Intramolecular C−H···π Interactions in Metal-Porphyrin Complexes
Abstract
:Introduction
Data Screening and Computational Methods
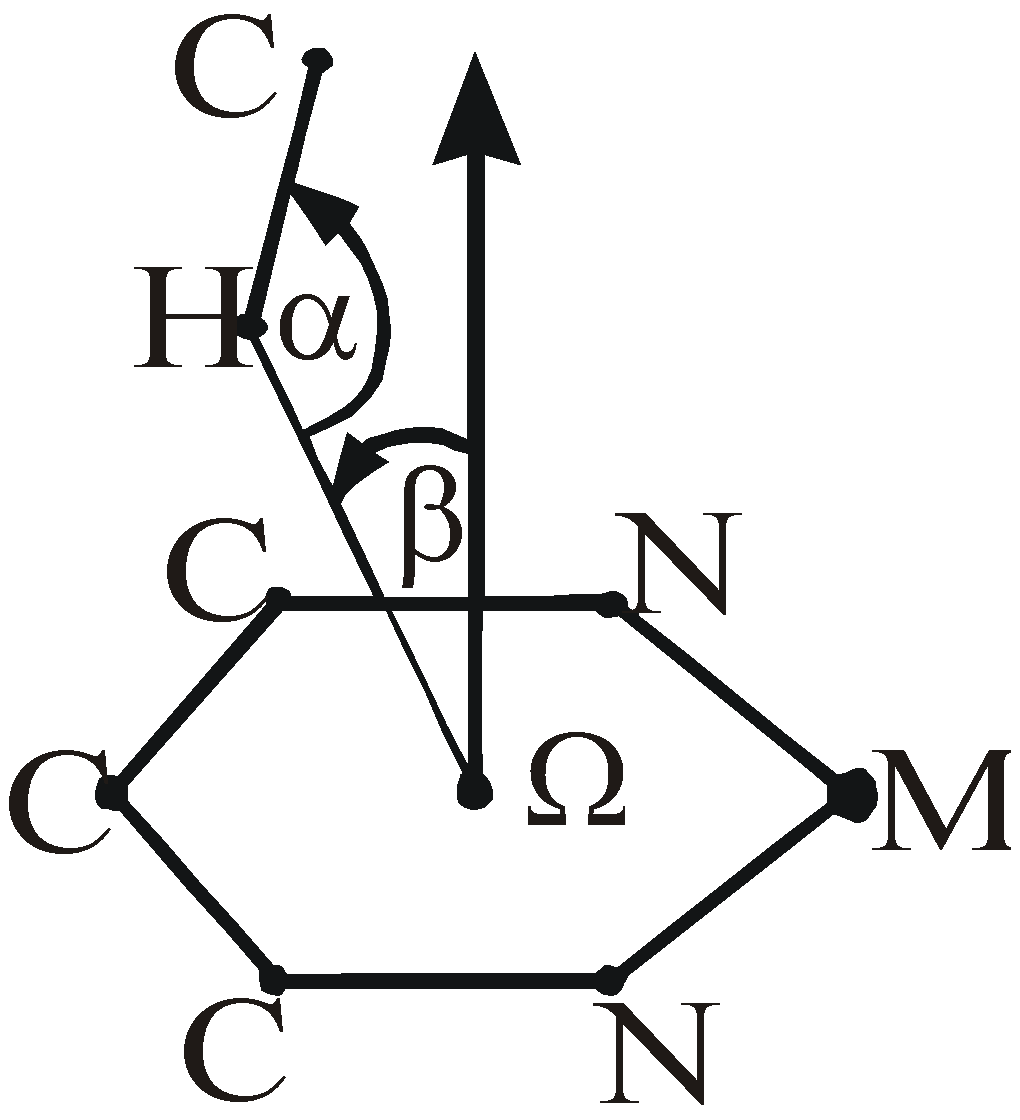
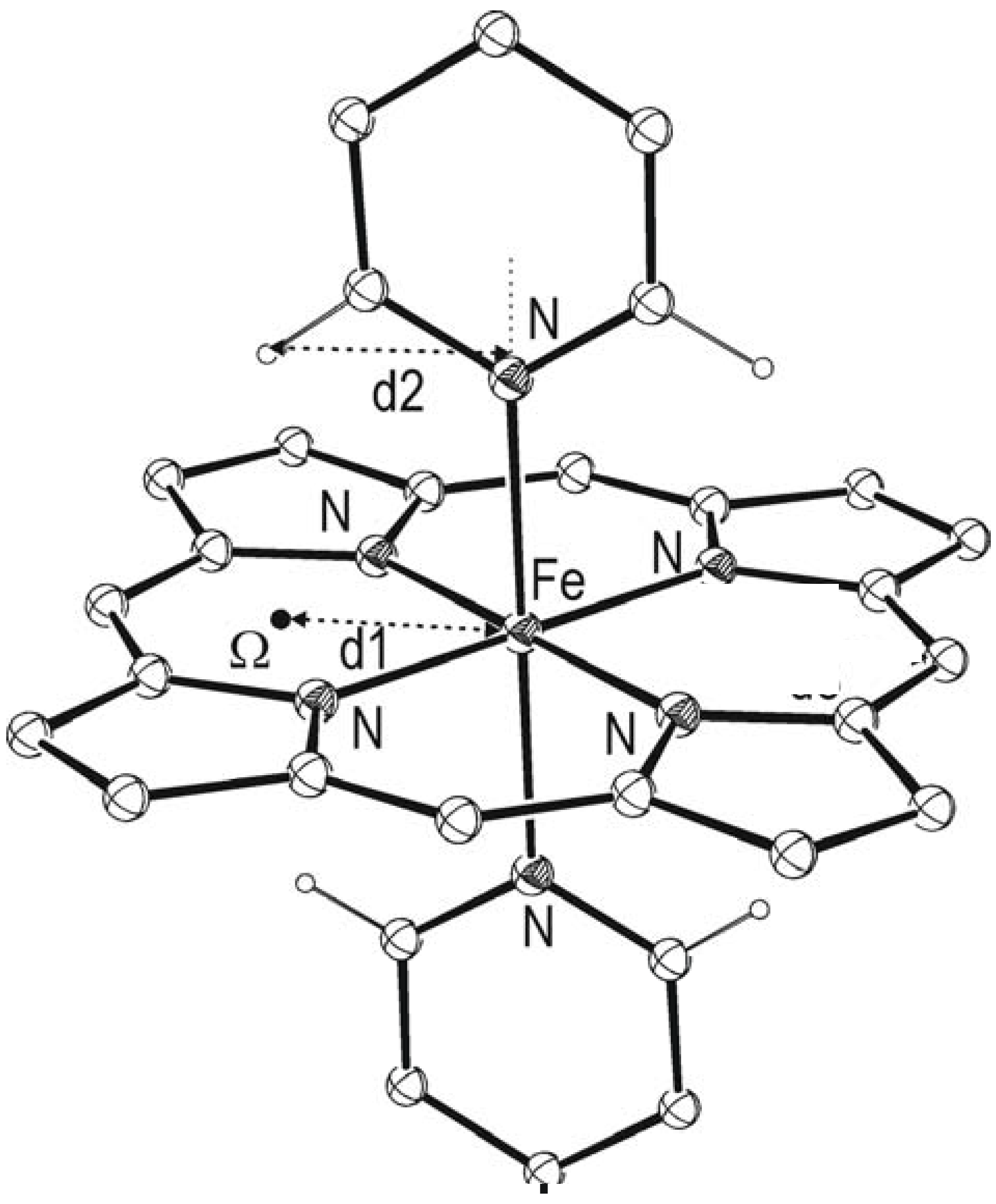
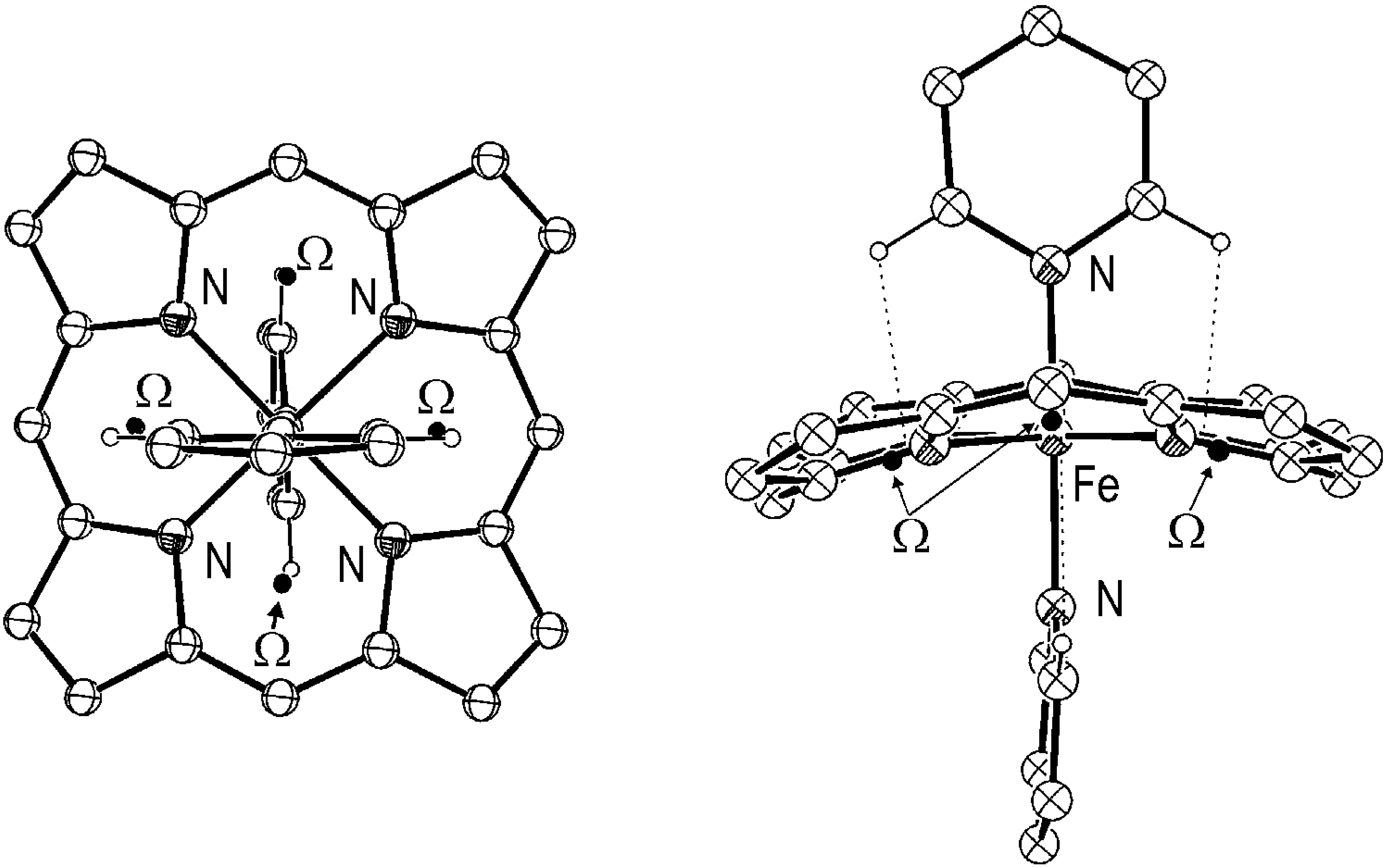
Results and discussion
| Refcode | Metal | β (o) | H… Ω (Å) | α(o) | Ref. No. | |
|---|---|---|---|---|---|---|
| 1 | DIQWOP | Ru4+ | 12.2 | 2.260 | 123.7 | 17 |
| 2 | HAMDII | Zn2+ | 5.8 | 2.396 | 118.9 | 18 |
| 3 | MAZVAKb | 2 Zn2+ | 9.8 | 2.397 | 125.5 | 19 |
| 4 | POZQIE | Co3+ | 7.0 | 2.344 | 139.0 | 20 |
| 11.2 | 2.240 | 135.1 | ||||
| 5 | VATXIX | Zn2+ | 14.6 | 2.324 | 144.8 | 21 |
| 6 | YIYPOLb | 2 Zn2+ | 3.9 | 2.145 | 153.1 | 22 |
| 7 | BAFKEZ | Ru2+ | 15.6 | 2.425 | 120.6 | 23 |
| 8 | LODQEA | Zn2+ | 1.9 | 2.466 | 125.6 | 24 |
| 9 | NACTUGb | 2 Ni2+ | 11.8 | 2.445 | 135.3 | 25 |
