Introduction
Intramolecular hydrogen bonds are often applied model systems in the study of the proton transfer reaction. An advantage of using such specific systems is the enhanced structural and thermodynamic stability of intramolecular hydrogen bond [
1]. The solvent or temperature modifications do not result in change the stoichiometry neither the structure of the complexes. This allows obtaining more clear description of the proton transfer equilibrium in comparison to intermolecular complexes, where in solution the situation becomes complicated, partially due to selfassociation of particular partners of the hydrogen bonded interactions [
2].
Because of internal self complexation of acid and base groups within the molecules forming the intramolecular hydrogen bonds, also specific interactions with active solvent molecules are reduced to high extent. In such a case also the thermodynamic characteristics of the proton transfer reaction appear to be more reliable than in intermolecular complexes, where they are strongly dependent on local interactions with a solvent.
On the other side, the intramolecular complexes reveal some specificity, resulting from hydrogen bridge bending [
3]. This leads to decrease of the strength of interactions, the ν(XH) frequency shift appears to be lower than in related intermolecular complexes, similarly drops the intensity of ν(XH) absorption [
4].
Additional specificity appears when intramolecular, so called
resonance assisted hydrogen bonds are studied [
5,
6]. Through–molecule direct electron coupling between acid and base centers leads to increase the acid base interaction and characteristic o-quinoid charge redistribution, with structural consequences of it [
7].
The aim of this work is to describe more in detail this feature in ortho hydroxy Schiff bases, while analogous Mannich bases shall be used as reference intramolecular hydrogen bonds (see
Scheme 1). In both these systems the intramolecular hydrogen bonds are bent in a similar way. Comparing both types of molecules we shall discuss, in majority, the effect of intramolecular electronic coupling on the character of tautomers obtained due to intramolecular proton transfer.
Schiff bases are of special interest in the literature; they show thermochromic and photochromic properties, which can be a basis for numerous practical applications [
8,
9,
10]. The features responsible for such properties are directly connected with ground state and excited state proton transfer. Theoretical studies on the ways of modification the shape of the potential for the proton transfer seem to have also some practical implications.
The most precise indication of the proton transfer in these types of hydrogen bond goes from UV-VIS spectra. The phenolate chromophor reveals as a rule significantly more red shifted absorption than related phenol chromophor. The bands are easy resolved and the concentration of both forms can be precisely determined.
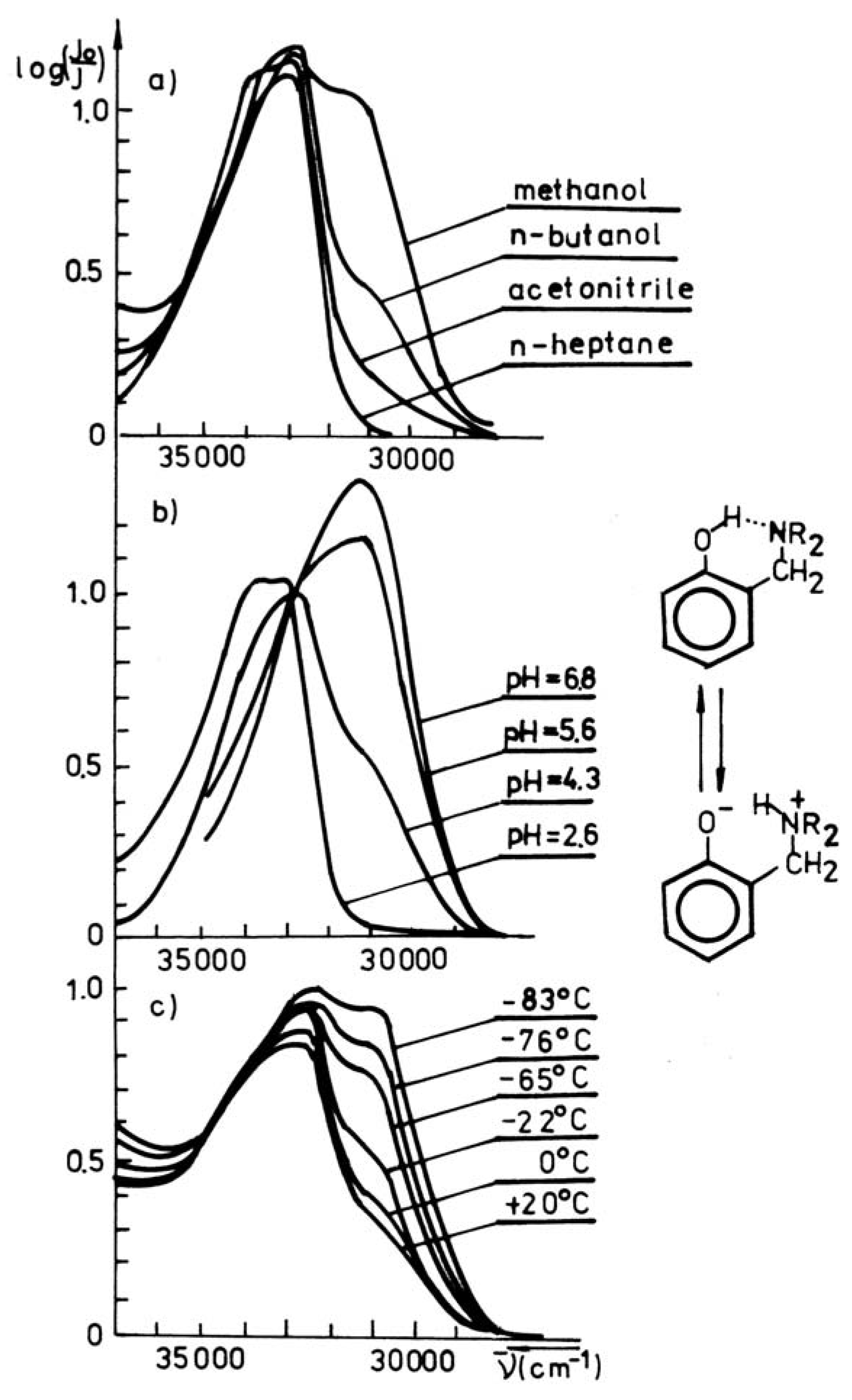
Figure 1 shows the UV-VIS spectra of Mannich base in dependence on temperature decrease and increase of the polarity of solvent. It is clearly seen, that such modifications of both parameters change the spectra in the same direction as pH increase, which shifts the equilibrium towards the phenolate form. The proton transfer equilibrium constant K
