Effect of Organic Solvents and Biologically Relevant Ions on the Light-Induced DNA Cleavage by Pyrene and Its Amino and Hydroxy Derivatives
Abstract
:Introduction

Materials and Methods
Reagents, chemicals, and instrumentation
Light-induced DNA single strand cleavage by PAHs in the presence of biologically relevant ions and in various organic solvents
Solubility tests
Results and Discussion
Solubility of pyrene, 1-AP, and 1-HP in methanol, DMF, and DMSO
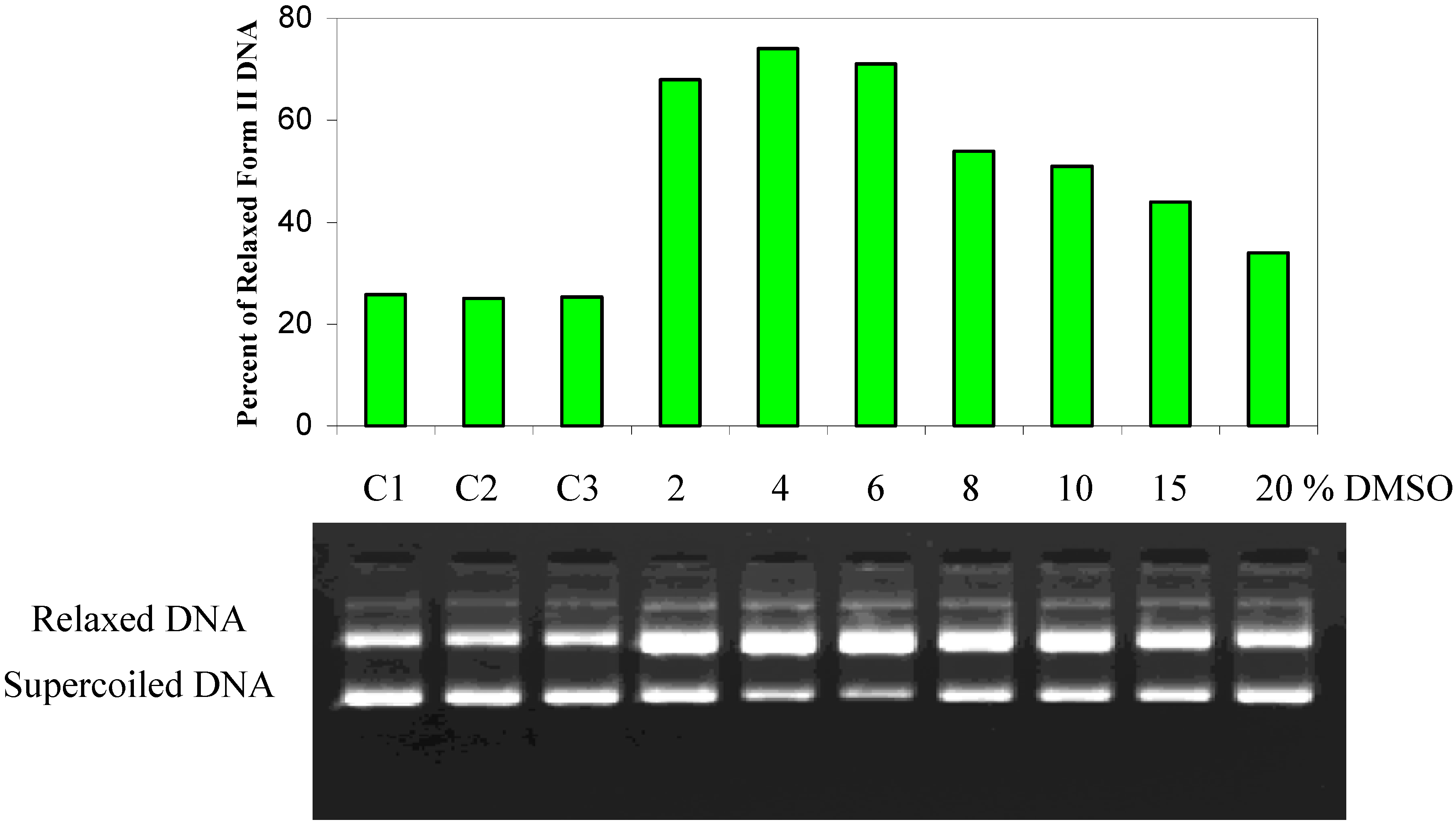
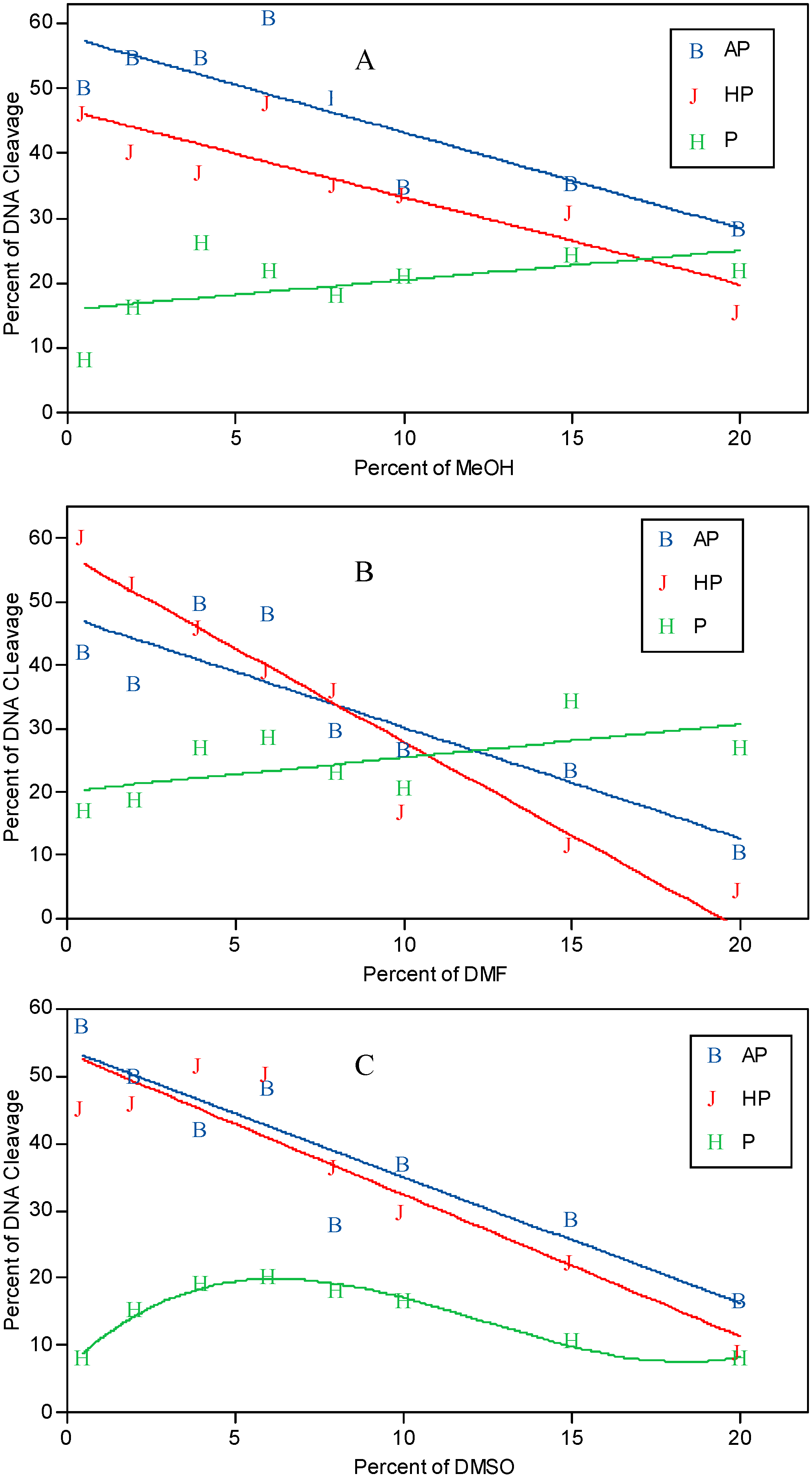
Effect of methanol, DMF, and DMSO on the light-induced DNA cleavage by PAHs
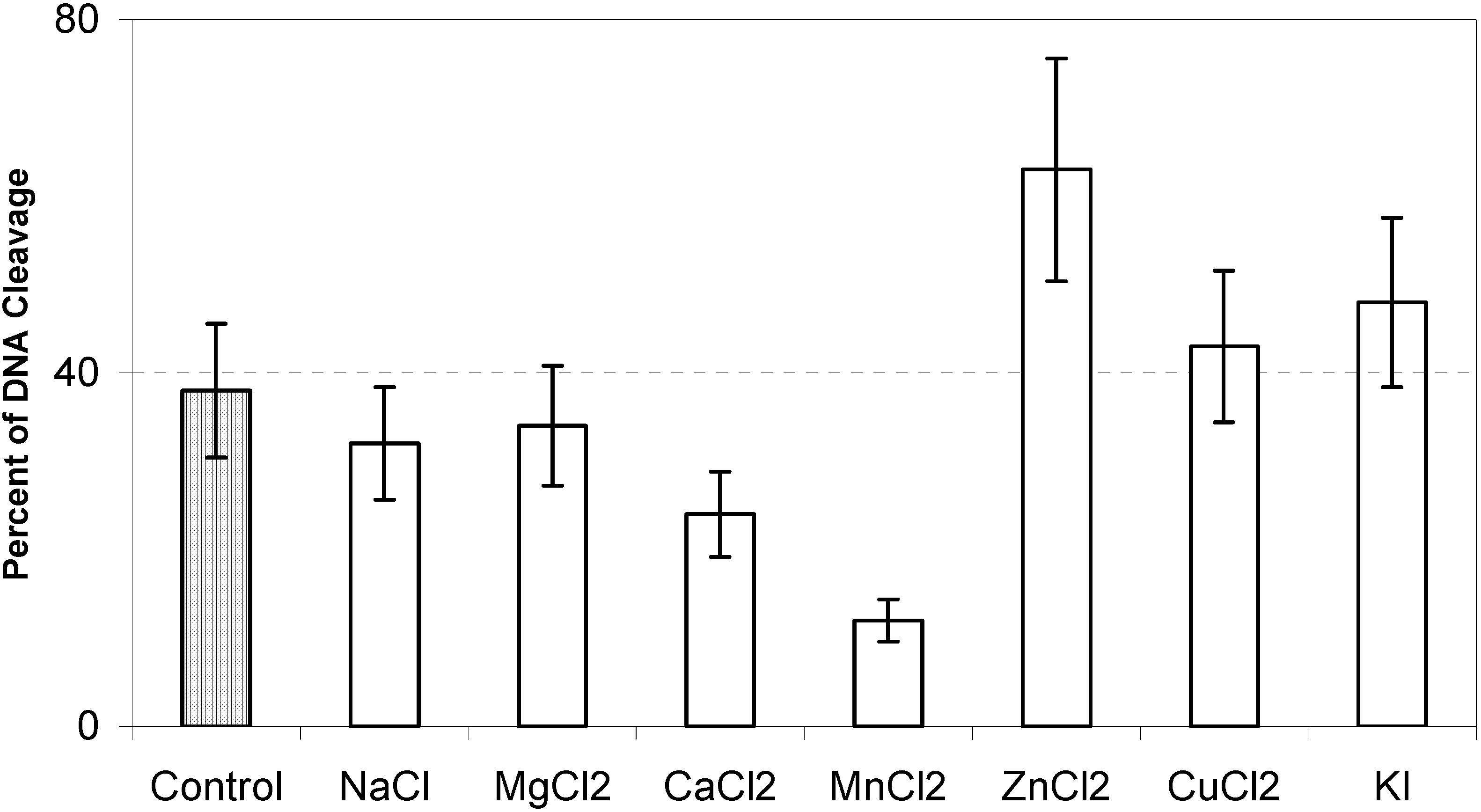
Effect of ions on photocleavage of DNA by 1-AP
Conclusions
Acknowledgements
References
- Baum, E. Occurrence and surveillance of polycyclic aromatic hydrocarbons. In Polycyclic Aromatic Hydrocarbons and Cancer; Gelboin, H., Ts'O, T., Eds.; 1978; Vol. 1, pp. 45–70. Academic Press: New York. [Google Scholar]
- Connell, D. W.; Hawker, D. W.; Warne, M. J.; Vowles, P. P. Polycyclic aromatic hydrocarbons (PAHs). In Introduction into Environmental Chemistry; McCombs, K., Starkweather, A. W., Eds.; 1997; pp. 205–217. CRC Press LLC: Boca Raton, FL. [Google Scholar]
- Pitot, H. C. Mechanisms of Chemical Carcinogenesis: Theoretical and Experimental Bases. In Chemical Carcinogenesis and Mutagenesis I. Handbook of Experimental Pharmacology; Cooper, C. S., Growler, P. L., Eds.; 1990; Vol. 94/I, pp. 2–29. Springer-Verlag: London. [Google Scholar]