| 10 | HUHLEB | Ru 2+ | 9.6 | 2.454 | 144.9 | 26 |
| 11 | BMPRCU | Cu2+ | 9.8 | 2.561 | 161.1 | 27 |
| 12 | GITLEA | Ni2+ | 2.4 | 2.501 | 112.0 | 28 |
| 13 | KACGIE | Fe2+ | 9.3 | 2.513 | 116.6 | 29 |
| 8.9 | 2.722 | 110.2 | ||||
| 14 | ZAWBAA01 | Fe3+ | 10.8 | 2.576 | 163.9 | 30 |
| 15 | BONREB | Zr4+ | 6.3 | 2.615 | 117.2 | 31 |
| 16 | CAFBANb | 2 Zn2+ | 12.9 | 2.647 | 139.9 | 32 |
| 17 | HAMMIR | Zn2+ | 15.2 | 2.824 | 117.2 | 33 |
| 18 | PAFTUL | Fe3+ | 13.0 | 2.734 | 161.6 | 34 |
| 19 | QOHWEP | Ti2+ | 15.6 | 2.601 | 129.7 | 35 |
| 20 | TAKVOQb | Cu2+ Fe3+ | 12.2 | 2.858 | 147.2 | 36 |
| 21 | VUPKUM | Ru2+ | 15.7 | 2.655 | 161.3 | 37 |
| 22 | WAVDOM10b | Cu2+ Fe3+ | 12.8 | 2.735 | 127.3 | 38 |
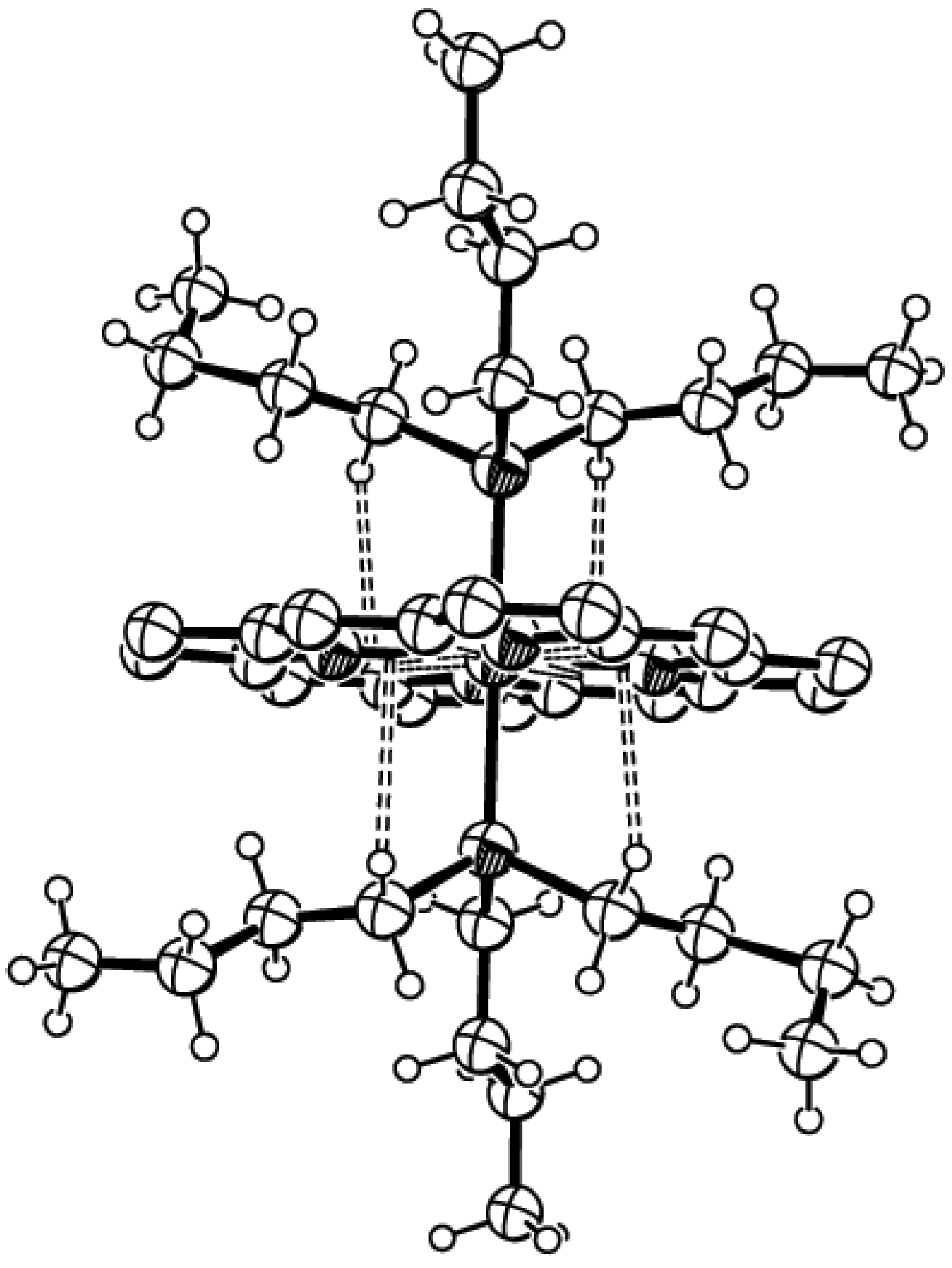


| Refcode | Metal | β (o) | H…Ω (Å) | α(o) | Fe…Ω (Å) | P/Nb | Ref. No. | |
|---|---|---|---|---|---|---|---|---|