PT ( = [zwitterionic form]/[enol form]) can be related, in two parameter correlation equation, to E
T (solvent polarity parameter) and Δp
Ka [(=p
Ka (BH
+) - p
Ka (AH)] [
1]:
In the case of Schiff base one can observe the similar changes of UV-VIS spectra with solvent polarity increase; proton transfer forms reveal the long wave absorption bands (
Figure 2). One can mention however, that these bands are shifted more to long waves than in Mannich bases. It appears that the proton transfer tautomer in Schiff bases have a different type of chromophore than in Mannich bases, where –CH
2- group prevents direct coupling between acid and base centers. With respect to this
Figure 1.
Spectroscopic manifestation of the intramolecular proton transfer and ionization of phenol group in 2-(pyperidinomethyl)-3,4,6-trichlor-phenol; a) solvent effect, b) pH effect, c) temperature effect [
11].
Figure 1.
Spectroscopic manifestation of the intramolecular proton transfer and ionization of phenol group in 2-(pyperidinomethyl)-3,4,6-trichlor-phenol; a) solvent effect, b) pH effect, c) temperature effect [
11].
Figure 2.
Dependence of electronic absorption spectra of 2-(N-methyl-α-iminoethyl)-4,6-dichlorophenol on solvent polarity; 1 – CCl
4, 2 – CH
2Cl
2, 3 – CH
3OH (upper picture) and temperature; 1 – 298 K, 2 – 273 K, 3 – 248 K, 4 – 233 K, 5 – 213 K, 6-183 (lower picture) [
12].
Figure 2.
Dependence of electronic absorption spectra of 2-(N-methyl-α-iminoethyl)-4,6-dichlorophenol on solvent polarity; 1 – CCl
4, 2 – CH
2Cl
2, 3 – CH
3OH (upper picture) and temperature; 1 – 298 K, 2 – 273 K, 3 – 248 K, 4 – 233 K, 5 – 213 K, 6-183 (lower picture) [
12].
problems important seems to be the observation of [
13], where it was found that the long-wave absorption in Schiff bases is observed in water solutions only at intermediate pH, while in strong acid and base solutions the long wave absorption disappears; in acids because enol form predominates and phenol chromophore is absorbing and in bases – because the phenolate chormophore. The long-wave absorption in water solutions at intermediate pH solvents appeared to be a specific one, and was explained by resonance interaction between zwitterionic and keto valence structures (
Scheme 2):
If such a resonance is responsible for the long-wave absorption in Schiff bases, the solvatochromic effects should be expected — the position of long–wave absorption band should depend on solvent polarity. Such a dependency was really found in experimental way [
12].
The solvatochromy was found to be negative. Such situation is observed if polar forms are more stable [
14]. Increase of the solvent polarity, enhances the difference in the energy of both states, which decreases the effectiveness of resonance stabilization and shifts the band position to shorter waves. In non polar solvents the content of zwitterionic valence structure should be at least 50%. One can expect the changes of the structure and properties of these species with the solvent or temperature modification, even if there is a complete proton transfer in it. In Mannich bases, with more localized charge distribution, the final state of the proton transfer is a defined form of phenolate.
