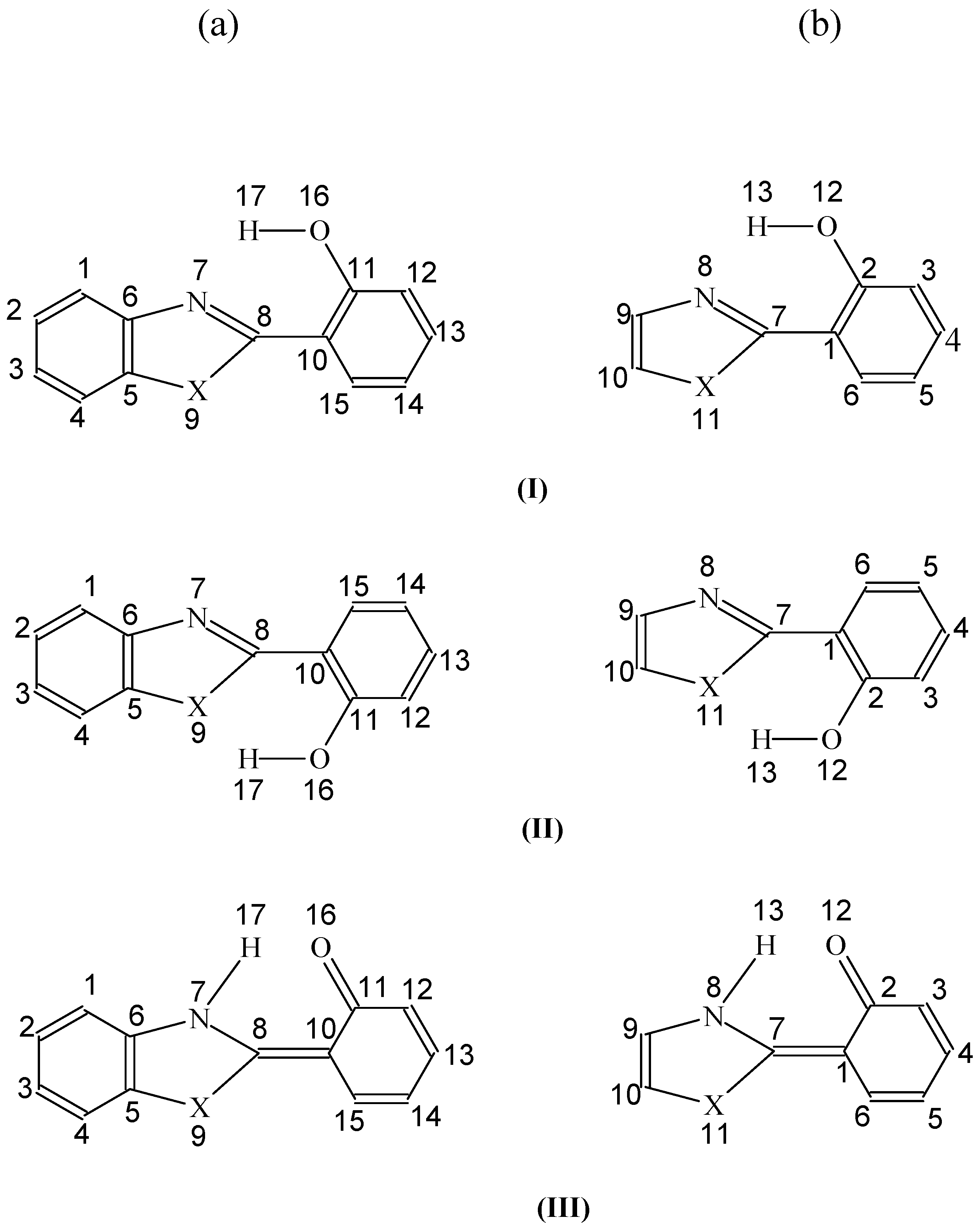
Scheme 2.
Structure of MHBI.
Intramolecular rotation
Table 2 presents the various calculated geometric parameters of the different rotameric and prototropic species of HBO, HBI, HBT and HPO, HPI, HPT in different electronic states. The table reflects the stability of the normal form (I) over the rotamer (II) and the tautomer (III) in the ground state for all the compounds. The calculated energies, E (in eV) and dipole moments, μ (in debye), of the different species have also been calculated in different electronic states. The torsional angles (in degrees) and the interatomic distances (in Å) at the proton transfer site of the optimized isomers have also been presented in the table.
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6 reflect the simulated energy profiles for the intramolecular rotation of the hydroxylphenyl moiety relative to the heterocyclic ring for the molecules of both the series in different electronic states to examine the existence of the different conformational isomers. The figures present the energy diagrams for the bare molecules along with their solvated species in ethanol.
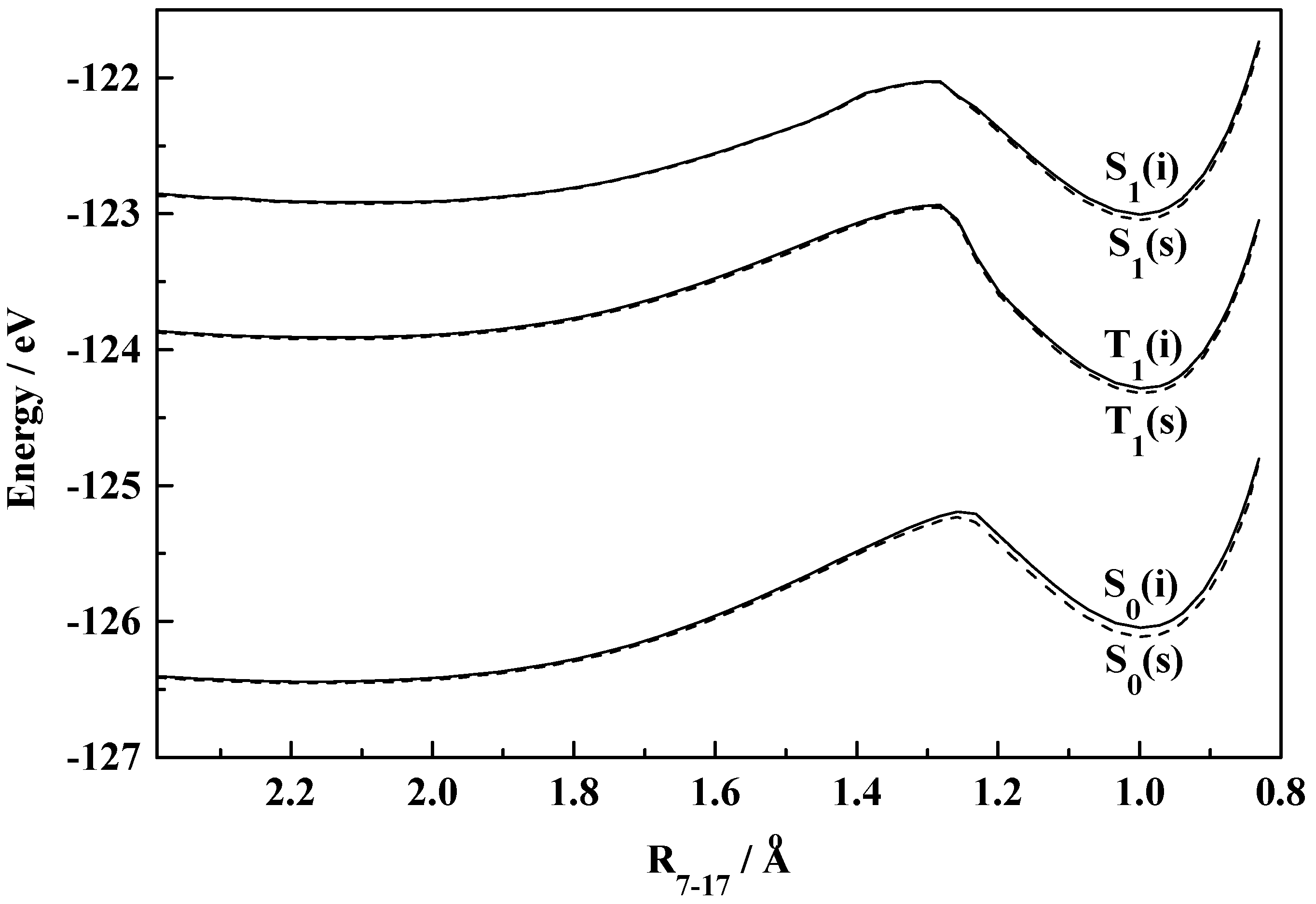
Figure 1 and
Figure 2 demonstrate the existence of the normal (I) and rotameric (II) forms of HBO and HBI in the ground state which is supported by the experimental observations [
16,
37,
39]. Nagaoka
et al. [
44] and Das
et al. [
16] also reported the existence of the two rotameric forms (I and II) of HBO in the ground state from their
ab initio (STO-3G) and semiempirical (CNDO/SCI) calculations respectively. However, slight discrepancy has crept into the reported ground state geometry of the rotational isomers. Present calculations show that the monitored dihedral angles (T
7-8-10-11) for I and II of HBO are 0° and 150° respectively. Nagaoka
et al. got them as 0° and 180° and Das
et al. found the values to be 30° and 180° respectively. The corresponding angles for the similar ground state rotameric species (I and II) of HBI are calculated to be 40° and 140° which match with those calculated by Das
et al. [
16].