| 1 | CPOEFE10c | Fe3+ | 3.7 | 2.40 | 119.3 | 1.98 | P | 39 |
| 6.2 | 2.21 | 123.0 | ||||||
| 2 | DAJJAZc | Fe2+ | 10.3 | 2.22 | 117.2 | 1.97 | P | 40 |
| 9.5 | 2.22 | 118.7 | ||||||
| 3 | FUXTUNc | Fe2+ | 6.6 | 2.16 | 120.2 | 1.91 | P | 41 |
| 3.3 | 2.17 | 119.2 | ||||||
| 4 | NIWLOUc | Fe2+ | 4.3 | 2.16 | 117.0 | 1.98 | P | 42 |
| 6.1 | 2.19 | 115.9 | ||||||
| 5 | NIWLUAc | Fe2+ | 5.6 | 2.15 | 117.3 | 1.95 | P | 42 |
| 2.7 | 2.22 | 116.5 | ||||||
| 6 | NIWMAHc | Fe2+ | 2.9 | 2.24 | 115.7 | 1.96 | P | 42 |
| 5.7 | 2.17 | 117.3 | ||||||
| 7 | VOFLORc | Fe3+ | 8.4 | 2.18 | 116.1 | 1.98 | P | 43 |
| 8.1 | 2.23 | 116.5 | ||||||
| 8 | GEWKOI | Fe3+ | 9.9 | 2.30 | 115.8 | 1.96 | N | 44 |
| 5.6 | 2.32 | 115.2 | ||||||
| 3.7 | 2.22 | 116.9 | ||||||
| 6.6 | 2.26 | 116.3 | ||||||
| 9 | HERZIN | Fe3+ | 9.8 | 2.38 | 115.6 | 1.93 | N | 45 |
| 10.1 | 2.42 | 114.6 | ||||||
| 8.1 | 2.32 | 116.6 | ||||||