A consequence of such conception of the nature of proton transfer in Schiff bases should be redistribution of ring bond lengths in direction of ortho quinoid scheme, characteristic for the keto resonance forms (see
Scheme 2). The bond lengths pattern can be a measure of the content of zwitterionic and keto forms in the resonance, deciding about the nature of proton transfer tautomers.
Such an approach was applied already in the discussion of crystallographic data. Woźniak et. al. [
15] using a set of resonance forms for description of the phenyl ring structure in 2-(N-methyl-iminomethyl)-4-nitrophenol, which crystallizes as a proton transfer tautomer, estimated the amount of keto form on 50 %. Similar result (47 %) was found [
16] in the crystal structure of proton transfer form of 2-(N-methyliminomethyl)-4,6-dichlorophenol by the analysis the geometry of chelate ring. In both approaches the standard single, double and aromatic bond lengths were applied [
17].
Results and discussion
Detailed analysis of the geometry of basic (the simplest) ortho hydroxy Schiff base, 2-(N-methyliminomethyl)-phenol (cf.
scheme 1, R = -CH
3) on the basis of
ab initio and DFT calculations was performed in this work. The aim of this study was establishing the content of keto and zwitterionic valence structures in the “real”-optimized structure of this Schiff base.
The use of
ab initio and DFT calculations in the study the interactions between substituents in phenyl ring becomes last times quite popular [
18,
19,
20,
21,
22]. In the paper by Hargittai et.al. [
21] it was stated that MP2/6-31G (d,p) method provide reliable molecular geometry of hydrogen-bonded systems (cf. also [
18]). According to the Authors, obtained in that way structures are free from random errors [
21]. If even there are systematic errors within the group of related molecules, the reliable trends can be predicted from such calculations.
In this work the DFT B3LYP calculations were performed with the 6-31G(d,p), 6-31+G(d,p) and 6-31++G(d,p) basis sets while ab initio, MP2 calculations with 6-31G(d,p) and 6-31+G(d,p) basis sets. The final stage of energy and structure optimization is a particular tautomer structure, which, on the other side, can be treated as a result of combination of two selected, border resonance structures. Separate resonance structures can not be obtained by quantum chemical optimization, because they do not form the potential energy minima.
To establish the content of particular resonance forms one should define the reference molecule for a selected resonance structure. It should not be a classical form with standard single, double and “aromatic” bond lengths [
17]. The calculations of standard structures should be consistent with the method used for the calculation of the final (minimum energy) structure. Applied in the calculations the reference structures are shown in
scheme 3.
The structure A [cf. 23] will be used to describe the geometry of the phenyl ring in valence keto resonance form, while structure B the geometry of the ring in zwitterionic “virtual” (cf.
Scheme 2) state. Selection of this structure allows consideration of the influence of –O
- substituent on the geometry of the ring and introducing the ortho methyl substituent – not complete axial symmetry of the phenolate ring. Structure C (syn and anti) will be used in description of enol forms.
To calculate the content of zwitterionic and keto forms in the Schiff base we have used the set of linear equations
where r
i is a given bond length in the Schiff base, a
i, b
i – the lengths of relative bonds in reference A and B states. Additional condition a + b = 1 results in:
Solving the set of these equations gives 35% content of keto forms in description the structure of phenyl ring in Schiff base (MP2/6-31G(d,p)), after the proton transfer. For the comparison sake one can also perform the calculations for Schiff bases in enol form. It allows understanding the differences between hydrogen bond in enol and proton transfer state in Schiff bases. The resonance in the enol hydrogen bonded Schiff bases can be presented like in
Scheme 4:
As a reference state we have selected structures C and A. The calculations performed by solving the set of appropriate linear equations give about 10% of keto form in Schiff base. The calculations show, that the proton transfer increases the content of keto form. This content, however, (estimated on 35 %) is far from the “pure” keto form (with the structure like A in
Scheme 3). In accordance to above reasoning the structure of the proton transfer form in resonance assisted hydrogen bonds, is rather in between of two: keto and zwitteionic forms than is pure keto forms, as it is traditionally named in theoretical [
10] papers on proton transfer in Resonance Assisted Hydrogen Bonds.
Table 1 contains the results of DFT calculations at different basis sets and also MP2
ab initio method, to find out to what extent results depend on the applied method. The presented data support the above conclusions; the content of keto form in open Schiff bases (non hydrogen bonded) of enol form is about 5 ± 1%.
Table 1.
Calculated content of keto structure in particular forms of Schift bases.
Table 1.