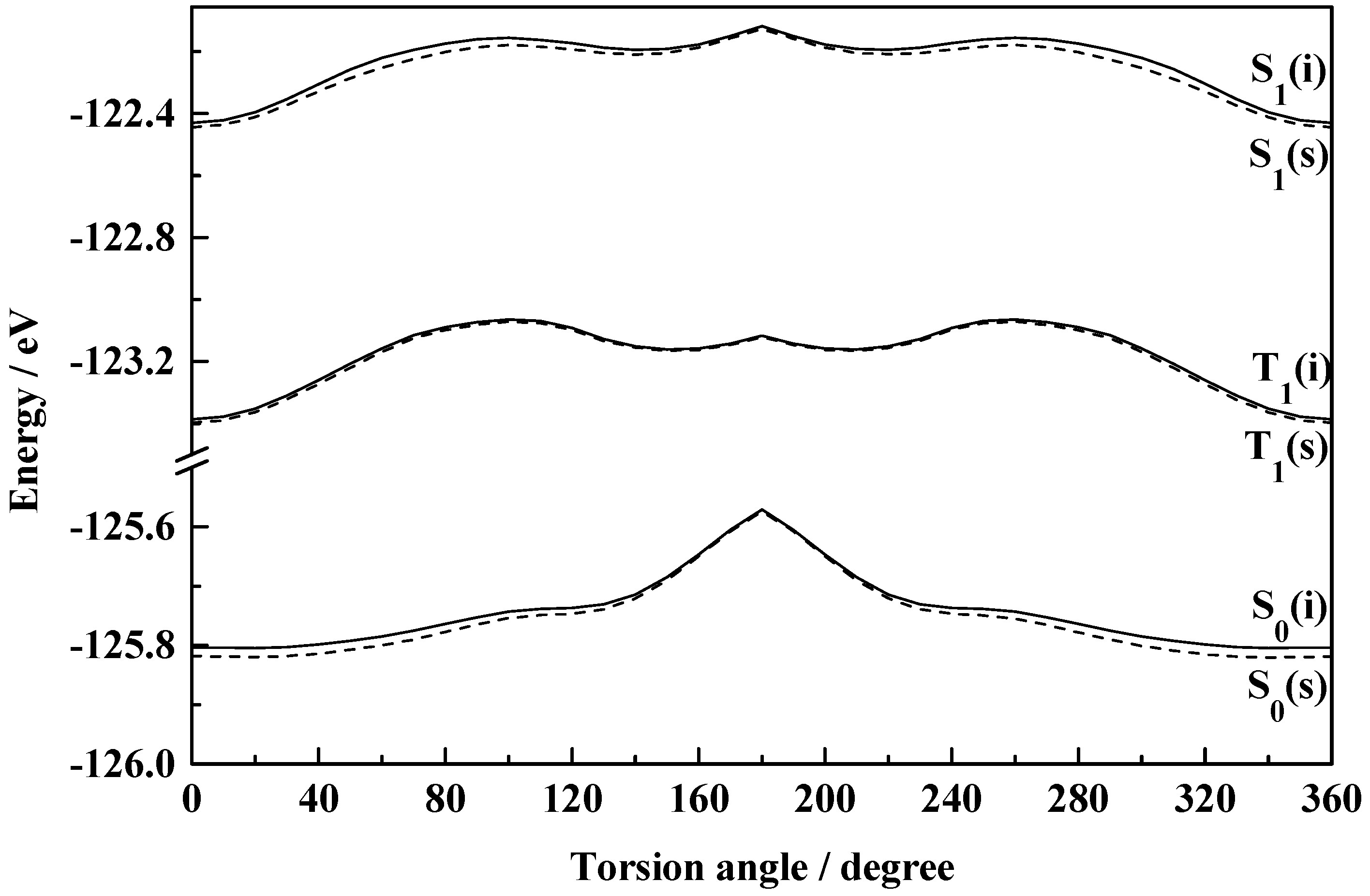
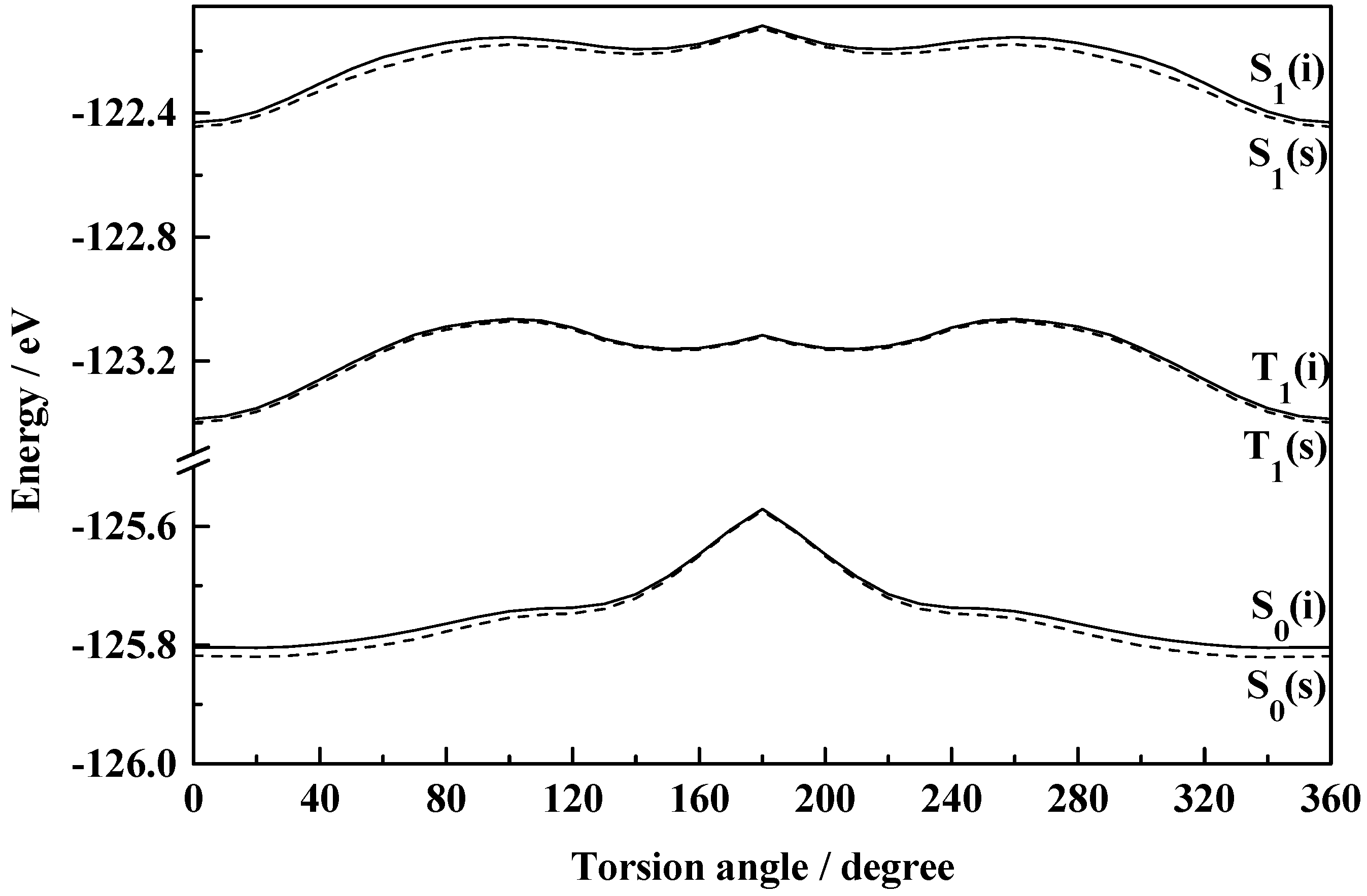
Figure 3 indicates the existence of only one stable form of HBT in the ground state, the normal (I) one. Calculation shows insignificant energy barrier for the interconversion between the normal and rotameric forms and thus rules out the independent existence of the rotamer. The instability of the rotamer of HBT has been supported by Nagaoka
et al. [
44] from their
ab initio calculations. The differential behaviour of HBT compared to that of HBO and HBI may be incurred due to the low electronegativity and the bulkier size of the sulfur atom [
44]. The energy of activation for the interconversion of I to II has been calculated to be 0.101 eV and 0.045 eV for HBO and HBI respectively in the isolated conditions which are calculated to be 0.113 eV and 0.054 eV in ethanol solution. The barrier heights for the reverse transformation for the said molecules are 0.096 eV and 0.021 eV in isolated condition and 0.099 eV and 0.005 eV in ethanol solution respectively. The low energy barriers make a clear suggestion for the existence of the rotameric forms I and II in equilibrium. There is practically no energy barrier for II → I transformation in the case of HBT resulting in the nonexistence of form II of this system. The calculations, thus, support the existence of two isomers (the normal (I) and the rotamer (II)) of HBO and HBI and only the normal (I) species of HBT in the ground state.