- Kennaway, E. L.; Hieger, I. Carcinogenic substance and fluorescence spectra. Brit. Med. J. 1930, 1, 1044–1046. [Google Scholar] [CrossRef]
- Cook, J. W.; Hewett, C. L.; Hieger, I. The isolation of a cancer producing hydrocarbon from coal tar. J. Chem. Soc. 1933, 395–405. [Google Scholar] [CrossRef]
- Angerer, J.; Mannschreck, C.; Gundel, J. Biological monitoring and biochemical effect monitoring of exposure to polycyclic aromatic hydrocarbons. Int. Arch. Occup. Environ. Health 1997, 70, 365–377. [Google Scholar] [CrossRef]
- Harvey, R. G. Polycyclic Aromatic Hydrocarbons: Chemistry and Carcinogenicity; 1991; Cambridge University Press: London. [Google Scholar]
- Hemminski, K.; Grzybowska, E.; Chorazy, M.; Twardowska-Saucha, K.; Sroczynski, J. W.; Putman, K. L.; Randrath, K.; Phillips, D. H.; Hewer, A.; Santella, R. M.; Perera, F. P. DNA adducts in humans related to occupational exposure to aromatic compounds. In Complex Mixtures and Cancer Risk; Vainio, H., Sorsa, M., McMichael, A. J., Eds.; 1990; Vol. 104, pp. 181–192. IARC Scientific Publisher: Lyon, France. [Google Scholar]
- Bjorseth, A.; Becher, G. PAH in work atmospheres: Occurence and determination; 1986; Boca Raton, FL. [Google Scholar]
- Bjorseth, A.; Ramdahl, T. Emission source and recent progress in analytical chemistry; Vol. 2, 1985; Marcel Dekker: New York. [Google Scholar]
- Grimmer, G.; Brune, H.; Dettbarn, G.; Jacob, J.; Misfeld, J.; Mohr, U.; Nanjack, K.; Timm, J.; Wenzel-Hartung, R. Relevance of polycyclic aromatic hydrocarbons as environmental carcinogens. Fresnius J. Anal. Chem. 1991, 339, 792–792. [Google Scholar] [CrossRef]