| 8.9 | 2.35 | 115.4 | ||||||
| 10 | KEFFOQ | Fe2+ | 7.6 | 2.40 | 116.4 | 1.93 | N | 46 |
| 6.3 | 2.37 | 116.3 | ||||||
| 6.8 | 2.38 | 116.2 | ||||||
| 7.2 | 2.39 | 116.9 | ||||||
| 11 | PALVED | Fe3+ | 4.5 | 2.30 | 116.6 | 1.94 | N | 47 |
| 2.5 | 2.25 | 116.0 | ||||||
| 2.0 | 2.23 | 117.5 | ||||||
| 7.1 | 2.42 | 115.4 | ||||||
| 12 | PALVIH | Fe3+ | 5.0 | 2.33 | 116.1 | 1.95 | N | 47 |
| 4.1 | 2.33 | 116.2 | ||||||
| 10.5 | 2.27 | 114.8 | ||||||
| 9.8 | 2.29 | 113.4 | ||||||
| 13 | PALVON | Fe3+ | 1.2 | 2.15 | 118.9 | 1.94 | N | 47 |
| 5.8 | 2.42 | 115.2 | ||||||
| 2.6 | 2.19 | 115.3 | ||||||
| 9.0 | 2.47 | 115.1 | ||||||
| 14 | TUBJAB | Fe2+ | 11.6 | 2.45 | 116.7 | 1.93 | N | 48 |
| 12.1 | 2.38 | 117.9 | ||||||
| 7.2 | 2.37 | 118.5 | ||||||
| 7.5 | 2.38 | 117.8 | ||||||
| 15 | VOFLUX | Fe3+ | 9.2 | 2.32 | 116.3 | 1.94 | N | 43 |
| 10.5 | 2.41 | 115.2 | ||||||