Table 3,
Table 4 and
Table 5 represent the calculated energy values of S
0, S
1 and T
1 states of the different possible isomers (I, II and III) of HBO, HBI and HBT respectively in isolated and solvated conditions.
Table 2.
Equilibrium parameters of different photoisomers of HBO, HBI, HBT and HPO, HPI, HPT in different electronic states.
R7-17 represents the interatomic distance between the two atoms for the first series and
R8-13 represents it for the other series referred to by the numbers (see
Scheme 1).
T7-8-10-11 is the torsional angle developed by the atoms of the first series and
T2-1-7-8 is that developed by those of the second series.
Table 2.
Equilibrium parameters of different photoisomers of HBO, HBI, HBT and HPO, HPI, HPT in different electronic states. R7-17 represents the interatomic distance between the two atoms for the first series and R8-13 represents it for the other series referred to by the numbers (see Scheme 1). T7-8-10-11 is the torsional angle developed by the atoms of the first series and T2-1-7-8 is that developed by those of the second series.
| Molecules | Parameters | Normal (I) | Rotamer (II) | Tautomer (III) |
|---|
| HBO | E (S0) | -126.4425 | -126.4369 | -126.0482 |
| | μ (S0) | 1.77 | 0.80 | 4.26 |
| | R7-17 | 2.17 | 3.70 | 0.996 |
| | T7-8-10-11 | 0 | 150 | 0 |
| | E (S1) | -122.9232 | -122.8528 | -123.0303 |
| | μ (S1) | 1.15 | 1.33 | 3.08 |
| | T7-8-10-11 | 0 | 180 | 0 |
| | E (T1) | -123.9292 | -123.8853 | -124.4109 |
| | μ (T1) | 1.84 | 1.19 | 2.22 |
| | T7-8-10-11 | 0 | 180 | 0 |
| HBI | E (S0) | -129.5468 | -129.5230 | -129.1756 |
| | μ (S0) | 3.36 | 1.67 | 5.49 |
| | R7-17 | 2.29 | 3.67 | 0.998 |
| | T7-8-10-11 | 40 | 140 | 0 |
| | E (S1) | -126.0484 | -125.9378 | -126.2733 |
| | μ (S1) | 2.65 | 1.97 | 4.00 |
| | T7-8-10-11 | 0 | 160 | 0 |
| | E (T1) | -126.9800 | -126.8966 | -127.4739 |
| | μ (T1) | 3.07 | 1.19 | 3.43 |
| | T7-8-10-11 | 0 | 160 | 0 |
| HBT | E (S0) | -125.8044 | — | -125.4224 |
| | μ (S0) | 2.11 | — | 4.03 |
| | R7-17 | 2.16 | — | 1.00 |
| | T7-8-10-11 | 0 | — | 0 |
| | E (S1) | -122.4303 | — | -122.7835 |
| | μ (S1) | 2.00 | — | 0.85 |
| | T7-8-10-11 | 0 | — | 0 |
| | E (T1) | -123.3868 | — | -124.0442 |
| | μ (T1) | 1.80 | — | 2.72 |
| | T7-8-10-11 | 0 | — | 0 |
| HPO | E (S0) | -93.0181 | -93.0060 | -92.5105 |
| | μ (S0) | 2.08 | 0.87 | 4.58 |
| | R8-13 | 2.18 | 3.69 | 0.997 |
| | T2-1-7-8 | 0 | 150 | 0 |
| | E (S1) | -89.4435 | -89.3764 | -89.4581 |
| | μ (S1) | 1.01 | 1.74 | 3.96 |
| | T2-1-7-8 | 0 | 170 | 0 |
| | E (T1) | -90.9033 | -90.8851 | -91.0296 |
| | μ (T1) | 1.84 | 0.80 | 2.42 |
| | T2-1-7-8 | 0 | 180 | 0 |
| HPI | E (S0) | -96.0248 | -96.0043 | -95.0158 |
| | μ (S0) | 3.88 | 1.97 | 6.33 |
| | R8-13 | 2.33 | 3.69 | 1.00 |
| | T2-1-7-8 | 40 | 140 | 0 |
| | E (S1) | -92.4601 | -92.2281 | -92.5831 |
| | μ (S1) | 2.91 | 2.57 | 5.36 |
| | T2-1-7-8 | 0 | 140 | 0 |
| | E (T1) | -93.7735 | -93.7452 | -93.9393 |
| | μ (T1) | 3.04 | 1.83 | 4.15 |
| | T2-1-7-8 | 0 | 180 | 0 |
| HPT | E (S0) | -92.2318 | — | -91.7456 |
| | μ (S0) | 2.54 | — | 4.00 |
| | R8-13 | 2.14 | — | 1.00 |
| | T2-1-7-8 | 10 | — | 0 |
| | E (S1) | -88.9889 | — | -89.3397 |
| | μ (S1) | 1.62 | — | 1.15 |
| | T2-1-7-8 | 0 | — | 0 |
| | E (T1) | -90.2039 | — | -90.4719 |
| | μ (T1) | 1.83 | — | 2.44 |
| | T2-1-7-8 | 0 | — | 0 |
Figure 1.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBO (i, isolated; s, solvated in ethanol).
Figure 1.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBO (i, isolated; s, solvated in ethanol).
Figure 2.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBI (i, isolated; s, solvated in ethanol).
Figure 2.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBI (i, isolated; s, solvated in ethanol).
Figure 3.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBT (i, isolated; s, solvated in ethanol).
Figure 3.
Plot of total molecular energy as a function of torsional angle (7-8-10-11) in S0, S1 and T1 states of HBT (i, isolated; s, solvated in ethanol).
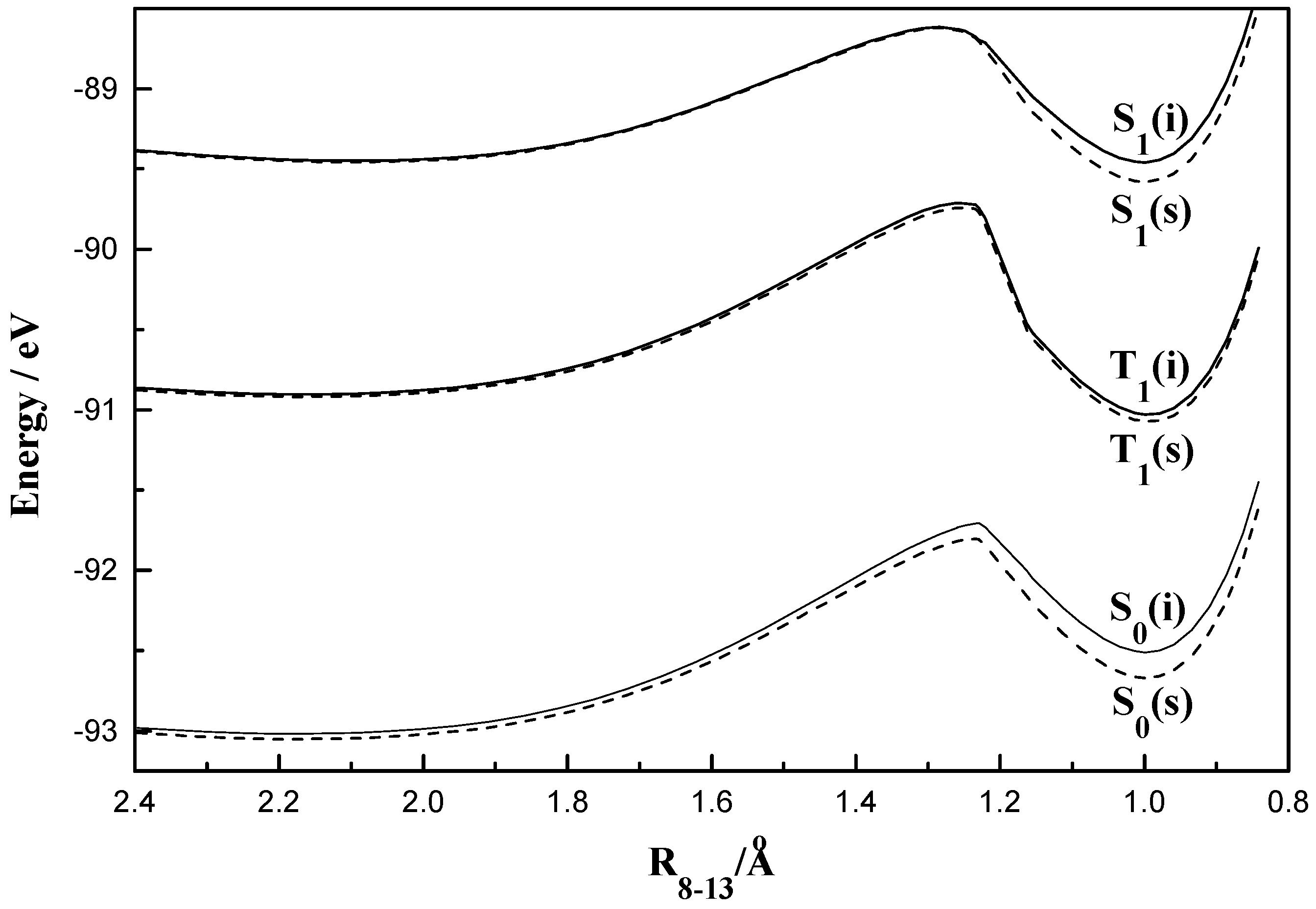
Figure 4.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPO (i, isolated; s, solvated in ethanol).
