
|---|
| 2-substituted pyridinesb | C-2 | CDCl3 | 8 | 148.9 | 0.97 | 0.996 |
| 2-substituted pyridinesc | C-2 | CDCl3 | 12 | 149.3 | 0.46 | 0.988 |
| 3-substituted pyridines | C-3 | CDCl3 | 15 | 124.7 | 0.96 | 0.998 |
| 4-substituted pyridines | C-4 | CDCl3 | 14 | 136.2 | 1.07 | 0.992 |
| 4-substituted pyridines | C-4 | DMSO | 4 | 137.5 | 0.89 | 0.984 |
| 2-substituted pyrazines | C-2 | CDCl3 | 5 | 145.1 | 0.67 | 0.977 |
| 2-substituted pyrazines | C-2 | CS2 | 5 | 145.4 | 0.49 | 0.985 |
| 2-substituted pyrimidines | C-2 | CDCl3 | 6 | 153.8 | 0.55 | 0.878 |
| 2-substituted pyrimidines | C-2 | Acetone | 8 | 157.6 | 0.47 | 0.907 |
| 2-substituted pyrimidines | C-2 | DMSO | 4 | 159.7 | 0.31 | 0.613 |
Carbon atoms two bonds away from a substituent
For carbon atoms two bonds away from a substituent, i.e. in position corresponding to ortho- in the benzene ring, both the values of both chemical shifts and substituent parameters vary within a much smaller range (20-25 ppm, and 24.5 ppm respectively), but still enabling one to draw reliable conclusions. In this case correlations with Ao parameters are of at least satisfactory quality (r > 0.90) and for C-2 in 3-substituted pyridines as well as C-3 in 2 substituted pyrazines correlations are even excellent.
Table 2.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms two bonds away from substituent with Ao parameters.
Table 2.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms two bonds away from substituent with Ao parameters.
| Series | Atom | Solvent | na | doC(k) | | r |
|---|
| 2-substituted pyridines | C-3b | CDCl3 | 8 | 123.2 | 1.39 | 0.901 |
| 2-substituted pyridines | C-3c | CDCl3 | 12 | 122.8 | 0.99 | 0.974 |
| 3-substituted pyridines | C-2 | CDCl3 | 14 | 149.5 | 0.86 | 0.985 |
| 3-substituted pyridines | C-4 | CDCl3 | 15 | 135.8 | 1.04 | 0.995 |
| 4-substituted pyridines | C-3 | CDCl3 | 15 | 123.4 | 0.62 | 0.909 |
| 4-substituted pyridines | C-3 | DMSO | 4 | 122.9 | 0.94 | 0.907 |
| 2-substituted pyrazines | C-3 | CDCl3 | 5 | 145.6 | 0.69 | 0.990 |
| 2-substituted pyrazines | C-3 | CS2 | 5 | 145.7 | 0.45 | 0.751 |
For these atoms again in most of the cases the slope of correlation line is considerably different from unity. It is worth to notice that for C-3 in 4-substituted pyridines the parameter
obtained for solutions in deuteriochloroform and DMSO are considerably different.
Carbon atoms four bonds away from a substituent
For carbon atoms four bonds away from a substituent, i.e. in a position corresponding to para- in a benzene ring, both the chemical shifts and substituent parameters Ap vary in a range similar to that for carbon atoms 3 bonds away. Correlations obtained for these carbon atoms are in most of the cases at least of good quality, only for C-5 in 2-substituted pyrazines recorded in CS2 and C-5 in 2-substituted pyrimidines recorded in acetone they are only satisfactory. Influence of a solvent on the slope of corelation line is the most evident in the case of pyrimidines: 0.62 in acetone, 1.07 in deuteriochloroform and 1.23 in dimethylsulfoxide. As the differences between chemical shifts for all compounds in a series recorded in these solvents are not identical, or even similar, they can be explained only by solvation effects.
Table 3.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms four bonds away from substituent with Ap parameters.
Table 3.
Parameters of linear regressions (equation 3) of the chemical shifts of carbon atoms four bonds away from substituent with Ap parameters.
| Series | Atom | Solvent | na | doC(k) | | r |
|---|
| 2-substituted pyridines | C-5 | CDCl3 | 19 | 123.1 | 0.83 | 0.949 |
| 3-substituted pyridines | C-6 | CDCl3 | 15 | 149.4 | 0.92 | 0.972 |
| 2-substituted pyrazines | C-5 | CDCl3 | 5 | 145.1 | 1.09 | 0.979 |
| 2-substituted pyrazines | C-5 | CS2 | 5 | 144.5 | 0.52 | 0.915 |
| 2-substituted pyrimidines | C-5 | CDCl3 | 6 | 121.7 | 1.07 | 0.974 |
| 2-substituted pyrimidines | C-5 | Acetone | 8 | 122.1 | 0.62 | 0.924 |
| 2-substituted pyrimidines | C-5 | DMSO | 4 | 122.5 | 1.23 | 0.993 |
Carbon atoms three bonds away from a substituent
The changes of chemical shifts of carbon atoms three bonds away from substituent i.e. in positions corresponding to the meta position in benzene, in all series of studied compounds they are about ten times smaller and in some instances amount to about one third (or even half) of the differences between the values reported in various sources. Moreover the values of substituent parameters Am vary in a very narrow range below 2 ppm, which is of the same order as the differences between the data for the same compound but from various sources. Therefore relations between chemical shifts of these atoms and the Am parameters are less evident, however, in some instances, the tendency is still noticeable.