- IARC. Polynuclear aromatic compounds. Part I: Chemical, Environmental and Experimental Data; 1983; International Agency for Research on Cancer: Lyon. [Google Scholar]
- Bleeker, E. A.; Wiegman, S.; de Voogt, P.; Kraak, M.; Leslie, H. A.; de Haas, E.; Admiraal, W. Toxicity of azaarenes. Rev. Environ. Contam. Toxic. 2002, 173, 39–83. [Google Scholar]
- Hemminki, K.; Koskinen, M.; Rajaniemi, H.; Zhao, C. DNA adducts, mutations, and cancer 2000. Regul. Toxicol. Pharmacol. 2000, 32, 264–275. [Google Scholar] [CrossRef]
- Shaw, G. R.; Connell, D. W. Prediction and monitoring of the carcinogenicity of polycyclic aromatic compounds (PACs). Rev. Environ. Contam. Toxic. 1994, 135, 1–62. [Google Scholar]
- Dabestani, R.; Ivanov, I. N. A comparison of physical, spectroscopic and photophysical properties of polycyclic aromatic hydrocarbons. Photochem. Photobiol 1999, 70, 10–34. [Google Scholar]
- Dong, S.; Hwang, H.-M.; Harrison, C.; Holloway, L.; Shi, X.; Yu, H. UVA light-induced DNA cleavage by selected polycyclic aromatic hydrocarbons. Bull. Environ. Contam. Toxicol. 2000, 64, 467–474. [Google Scholar] [CrossRef]
- Huang, X.-D.; Dixon, D. G.; Greenberg, B. M. Impact of UV radiation and photomodification on the toxicity of PAHs to the higher plant Lemna gibba (Duckweed). Envin. Toxicol. Chem. 1993, 12, 1067–1077. [Google Scholar]