Figure 4.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPO (i, isolated; s, solvated in ethanol).
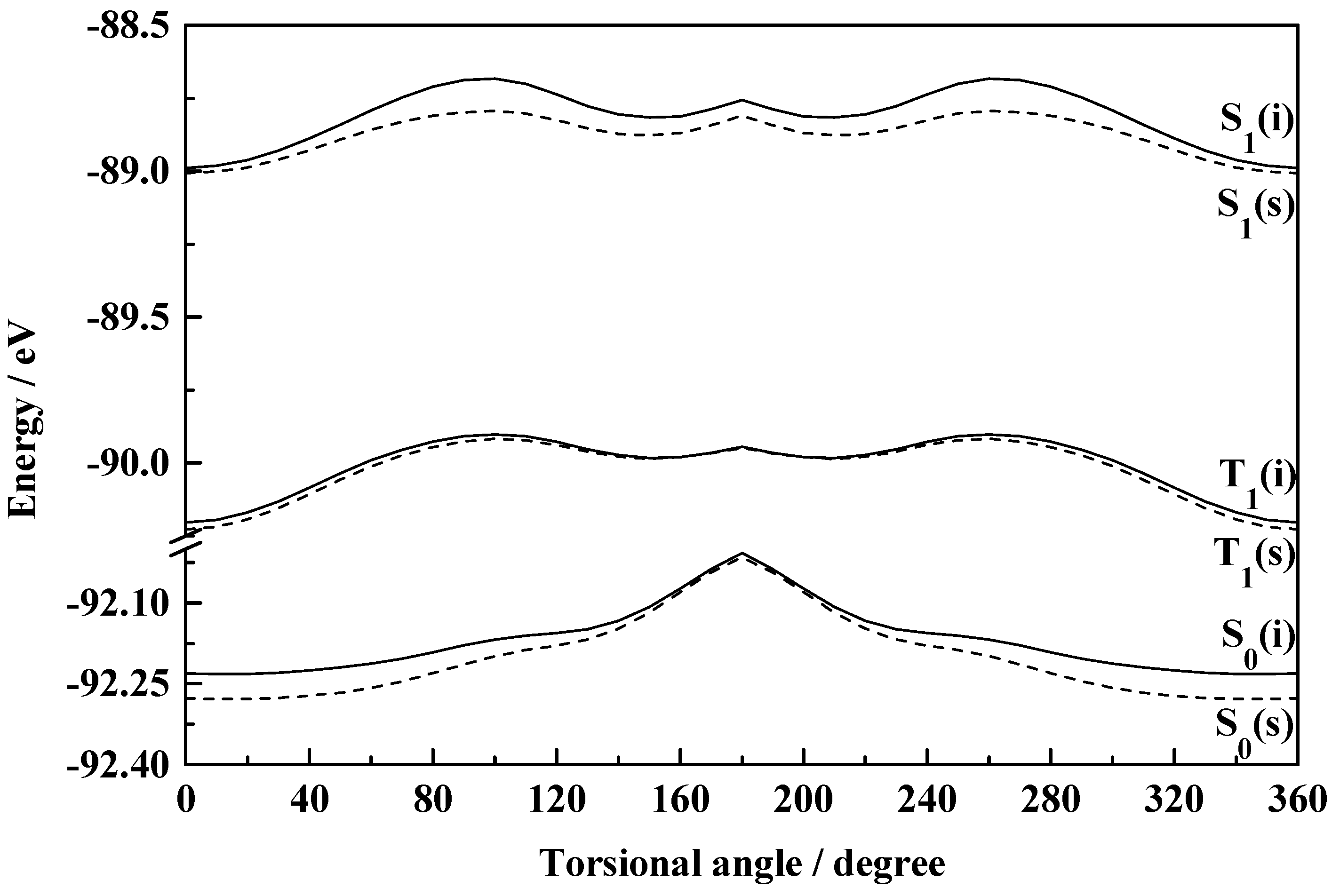
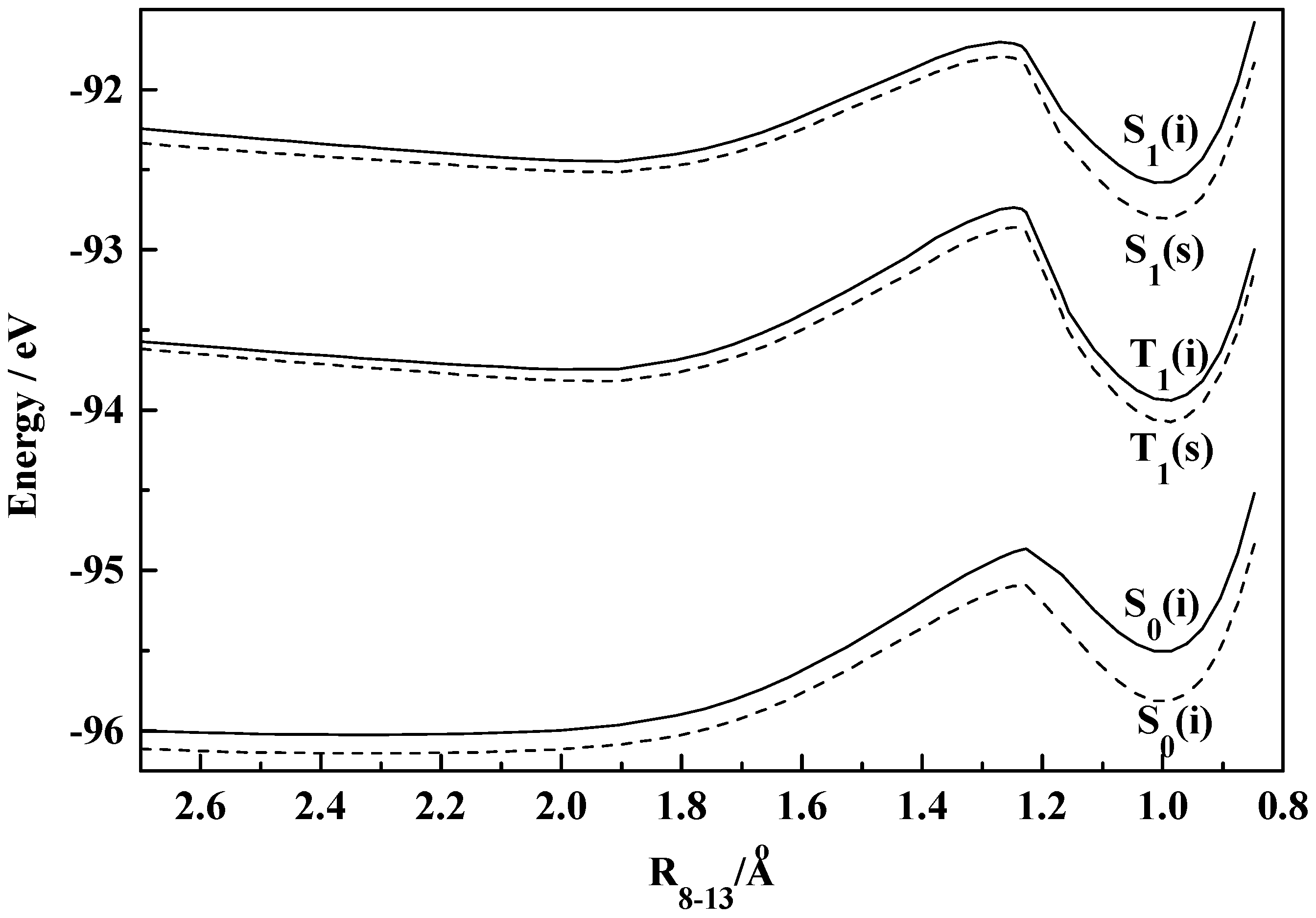
Figure 5.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPI (i, isolated; s, solvated in ethanol).