| 5.2 | 2.41 | 114.3 | ||||||
| 6.0 | 2.32 | 117.1 |
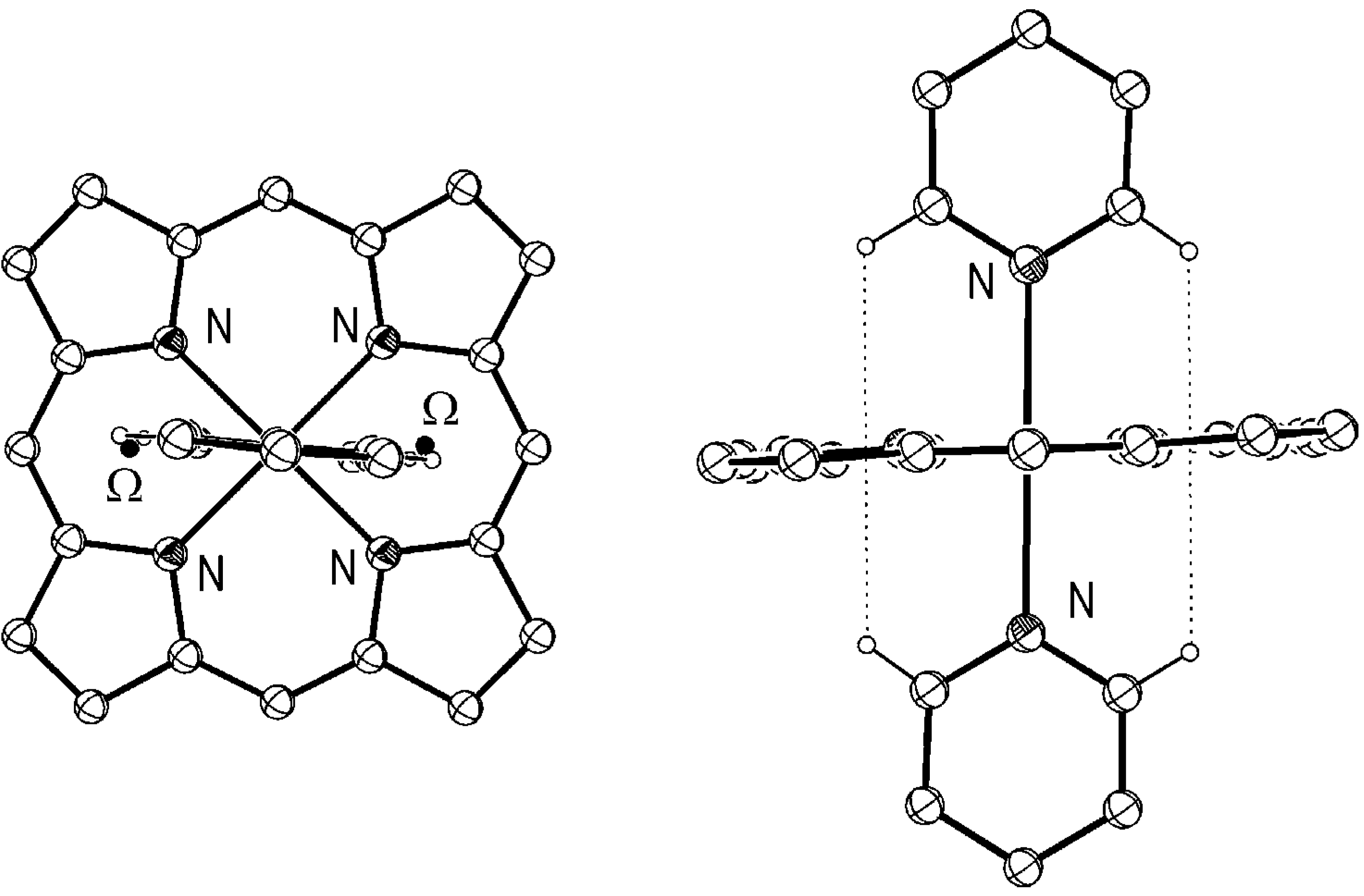

Conclusion
References
- Ma, J. C.; Daugherty, D. A. Chem. Rev. 1997, 97, 1303. Daugherty, D. A. Science 1996, 271, 163. Steiner, T. Angew. Chem. Int. Ed. 2002, 41, 48. Yamauchi, O. O.; Takani, M. J. Chem. Soc.-Dalton Trans. 2002, 3411. Meyer, E. A.; Castellano, R. K.; Diederich, F. Angew. Chem.-Int. Edit. 2003, 42, 1210. Schmitt, W.; Anson, C. E.; Hill, J. P.; Powell, A. K. J. Am. Chem. Soc. 2003, 125, 11142. Amunugama, R.; Rodgers, M. T. Int. J. Mass Spectrom. 2003, 227, 339. Zhu, W. L.; Tan, X. J.; Shen, J. H.; Luo, X. M.; Cheng, F.; Mok, P. C.; Ji, R. Y.; Chen, K. X.; Jiang, H. L. J. Phys. Chem. A 2003, 107, 229. Pletneva, E. V.; Laederach, A. T.; Fulton, D. B.; Kostić, N. M. J. Am. Chem. Soc. 2001, 123, 623. Burghardt, T. P.; Juranić, N.; Macura, S.; Ajtai, K. Biopolimers 2002, 63, 261. Kim, D.; Hu, S.; Tarakeshwar, P.; Kim, K.S.; Lisy, J.M. J. Phys. Chem. A 2003, 107, 1228.
- Zarić, S. D. Eur. J. Inorg. Chem. 2003, 2197. Zarić, S. D. Chem. Phys. Lett. 1999, 311, 77. Zarić, S. D. Chem. Phys. 2000, 256, 213. Zarić, S. D.; Popović, D. M.; Knapp, E. W. Chemistry 2000, 6, 3935. Milčić, M.; Zarić, S. D. Eur. J. Inorg. Chem. 2001, 2143. Žmirić, A.; Milčić, M.; Zarić, S. D. Int. J. Quantum Chem. 2002, 87, 354. McFail-Isom, L.; Shui, X.; Williams, L. D. Biochemistry 1998, 37, 17105. Kumita, H.; Kato, T.; Jitsukawa, K.; Einaga, H.; Masuda, H. Inorg. Chem. 2001, 40, 393. Suezawa, H.; Yoshida, T.; Umezawa, Y.; Tsuboyama, S.; Nishio, M. Eur. J. Inorg. Chem. 2002, 3148. Sénèque, O.; Giorgi, M.; Reinaud, O. Chem. Commun. 2001, 984. [poner, J.; [poner, J. E.; Leszczynski, J. J. Biomol. Struct. Dyn. 2000, 17, 1087.