Calculated content of keto structure in particular forms of Schift bases.
| Method | enol-open | enol-close | PT-close | PT-open |
| MP2/6-31G(d,p) | 6.1 % | 10.4 % | 34.5 % | 69.8 % |
| B3LYP/6-31G(d,p)* | 6.2% | 13.2% | 44.7% | 70.4% |
| MP2/6-31+G(d,p) | 4.1% | 10.2% | 33.5% | 74.0% |
| B3LYP/6-31+G(d,p) | 5.6% | 12.4% | 44.4% | 68.5% |
| B3LYP/6-31++G(d,p) | 4.3% | 10.7% | 45.6% | 69.0% |
Formation of intramolecular hydrogen bond gives in average the 11±2% of keto form. Proton transfer reaction leads to increase the content of keto form to 40±5%.
Table contains also results of calculations for the open (with broken hydrogen bond), proton transfer form of Schiff base. Only in this case one obtains the predominance of keto form, (about 70%). It shows that the condition for resonance stabilization of intramolecular hydrogen bond is participation of non negligible content of zwitterionic form.
Figure 3 present the results of the calculations of the potential energy modification upon the extension of OH distance for Mannich (2) and Schiff bases (1). The differences in the character of proton transfer reaction in Mannich and Schiff bases are clear. In the gas phase, the second minimum is not formatted in Mannich bases at all. Assuming that the OH bond length is there similar to that in Schiff base one can estimate the energy of proton transfer for the Mannich base on 15 kcal/mol or more, while calculated value for the Schiff base is 8 kcal/mol.
Very interesting are large differences in the increase of dipole moment on proton transfer.
Figure 4 shows the dependencies of dipole moments calculated by MP2/6-31+G(d,p) method on the O-H bond extension. For the Schiff base for which the proton can be localized in the second minimum the increase of dipole moment upon the proton transfer is 2.14D. Estimated for Mannich base, for the same (1.6Å) O…H distance, is 4.87 D. The vector increase of dipole moment (
) was found in Mannich bases to be similar to that in intermolecular complexes (up to 10D) [
24].
In the case of molecules with π-electron coupling there is intramolecular compensation of the charge redistribution which results from the formation of intramolecular hydrogen bonding. This can be also presented as a result of resonance in Schiff bases.
Figure 3.
Potential energy (MP2/6-31+G(d,p)) dependence on O-H bond stretching in (1) Schiff and (2) Mannich bases.
Figure 3.
Potential energy (MP2/6-31+G(d,p)) dependence on O-H bond stretching in (1) Schiff and (2) Mannich bases.
Figure 4.
Dependence of calculated values of dipole moments on O…H distances in (1) Mannich base and (2)Schiff base.
Figure 4.
Dependence of calculated values of dipole moments on O…H distances in (1) Mannich base and (2)Schiff base.
The work of charge separation upon the proton transfer in Schiff bases is considerably less than in Mannich bases or intermolecular complexes. This is a source of decrease of the energy of proton transfer state in Schiff bases and formation the second minimum on the potential energy curve. In Mannich bases the proton transfer state is accessible only due to interaction with solvent molecules stabilizing the polar, proton transfer form.
Conclusions
The intramolecular proton transfer reaction in Schiff bases reveals specificity in relation to Mannich bases. It results from possibility of direct π-electronic coupling, which leads to partial compensation of charge redistribution upon the formation intramolecular hydrogen bond. Increase of dipole moment (Δμ) resulting from the proton transfer is here considerably lower than in related Mannich bases, where the π-electronic coupling is very limited. Smaller Δμ in Schiff bases reduces the work of charge separation in nonpolar solvents and in the gas phase. The energy of proton transfer state in Schiff bass is of order of a 8 kcal/mol, when in Mannich bases more than 15 kcal/mol.
The proton transfer form in Schiff bases appears to be much easier accessible, what also goes from experimentally found thermochromic properties of Schiff bases. The enol and proton transfer tautomers appear here to be of different character, than in intermolecular complexes and Mannich bases. In Schift bases each form reveals features of resonance between at least two border structures. Calculated amount of keto resonance structure is about 11% in enol form of hydrogen bond and about 40% in proton transfer state. This is not a keto form, as it is declared in literature, but consists at least with the same amount of zwitterionic form. Calculations show, that predominating amount of keto form one can obtain only by opening the intramolecular hydrogen bond of PT form. Interactions in intramolecular hydrogen bonds lead to some kind of equalization of the content of keto and zwitterionic forms. Interaction with a solvent modulates the energy difference between both resonance border forms and influences the properties of tautomers. For example the UV-vis absorption shows distinct, negative solvatochromy, while in Mannich bases “pure” phenolate absorption is observed. In Schiff bases the structure of tautomeric forms is dependent on resonance and show quite large flexibility on the solvent change. Namely, such resonance as discussed here, is responsible for the increased stability of so called resonance assisted hydrogen bonds.