Figure 5.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPI (i, isolated; s, solvated in ethanol).
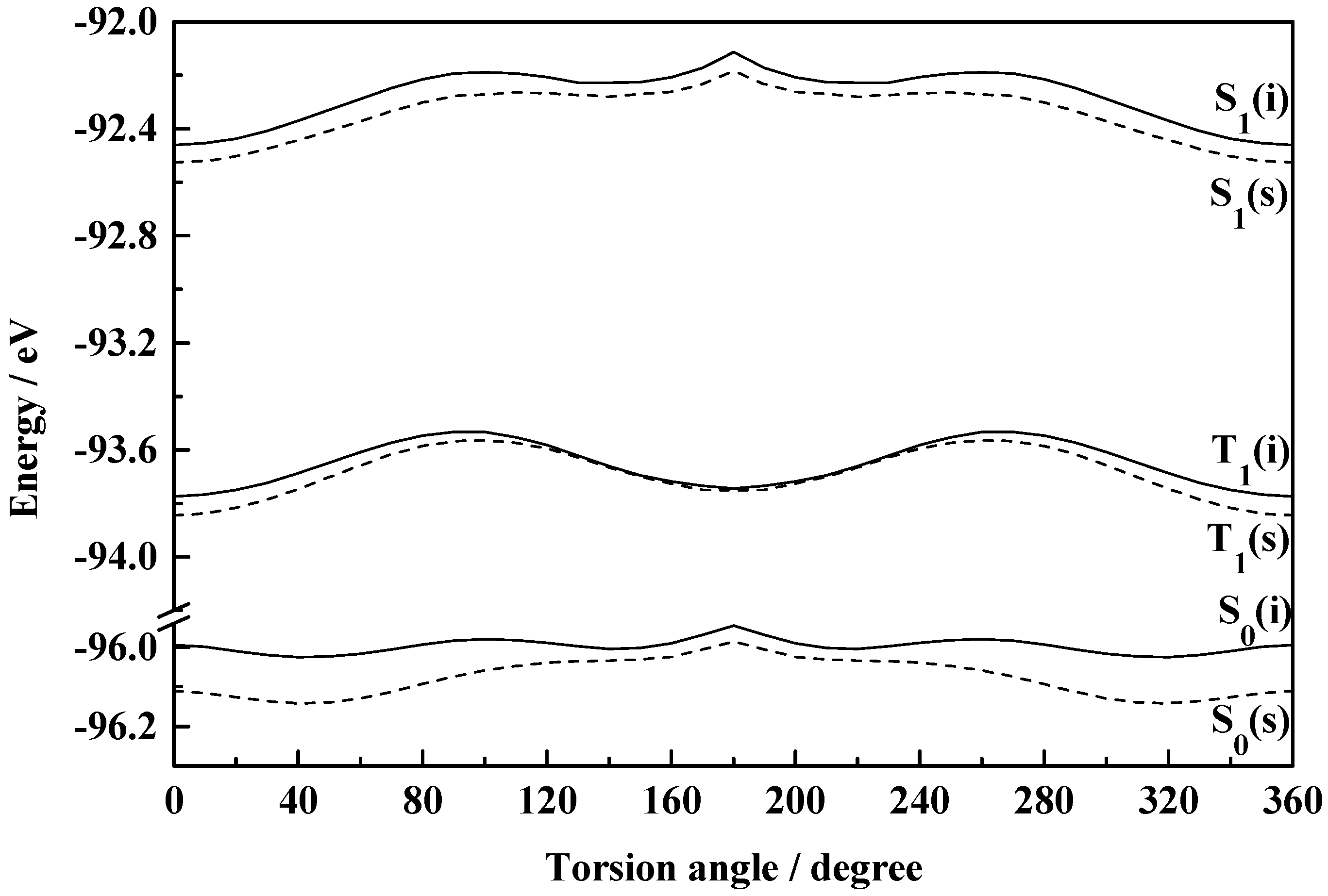
Figure 6.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPT (i, isolated; s, solvated in ethanol).
Figure 6.
Plot of total molecular energy as a function of torsional angle (2-1-7-8) in S0, S1 and T1 states of HPT (i, isolated; s, solvated in ethanol).
Table 3.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HBO in isolated and solvated conditions (Onsager’s cavity radius, a = 5.46 Å).
Table 3.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HBO in isolated and solvated conditions (Onsager’s cavity radius, a = 5.46 Å).
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -126.4425 | -122.9232 | -123.9292 |
| | | II | -126.4369 | -122.8528 | -123.8853 |
| | | III | -126.0482 | -123.0303 | -124.4109 |
| cyclohexane | 2.0 | I | -126.4474 | -122.9253 | -123.9345 |
| | | II | -126.4379 | -122.8553 | -123.8871 |
| | | III | -126.0766 | -123.0450 | -124.4185 |
| p-dioxane | 2.2 | I | -126.4478 | -122.9255 | -123.9350 |
| | | II | -126.4380 | -122.8553 | -123.8876 |
| | | III | -126.0793 | -123.0450 | -124.4193 |
| ethanol | 24.3 | I | -126.4538 | -122.9280 | -123.9415 |
| | | II | -126.4392 | -122.8586 | -123.8895 |
| | | III | -126.1140 | -123.0645 | -124.4287 |
| acetonitrile | 38 | I | -126.4540 | -122.9281 | -123.9418 |
| | | II | -126.4393 | -122.8588 | -123.8896 |
| | | III | -126.1155 | -123.0653 | -124.4291 |
| water | 80 | I | -126.4543 | -122.9282 | -123.9421 |
| | | II | -126.4393 | -122.8589 | -123.8897 |
| | | III | -126.1169 | -123.0660 | -124.4295 |
Table 4.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HBI in isolated and solvated conditions (Onsager’s cavity radius, a = 5.47 Å).
Table 4.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HBI in isolated and solvated conditions (Onsager’s cavity radius, a = 5.47 Å).
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -129.5468 | -126.0484 | -126.9800 |
| | | II | -129.5230 | -125.9378 | -126.8966 |
| | | III | -129.1756 | -126.2733 | -127.4739 |
| cyclohexane | 2.0 | I | -129.5641 | -126.0592 | -126.9944 |
| | | II | -129.5273 | -125.9438 | -126.8988 |
| | | III | -129.2220 | -126.2980 | -127.4919 |
| p-dioxane | 2.2 | I | -129.5661 | -126.0604 | -126.9960 |
| | | II | -129.5278 | -125.9445 | -126.8991 |
| | | III | -129.2271 | -126.3007 | -127.4939 |
| ethanol | 24.3 | I | -129.5875 | -126.0738 | -127.0139 |
| | | II | -129.5330 | -125.9518 | -126.9018 |
| | | III | -129.2845 | -126.3312 | -127.5163 |
| acetonitrile | 38 | I | -129.5885 | -126.0744 | -127.0147 |
| | | II | -129.5333 | -125.9522 | -126.9019 |
| | | III | -129.2870 | -126.3325 | -127.5173 |
| water | 80 | I | -129.5896 | -126.0751 | -127.0156 |
| | | II | -129.5336 | -125.9525 | -126.9020 |
| | | III | -129.2901 | -126.3341 | -127.5185 |
Table 5.
Calculated S0, S1 and T1 energies (eV) of the normal (I) and tautomer (III) of HBT in isolated and solvated conditions (Onsager’s cavity radius, a = 5.57 Å)
Table 5.
Calculated S0, S1 and T1 energies (eV) of the normal (I) and tautomer (III) of HBT in isolated and solvated conditions (Onsager’s cavity radius, a = 5.57 Å)
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -125.8044 | -122.4303 | -123.3868 |
| | | III | -125.4224 | -122.7835 | -124.0442 |
| cyclohexane | 2.0 | I | -125.8110 | -122.4362 | -123.3916 |
| | | III | -125.4462 | -122.7845 | -124.0551 |
| p-dioxane | 2.2 | I | -125.8116 | -122.4368 | -123.3920 |
| | | III | -125.4486 | -122.7846 | -124.0561 |
| ethanol | 24.3 | I | -125.8196 | -122.4440 | -123.3978 |
| | | III | -125.4777 | -122.7859 | -124.0694 |
| acetonitrile | 38 | I | -125.8199 | -122.4443 | -123.3981 |
| | | III | -125.4790 | -122.7860 | -124.0699 |
| water | 80 | I | -125.8203 | -122.4446 | -123.3983 |
| | | III | -125.4802 | -122.7860 | -124.0705 |
The fact that the rotational behaviours of HPO, HPI and HPT are grossly similar to those of the corresponding benzo analogues is obvious from figures 1-6.