- Bogdanović, G. A.; Bire, A. S.; Zarić, S. D. Eur. J. Inorg. Chem. 2002, 599.
- Tomić, Z. D.; Leovac, V. M.; Pokorni, S. V.; Zobel, D.; Zarić, S. D. Eur. J. Inorg. Chem. 2003, 1222.
- Castineiras, A.; Sicilia-Zafra, A. G.; Gonzalez-Perez, J. M.; Choquesillo-Lazarte, D.; Niclos-Gutierrez, J. Inorg. Chem. 2002, 41, 6956.
- Nishio, M.; Hirota, M.; Umezawa, Y. The CH/р Interaction, Evidence, Nature and Consequences; John Wiley & Sons, Inc.: New York, 1998. [Google Scholar]
- Walker, F. A.; Huynh, B.H.; Schedt, W.R.; Osvath, S.R. J. Am. Chem. Soc. 1986, 108, 5288. Walker, F. A. Chem. Rev. 2004, 104, 589. Menyhard, D.K.; Keseru, G.M. J. Am. Chem. Soc. 1998, 120, 7991.
- Zarić, S. D.; Popović, D. M.; Knapp, E. W. Biochemistry 2000, 40, 7914. [CrossRef]
- Medaković, V. B.; Zarić, S. D. Inorg. Chim. Acta 2003, 349, 1.
- Ghosh, A.; Gonzales, E.; Vangberg, T. J. Phys. Chem. 1999, 103, 1363.
- Allen, F. H.; Bellard, S.; Brice, M. D.; Cartwright, B. A.; Doubleday, A.; Higgs, H.; Hummelink, T.; Hummelink-Peters, B. G.; Kennard, O.; Motherwell, W. D. S.; Rodgers, J. R.; Watson, D. G. Acta Cryst. 1979, B35, 2331.
- Ciunik, Z.; Desiraju, G. R. Chem. Commun. 2001, 703.
- Steiner, T.; Desiraju, G. R. Chem. Commun. 1998, 891.
- Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
- Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. [CrossRef]
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M.A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, J. A.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P.Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B.B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, Revision A.6, Gaussian, Inc., Pittsburgh PA 1998.
- Huang, J.S.; Sun, X.R.; Leung, S.K.Y.; Cheung, K.K.; Che, C.M. Chem. Eur. J. 2000, 6, 334.
- Byrn, M.P.; Curtis, C.J.; Hsiou, Y.; Khan, S.I.; Sawin, P.A.; Tendick, S.K.; Terzis, A.; Strouse, C.E. J.Am.Chem.Soc. 1993, 115, 9480. [CrossRef]
- Nakash, M.; Clyde-Watson, Z.; Feeder, N.; Teat, S.J.; Sanders, J.K.M. Chem.-Eur.J. 2000, 6, 2112.
- Simonato, J.P.; Pecaut, J.; Marchon, J.C. J.Am.Chem.Soc. 1998, 120, 7363. [CrossRef]
- Jagessar, R.C.; Shang, M.; Scheidt, W.R.; Burns, D.H. J.Am.Chem.Soc. 1998, 120, 11684. [CrossRef]
- Bag, N.; Chern, S.S.; Peng, S.M.; Chang, C.K. Inorg.Chem. 1995, 34, 753.
- Lee, J.; Twamley, B.; Richter-Addo, G.B. Chem.Commun. 2002, 380.
- Senge, M.O.; Speck, M.; Wiehe, A.; Dieks, H.; Aguirre, S.; Kurreck, H. Photochem.Photobiol. 1999, 70, 206. [CrossRef]
- Jaquinod, L.; Nurco, D.J.; Medforth, C.J.; Pandey, R.K.; Forsyth, T.P.; Olmstead, M.M.; Smith, K.M. Angew.Chem.,Int.Ed.Engl. 1996, 35, 1013. [CrossRef]
- Stulz, E.; Maue, M.; Feeder, N.; Teat, S.J.; Ng, Y. F.; Bond, A.D.; Darling, S.L.; Sanders, J.K.M. Inorg.Chem. 2002, 41, 5255.