- Huang, X.-D.; Krylov, S. N.; Ren, L.; McKonkey, B. J.; Dixon, D. G.; Greenberg, B. M. Mechanistic quantitative structure-activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: II. An empirical model for the toxicity of 16 polycyclic aromatic hydrocarbons to the duckweek lemna gibba L. G-3. Environ. Toxicol. Chem. 1997, 16, 2296–2303. [Google Scholar]
- Pelletier, M. C.; Burgess, R. M.; Ho, K. T.; Kuhn, A.; McKinney, R. A.; Ryba, S. A. Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrae lavae and juveniles. Envin. Toxicol. Chem. 1997, 16, 2190–2199. [Google Scholar] [CrossRef]
- Arfsten, D. P.; Schaeffer, D. J.; Mulveny, D. C. The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: A review. Ecotoxicol. Environ. Safety 1996, 33, 1–24. [Google Scholar] [CrossRef]
- Dong, S.; Fu, P. P.; Hwang, H.-M.; Yu, H. Effects of histidine on light-induced DNA single strand cleavage by selected polycyclic aromatic hydrocarbons. Polycycl. Arom. Compd. 2002. In press. [Google Scholar]
- Dong, S.; Fu, P. P.; Shirsat, R. N.; Hwang, H.-M.; Leszczynski, J.; Yu, H. UVA light-induced DNA cleavage by isomeric methylbenz[a]anthracenes. Chem. Res. Toxicol. 2002, 15, 400–409. [Google Scholar] [CrossRef]
- Dong, S.; Hwang, H.-M.; Shi, X.; Holloway, L.; Yu, H. UVA-induced DNA single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem. Res. Toxicol. 2000, 13, 585–593. [Google Scholar] [CrossRef]
- Yu, H.; Dong, S.; Fu, P. P.; Hwang, H.-M. UVA light-induced DNA single strand cleavage by hydroxybenzo[a]pyrenes. Polycycl. Arom. Compd. 2002. In press. [Google Scholar]