Figure 4 demonstrates the existence of both normal (I) and the rotameric (II) forms for HPO in the ground state. The calculated values of the dihedral angles for the stable normal and rotameric forms in the ground state are found to be 0° and 150° respectively which resemble with those found for HBO through similar calculations. The intrinsic steric effect due to the lone electron pair on the oxygen atom in the heterocyclic ring and its effective size is probably responsible for the deviation of the torsional angle from 180°. It is thus, suggested that HPO and HBO have similar geometric arrangements. A comparison of the difference in the energies of the S
0 and S
1 states for HPO and HBO points to a red-shift of the observed absorption band in HBO relative to that in HPO and is ascribed to the extension of the conjugated system [
27]. The rotamers (II) for both the compounds are less stable than the corresponding normal forms (I). The activation energy for the rotameric transformation I → II for HPO is about 0.108 eV in vacuum while that for HBO was calculated to be 0.101 eV. For the reverse transformation II → I the same has a magnitude of 0.105 eV and 0.096 eV for the two compounds respectively. The low energy barriers suggest that the species I and II remain in equilibrium at ambient temperature.
The existence of the normal (I) as well as the rotamer (II) of HPI in the ground state is suggested from figure 5. In low polarity solvents like cyclohexane and 1,4-dioxane both the species coexist. However, in more polar solvents, like ethanol, acetonitrile and water, the barrier for the stabilization of the two rotational isomers hardly exists. Although a near stability zone can be achieved as shown in the figure, but the distinct stability of both the species becomes a question in solvents of higher polarity. This leads to the nonexistence of the rotamer (II) of HPI in polar solvents in contrast to the situation for HBI where both the conformers exist in solvents of all polarity, the barrier for the interconversion, of course, decreases in polar solvents. The additional steric factor developed due to the presence of the ‘N−H’ group in HPI or HBI compared to ‘−O−’ in HPO or HBO probably restricts the formation of the rotamer. The activation energies for the interconversion of I → II in HPI and HBI are found to be same (0.045 eV) in vacuum and those for the reverse process, i.e., II → I are 0.024 eV and 0.021 eV respectively. The dihedral angles corresponding to the normal (I) and the rotamer (II) of HPI and HBI are calculated to be 40° and 140° respectively.
Figure 6 demonstrates a considerable difference in the rotamerization characteristics of HPT in comparison with the other two members in the series. The rotamer (II) gets stabilization in neither isolated nor solvated conditions. The recent experimental study of Le Gourriérec
et al. [
27] rules out the existence of II and corroborates our proposition. The non-existence of the rotamer of HPT has been rationalized from a greater single bond character of the bond joining the phenol and the azole rings and thus allowing for more twisting vibrations whereby the rotamer is unable to get any well-defined stability. HBT and HPT behave similarly in this respect [
44].
Table 6,
Table 7 and
Table 8 report the calculated parameters for HPO, HPI and HPT respectively.
Table 6.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPO in isolated and solvated conditions (Onsager’s cavity radius, a = 4.25 Å).
Table 6.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPO in isolated and solvated conditions (Onsager’s cavity radius, a = 4.25 Å).
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -93.0181 | -89.4435 | -90.9033 |
| | | II | -93.0060 | -89.3764 | -90.8851 |
| | | III | -92.5105 | -89.4581 | -91.0296 |
| cyclohexane | 2.0 | I | -93.0322 | -89.4467 | -90.9099 |
| | | II | -93.0085 | -89.3784 | -90.8867 |
| | | III | -92.5790 | -89.5094 | -91.0487 |
| p-dioxane | 2.2 | I | -93.0338 | -89.4471 | -90.9106 |
| | | II | -93.0088 | -89.3855 | -90.8869 |
| | | III | -92.5866 | -89.5151 | -91.0508 |
| ethanol | 24.3 | I | -93.0511 | -89.4512 | -90.9188 |
| | | II | -93.0119 | -89.3966 | -91.0322 |
| | | III | -92.6713 | -89.5786 | -91.0744 |
| acetonitrile | 38 | I | -93.0519 | -89.4514 | -90.9191 |
| | | II | -93.0120 | -89.3970 | -91.0336 |
| | | III | -92.6750 | -89.5814 | -91.0755 |
| water | 80 | I | -93.0527 | -89.4516 | -90.9194 |
| | | II | -93.0121 | -89.4006 | -91.0358 |
| | | III | -92.6785 | -89.5840 | -91.0764 |
Table 7.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPI in isolated and solvated conditions (Onsager’s cavity radius, a = 4.23 Å).
Table 7.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPI in isolated and solvated conditions (Onsager’s cavity radius, a = 4.23 Å).
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -96.0248 | -92.4601 | -93.7735 |
| | | II | -96.0043 | -92.2281 | -93.7452 |
| | | III | -95.0158 | -92.5831 | -93.9393 |
| cyclohexane | 2.0 | I | -96.0748 | -92.4882 | -93.8041 |
| | | II | -96.0171 | -92.2500 | -93.7563 |
| | | III | -95.1484 | -92.6783 | -93.9963 |
| p-dioxane | 2.2 | I | -96.0803 | -92.4913 | -93.8075 |
| | | II | -96.0186 | -92.2524 | -93.7575 |
| | | III | -95.1615 | -92.6888 | -94.0026 |
| ethanol | 24.3 | I | -96.1418 | -92.526 | -93.8454 |
| | | II | -96.0344 | -92.2795 | -93.7713 |
| | | III | -95.3273 | -92.8066 | -94.0731 |
| acetonitrile | 38 | I | -96.1443 | -92.5275 | -93.8471 |
| | | II | -96.0351 | -92.2807 | -93.7719 |
| | | III | -95.3344 | -92.8117 | -94.0762 |
| water | 80 | I | -96.1470 | -92.5290 | -93.8487 |
| | | II | -96.0358 | -92.2818 | -93.7724 |
| | | III | -95.3412 | -92.8166 | -94.0790 |
Table 8.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPT in isolated and solvated conditions (Onsager’s cavity radius, a = 4.36 Å).
Table 8.
Calculated S0, S1 and T1 energies (eV) of the normal (I), rotamer (II) and tautomer (III) of HPT in isolated and solvated conditions (Onsager’s cavity radius, a = 4.36 Å).
| Medium / Solvent | Relative permittivity (εr) | Species | E (S0) | E (S1) | E (T1) |
|---|
| vacuum | — | I | -92.2318 | -88.9889 | -90.2039 |
| | | III | -91.7456 | -89.3397 | -90.4719 |
| cyclohexane | 2.0 | I | -92.2516 | -88.9967 | -90.2138 |
| | | III | -91.7934 | -89.3436 | -90.4897 |
| p-dioxane | 2.2 | I | -92.2537 | -88.9976 | -90.215 |
| | | III | -91.7987 | -89.3441 | -90.4916 |
| ethanol | 24.3 | I | -92.2781 | -89.0073 | -90.2274 |
| | | III | -91.8578 | -89.3490 | -90.5136 |
| acetonitrile | 38 | I | -92.2791 | -89.0077 | -90.2279 |
| | | III | -91.8604 | -89.3492 | -90.5146 |
| water | 80 | I | -92.2801 | -89.0081 | -90.2284 |
| | | III | -91.8628 | -89.3494 | -90.5155 |
Assignment of the electronic spectra
The excitation, fluorescence and phosphorescence spectra of HBO, HBI, HBT and HPO, HPI, HPT in some common solvents differing in polarity have been calculated and discussed in this section. In pure and homogeneous solvents the solvation dynamics is faster than the fluorescence decay rate [
61,
62]. This leads the probe molecule to get solvated before it fluoresces. Correspondence between the fluorescence and the transition from the solvated S
1 state to the corresponding Franck-Condon S
0 state is, therefore, justified. Similar correlation is also viable for the excitation spectra with the transition between the solvated S
0 state and the Franck-Condon S
1 state. A crude assignment of the phosphorescence emission of the compounds, which obviously gets modified in solid matrix, has been made by calculating the transition energies from the solvated T
1 state to the corresponding Franck-Condon S
0 state. The assignments for the excitation, fluorescence and phosphorescence spectra of HBO, HBI and HBT fluorophores are presented in
Table 9,
Table 10 and
Table 11 respectively.
Similar assignments have been made for HPO, HPI and HPT and demonstrated in
Table 12,
Table 13 and
Table 14 respectively. It is pertinent to mention here that the positions of the experimental absorption bands are always at a little higher energy compared to the calculated absorption positions. This is because the calculated spectra represent the 0-0 transition only between the S
0 and S
1 states, while the experiments give rise to absorption bands with broad maxima leading to the transition to the upper vibrational levels of S
1 as well. From the proximity of the spectral data and our calculated transitions, we ascribe that the S
1 and T
1 states effective for the ESIPT process are both of
ππ* nature. This is consistent with the literature reports [
16,
25,
27,
33,
50].