- Collmann, J.P.; Chong, A.O.; Jameson, G.B.; Oakley, R.T.; Rose, E.; Schmittou, E.R.; Ibers, J.A. J.Am.Chem.Soc. 1981, 103, 516. [CrossRef]
- Jia, S.L.; Jentzen, W.; Shang, M.; Song, X.Z.; Ma, J.G.; Scheidt, W.R.; Shelnutt, J.A. Inorg.Chem. 1998, 37, 4402.
- Belani, R.M.; James, B.R.; Dolphin, D.; Rettig, S.J. Can.J.Chem. 1988, 66, 2072.
- Chen, L.; Yi, G.B.; Wang, L.S.; Dharmawardana, U.R.; Dart, A.C.; Khan, M.A.; Richter-Addo, G.B. Inorg.Chem. 1998, 37, 4677.
- Kim, H.J.; Kim, K. Acta Crystallogr.,Sect.C:Cryst.Struct.Commun. 1999, 55, 1814.
- Fletcher, J.T.; Therien, M.J. Inorg.Chem. 2002, 41, 331.
- Byrn, M.P.; Curtis, C.J.; Hsiou, Y.; Khan, S.I.; Sawin, P.A.; Tendick, S.K.; Terzis, A.; Strouse, C.E. J.Am.Chem.Soc. 1993, 115, 9480. [CrossRef]
- Schulz, L.D.; Fallon, G.D.; Moubaraki, B.; Murray, K.S.; West, B.O. Chem.Commun. 1992, 971.
- Thorman, J.L.; Young Junior, V.G.; Boyd, P.D.W.; Guzei, I.A.; Woo, L.K. Inorg.Chem. 2001, 40, 499.
- Scott, M.J.; Goddard, C.A.; Holm, R.H. Inorg.Chem. 1996, 35, 2558.
- Seyler, J.W.; Fanwick, P.E.; Leidner, C.R. Inorg.Chem. 1992, 31, 3699.
- Lee, S.C.; Holm, R.H. Inorg.Chem. 1993, 32, 4745.
- Scheidt, W.R.; Geiger, D.K.; Haller, K.J. J.Am.Chem.Soc. 1982, 104, 495.
- Naiyin, L.; Petricek, V.; Coppens, P.; Landrum, J. Acta Crystallogr., Sect.C (Cr.Str.Comm.) 1985, 41, 902. [CrossRef] [Green Version]
- Naiyin, L.; Coppens, P.; Landrum, J. Inorg.Chem. 1988, 27, 482.
- Safo, M.K.; Nesset, M.J.M.; Walker, F.A.; Debrunner, P.G.; Scheidt, W.R. J.Am.Chem.Soc. 1997, 119, 9438. [CrossRef]
- Safo, M.K.; Gupta, G.P.; Walker, F.A.; Scheidt, W.R. J.Am.Chem.Soc. 1991, 113, 5497. [CrossRef]
- Inniss, D.; Soltis, S.M.; Strouse, C.E. J.Am.Chem.Soc. 1988, 110, 5644. [CrossRef]
- Safo, M.K.; Walker, F.A.; Raitsimring, A.M.; Walters, W.P.; Dolata, D.P.; Debrunner, P.G.; Scheidt, W.R. J.Am.Chem.Soc. 1994, 116, 7760. [CrossRef]
- Moore, K.T.; Fletcher, J.T.; Therien, M.J. J.Am.Chem.Soc. 1999, 121, 5196. [CrossRef]
- Safo, M.K.; Gupta, G.P.; Watson, C.T.; Simonis, U.; Walker, F.A.; Scheidt, W.R. J.Am.Chem.Soc. 1992, 114, 7066.
- Balch, A.L.; Noll, B.C.; Olmstead, M.M.; Phillips, S.L. Inorg.Chem. 1996, 35, 6495.
© 2004 by MDPI (http://www.mdpi.org).
Share and Cite
Bogdanović, G.A.; Medaković, V.; Milčić, M.K.; Zarić, S.D. Intramolecular C−H···π Interactions in Metal-Porphyrin Complexes. Int. J. Mol. Sci. 2004, 5, 174-185. https://doi.org/10.3390/i5040174
Bogdanović GA, Medaković V, Milčić MK, Zarić SD. Intramolecular C−H···π Interactions in Metal-Porphyrin Complexes. International Journal of Molecular Sciences. 2004; 5(4):174-185. https://doi.org/10.3390/i5040174
Chicago/Turabian StyleBogdanović, Goran A., Vesna Medaković, Miloš K. Milčić, and Snežana D. Zarić. 2004. "Intramolecular C−H···π Interactions in Metal-Porphyrin Complexes" International Journal of Molecular Sciences 5, no. 4: 174-185. https://doi.org/10.3390/i5040174