- Hwang, H.-M.; Shi, X.; Ero, I.; Jayasinghe, A.; Dong, S.; Yu, H. Microbial ecotoxicity and mutagenicity of 1-hydroxypyrene and its photoproducts. Chemosphere 2001, 45, 445–451. [Google Scholar] [CrossRef]
- Kochany, J.; Maguire, R. J. Abiotic transformations of polynuclear aromatic hydrocarbons and polynuclear aromatic nitrogen heterocycles in aquatic environments. Sci. Total Environ. 1994, 144, 17–31. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W. Y. Aqueous solubility of polynuclear aromatic hydrocarbons. J. Chem. Eng. Data 1977, 22, 399–402. [Google Scholar] [CrossRef]
© 2002 by MDPI (http://www.mdpi.org).
Share and Cite
Dong, S.; Wang, S.; Stewart, G.; Hwang, H.-M.; Fu, P.P.N.; Yu, H. Effect of Organic Solvents and Biologically Relevant Ions on the Light-Induced DNA Cleavage by Pyrene and Its Amino and Hydroxy Derivatives. Int. J. Mol. Sci. 2002, 3, 937-947. https://doi.org/10.3390/i3090937
Dong S, Wang S, Stewart G, Hwang H-M, Fu PPN, Yu H. Effect of Organic Solvents and Biologically Relevant Ions on the Light-Induced DNA Cleavage by Pyrene and Its Amino and Hydroxy Derivatives. International Journal of Molecular Sciences. 2002; 3(9):937-947. https://doi.org/10.3390/i3090937
Chicago/Turabian StyleDong, Shiming, Shuguang Wang, Gernerique Stewart, Huey-Min Hwang, Peter P. N. Fu, and Hongtao Yu. 2002. "Effect of Organic Solvents and Biologically Relevant Ions on the Light-Induced DNA Cleavage by Pyrene and Its Amino and Hydroxy Derivatives" International Journal of Molecular Sciences 3, no. 9: 937-947. https://doi.org/10.3390/i3090937