Table 9.
Assignment of excitation, fluorescence and phosphorescence spectra of HBO in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 9.
Assignment of excitation, fluorescence and phosphorescence spectra of HBO in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt (ref) | Calc. | Expt (ref) | Calc. | Expt (ref) |
|---|
| cyclohexane | I | 3.52 | 3.71 (39) | 3.52 | n.a. | 2.51 | n.a. |
| | II | 3.59 | 3.88 (39) | 3.58 | 3.40 (39) | 2.55 | n.a. |
| | III | — | — | 3.00 | 2.60 (39) | 1.63 | n.a. |
| p-dioxane | I | 3.52 | n.a. | 3.52 | n.a. | 2.51 | n.a. |
| | II | 3.59 | n.a. | 3.58 | n.a. | 2.55 | n.a. |
| | III | — | — | 2.98 | n.a. | 1.63 | n.a. |
| ethanol | I | 3.53 | 3.77 (39) | 3.51 | n.a. | 2.50 | 2.30 (8) |
| | II | 3.59 | 3.94 (39) | 3.58 | 3.40 (39) | 2.55 | 2.80 (8) |
| | III | — | — | 2.98 | 2.64 (39) | 1.62 | n.a. |
| acetonitrile | I | 3.53 | 3.77 (39) | 3.51 | n.a. | 2.50 | n.a. |
| | II | 3.59 | 3.94 (39) | 3.58 | 3.45 (39) | 2.55 | n.a. |
| | III | — | — | 2.98 | 2.62 (39) | 1.62 | n.a. |
| water | I | 3.53 | 3.77 (9) | 3.51 | n.a. | 2.50 | n.a. |
| | II | 3.59 | 3.94 (9) | 3.58 | 3.45 (9) | 2.55 | n.a. |
| | III | — | — | 2.98 | 2.59 (9) | 1.62 | n.a. |
Table 10.
Assignment of excitation, fluorescence and phosphorescence spectra of HBI in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 10.
Assignment of excitation, fluorescence and phosphorescence spectra of HBI in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt (ref) | Calc. | Expt (ref) | Calc. | Expt (ref) |
|---|
| cyclohexane | I | 3.52 | 3.70 (53) | 3.49 | n.a. | 2.55 | n.a. |
| | II | 3.59 | 3.88 (53) | 3.58 | n.a. | 2.49 | n.a. |
| | III | --- | --- | 2.88 | n.a. | 1.68 | n.a. |
| p-dioxane | I | 3.52 | n.a. | 3.49 | n.a. | 2.55 | n.a. |
| | II | 3.59 | n.a. | 3.58 | 3.55 (16) | 2.49 | n.a. |
| | III | --- | --- | 2.87 | 2.64 (16) | 1.68 | n.a. |
| ethanol | I | 3.54 | 3.75 (37) | 3.47 | n.a. | 2.53 | n.a. |
| | II | 3.60 | 3.80 (37) | 3.57 | 3.60 (37) | 2.47 | n.a. |
| | III | --- | --- | 2.84 | 3.10 (37) | 1.66 | n.a. |
| acetonitrile | I | 3.54 | n.a. | 3.47 | n.a. | 2.53 | n.a. |
| | II | 3.60 | n.a. | 3.57 | n.a. | 2.47 | n.a. |
| | III | --- | --- | 2.84 | n.a. | 1.66 | n.a. |
| water | I | 3.54 | n.a. | 3.47 | n.a. | 2.53 | n.a. |
| | II | 3.60 | n.a. | 3.57 | 3.55 (16) | 2.47 | n.a. |
| | III | --- | --- | 2.84 | 2.86 (16) | 1.66 | n.a. |
Table 11.
Assignment of excitation, fluorescence and phosphorescence spectra of HBT in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 11.
Assignment of excitation, fluorescence and phosphorescence spectra of HBT in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt (ref) | Calc. | Expt (ref) | Calc. | Expt (ref) |
|---|
| cyclohexane | I | 3.38 | n.a. | 3.37 | n.a. | 2.41 | n.a. |
| | III | — | — | 2.64 | n.a. | 1.37 | n.a. |
| p-dioxane | I | 3.38 | n.a. | 3.37 | n.a. | 2.41 | n.a. |
| | III | — | — | 2.64 | n.a. | 1.37 | n.a. |
| ethanol | I | 3.39 | 3.77 (41) | 3.36 | 3.36 (41) | 2.41 | n.a. |
| | III | — | — | 2.64 | 2.70 (41) | 1.35 | n.a. |
| acetonitrile | I | 3.39 | n.a. | 3.36 | n.a. | 2.41 | n.a. |
| | III | — | — | 2.64 | n.a. | 1.35 | n.a. |
| water | I | 3.39 | n.a. | 3.36 | n.a. | 2.41 | n.a. |
| | III | — | — | 2.64 | n.a. | 1.35 | n.a. |
Table 12.
Assignment of excitation, fluorescence and phosphorescence spectra of HPO in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 12.
Assignment of excitation, fluorescence and phosphorescence spectra of HPO in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt.* | Calc. | Expt.* | Calc. | Expt.* |
|---|
| cyclohexane | I | 3.81 | 3.88 | 3.57 | n.a. | 2.11 | n.a |
| | II | 3.87 | 4.00 | 3.60 | 3.66 | 2.09 | n.a. |
| | III | — | — | 2.99 | 2.60 | 1.46 | n.a. |
| p-dioxane | I | 3.82 | n.a. | 3.57 | n.a. | 2.11 | n.a. |
| | II | 3.87 | n.a. | 3.60 | n.a. | 2.09 | n.a. |
| | III | — | — | 2.99 | n.a. | 1.46 | n.a. |
| ethanol | I | 3.83 | 3.93 | 3.57 | n.a. | 2.10 | 2.30 (63) |
| | II | 3.87 | 4.06 | 3.59 | 3.60 | 2.09 | 2.80 (63) |
| | III | — | — | 2.93 | 2.70 | 1.44 | n.a. |
| acetonitrile | I | 3.83 | 3.96 | 3.57 | n.a. | 2.10 | n.a. |
| | II | 3.87 | 4.06 | 3.59 | 3.60 | 2.09 | n.a. |
| | III | — | — | 2.93 | 2.70 | 1.43 | n.a. |
| water | I | 3.83 | 3.98 | 3.57 | n.a. | 2.10 | n.a. |
| | II | 3.87 | 4.10 | 3.59 | 3.55 | 2.09 | n.a. |
| | III | — | — | 2.93 | 2.79 | 1.43 | n.a. |
Table 13.
Assignment of excitation, fluorescence and phosphorescence spectra of HPI in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 13.
Assignment of excitation, fluorescence and phosphorescence spectra of HPI in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt. | Calc. | Expt. | Calc. | Expt. |
|---|
| cyclohexane | I | 3.96 | 3.88 | 3.51 | n.a. | 2.19 | n.a |
| | II | 3.87 | 4.00 | 3.75 | n.a. | 2.19 | n.a. |
| | III | — | — | 2.83 | n.a. | 1.51 | n.a. |
| p-dioxane | I | 3.97 | n.a. | 3.50 | n.a. | 2.19 | n.a. |
| | II | 3.87 | n.a. | 3.75 | n.a. | 2.19 | n.a. |
| | III | — | — | 2.82 | n.a. | 1.51 | n.a. |
| ethanol | I | 4.03 | n.a. | 3.47 | n.a. | 2.15 | n.a. |
| | II | 3.88 | — | — | — | — | — |
| | III | — | — | 2.70 | n.a. | 1.43 | n.a. |
| acetonitrile | I | 4.03 | n.a. | 3.47 | n.a. | 2.15 | n.a. |
| | II | 3.89 | — | — | — | — | — |
| | III | — | — | 2.70 | n.a. | 1.43 | n.a. |
| water | I | 4.03 | n.a. | 3.46 | n.a. | 2.15 | n.a. |
| | II | 3.89 | — | — | — | — | — |
| | III | — | — | 2.69 | n.a. | 1.43 | n.a. |
Table 14.
Assignment of excitation, fluorescence and phosphorescence spectra of HPT in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
Table 14.
Assignment of excitation, fluorescence and phosphorescence spectra of HPT in different solvents in terms of calculated energies (eV). Numbers within parentheses refer to the references corresponding to the experimental data. (n.a. indicates non-availability of data).
| Solvent | Species | Excitation | Fluorescence | Phosphorescence |
|---|
| | | Calc. | Expt.* | Calc. | Expt.* | Calc. | Expt.* |
|---|
| cyclohexane | I | 3.45 | 3.65 | 3.23 | n.a. | 2.02 | n.a |
| | III | — | — | 2.40 | 2.37 | 1.26 | n.a. |
| p-dioxane | I | 3.45 | n.a. | 3.23 | n.a. | 2.02 | n.a. |
| | III | — | — | 2.40 | n.a. | 1.25 | n.a. |
| ethanol | I | 3.47 | 3.72 | 3.22 | n.a. | 2.00 | n.a. |
| | III | — | — | 2.40 | 2.46 | 1.23 | n.a. |
| acetonitrile | I | 3.47 | 3.72 | 3.22 | n.a. | 2.00 | n.a. |
| | III | — | — | 2.40 | 2.46 | 1.23 | n.a. |
| water | I | 3.48 | n.a. | 3.22 | n.a. | 2.00 | n.a. |
| | III | — | — | 2.40 | 2.48 | 1.23 | n.a. |
Intramolecular proton transfer
The potential energy curves (PEC) for the intramolecular proton transfer (IPT) process of the probes have been generated in S
0, S
1 and T
1 states, considering the distance between the migrating hydrogen atom and the relevant heteroatom to which the hydrogen is joined after proton transfer.
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11 and
Figure 12 represent the simulated PECs for the IPT process of the fluorophores HBO, HBI, HBT, HPO, HPI and HPT respectively in the three electronic states in isolated as well as in ethanolic solutions.
Figure 7.
Simulated PECs for IPT process of HBO in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 7.
Simulated PECs for IPT process of HBO in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 8.
Simulated PECs for IPT process of HBI in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 8.
Simulated PECs for IPT process of HBI in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 9.
Simulated PECs for IPT process of HBT in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 9.
Simulated PECs for IPT process of HBT in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 10.
Simulated PECs for IPT process of HPO in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 10.
Simulated PECs for IPT process of HPO in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 11.
Simulated PECs for IPT process of HPI in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 11.
Simulated PECs for IPT process of HPI in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 12.
Simulated PECs for IPT process of HPT in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
Figure 12.
Simulated PECs for IPT process of HPT in S0, S1 and T1 states (i, isolated; s, solvated in ethanol).
The figures clearly reveal that for all the fluorophores the formation of the tautomer via intramolecular proton transfer (IPT) in the ground state leads to endothermicity. However, the reaction becomes exothermic in S
1 as well as in T
1 states. This points to an unfavourable situation for the process in the ground state and a thermodynamic favour for the reaction in the lowest excited singlet and triplet states. Considering the kinetic aspect for the process, the present calculations reveal that the activation barrier for the process is quite high for all the molecular systems in the ground state leading to impose a restriction on the process. A considerable lowering of the activation barrier in the lowest excited singlet and triplet electronic states indicates that the IPT process is favoured in the excited states compared to the ground state.
Table 15 and
Table 16 present the calculated kinetic (
Eact) and thermodynamic (
ΔH) parameters for the IPT process in different electronic states of the molecular systems of the two series.
Calculations on all the molecular systems, thus, indicate that the activation barrier for the IPT reaction in the ground state is appreciably higher than those obtained in the lowest excited singlet (S
1) and triplet (T
1) states. As has been discussed before, the ΔH parameter also favours the proton transfer reaction in the excited states compared to the S
0 state. Hence, although the IPT process in infeasible in the ground state, the photoinduced proton transfer reaction is feasible in the S
1 and the T
1 states from both thermodynamic and kinetic reasons. The activation barriers from our calculations appear to be a little higher than the experimental values [
11,
16,
25,
27,
37,
48,
53]. This deviation may be because of the fact that the short range specific interactions, like hydrogen bonding, have not been considered in the present work. In any case, for all the systems the predicted trends of the occurrence of the photoprocesses and the spectral patterns match well with the experimental results.
Table 15.
Calculated activation energies (Eact in eV) and reaction enthalpies (ΔH in eV) for the intramolecular proton transfer reaction of HBO, HBI and HBT in S0, S1 and T1 states.
Table 15.
Calculated activation energies (Eact in eV) and reaction enthalpies (ΔH in eV) for the intramolecular proton transfer reaction of HBO, HBI and HBT in S0, S1 and T1 states.
| Medium / Solvent | State | HBO | HBI | HBT |
|---|
| | | Eact. | ΔH | Eact. | ΔH | Eact. | ΔH |
|---|
| vacuum | S0 | 1.251 | + 0.394 | 1.075 | + 0.335 | 1.187 | + 0.380 |
| | S1 | 0.892 | - 0.088 | 0.781 | - 0.236 | 0.722 | - 0.338 |
| | T1 | 0.975 | - 0.375 | 0.829 | - 0.448 | 0.806 | - 0.627 |
| cyclohexane | S0 | 1.239 | + 0.371 | 1.050 | + 0.306 | 1.170 | + 0.362 |
| | S1 | 0.892 | - 0.102 | 0.777 | - 0.250 | 0.722 | - 0.333 |
| | T1 | 0.972 | - 0.385 | 0.820 | - 0.460 | 0.806 | - 0.639 |
| p-dioxane | S0 | 1.238 | + 0.369 | 1.047 | + 0.302 | 1.168 | + 0.361 |
| | S1 | 0.892 | - 0.103 | 0.777 | - 0.252 | 0.722 | - 0.332 |
| | T1 | 0.972 | - 0.386 | 0.819 | - 0.462 | 0.806 | - 0.641 |
| ethanol | S0 | 1.223 | + 0.340 | 1.015 | + 0.266 | 1.146 | + 0.339 |
| | S1 | 0.893 | - 0.121 | 0.772 | - 0.269 | 0.723 | - 0.326 |
| | T1 | 0.968 | - 0.399 | 0.807 | - 0.477 | 0.805 | - 0.656 |
| acetonitrile | S0 | 1.223 | + 0.339 | 1.014 | + 0.265 | 1.145 | + 0.338 |
| | S1 | 0.893 | - 0.121 | 0.772 | - 0.270 | 0.723 | - 0.325 |
| | T1 | 0.968 | - 0.400 | 0.807 | - 0.477 | 0.805 | - 0.656 |
| water | S0 | 1.222 | + 0.338 | 1.013 | + 0.263 | 1.144 | + 0.337 |
| | S1 | 0.893 | - 0.122 | 0.772 | - 0.270 | 0.723 | - 0.325 |
| | T1 | 0.967 | - 0.400 | 0.807 | - 0.478 | 0.805 | - 0.657 |
Table 16.
Calculated activation energies (Eact in eV) and reaction enthalpies (ΔH in eV) for the intramolecular proton transfer reaction of HPO, HPI and HPT in S0, S1 and T1 states.
Table 16.
Calculated activation energies (Eact in eV) and reaction enthalpies (ΔH in eV) for the intramolecular proton transfer reaction of HPO, HPI and HPT in S0, S1 and T1 states.
| Medium / Solvent | State | HPO | HPI | HPT |
|---|
| | | Eact. | ΔH | Eact. | ΔH | Eact. | ΔH |
|---|
| vacuum | S0 | 1.311 | + 0.508 | 1.159 | + 0.523 | 1.212 | + 0.491 |
| | S1 | 0.834 | - 0.010 | 0.744 | - 0.132 | 0.834 | - 0.361 |
| | T1 | 1.193 | - 0.126 | 1.009 | - 0.193 | 0.962 | - 0.284 |
| cyclohexane | S0 | 1.283 | + 0.453 | 1.113 | + 0.440 | 1.179 | + 0.462 |
| | S1 | 0.835 | - 0.058 | 0.735 | - 0.197 | 0.839 | - 0.356 |
| | T1 | 1.186 | - 0.138 | 0.987 | - 0.221 | 0.957 | - 0.292 |
| p-dioxane | S0 | 1.279 | + 0.447 | 1.108 | + 0.431 | 1.175 | + 0.459 |
| | S1 | 0.835 | - 0.063 | 0.733 | - 0.204 | 0.840 | - 0.355 |
| | T1 | 1.185 | - 0.140 | 0.985 | - 0.224 | 0.956 | - 0.293 |
| ethanol | S0 | 1.244 | + 0.380 | 1.051 | + 0.329 | 1.135 | + 0.423 |
| | S1 | 0.835 | - 0.122 | 0.722 | - 0.287 | 0.846 | - 0.348 |
| | T1 | 1.177 | - 0.155 | 0.957 | - 0.257 | 0.950 | - 0.303 |
| acetonitrile | S0 | 1.242 | + 0.377 | 1.048 | + 0.324 | 1.133 | + 0.421 |
| | S1 | 0.835 | - 0.125 | 0.722 | - 0.287 | 0.846 | - 0.348 |
| | T1 | 1.177 | - 0.156 | 0.956 | - 0.259 | 0.950 | - 0.303 |
| water | S0 | 1.241 | + 0.374 | 1.046 | + 0.320 | 1.131 | + 0.420 |
| | S1 | 0.835 | - 0.127 | 0.716 | - 0.295 | 0.847 | - 0.347 |
| | T1 | 1.177 | -0.156 | 0.955 | -0.260 | 0.950 | -0.304 |