Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Quantification of Glucosinolates in Chinese Cabbage
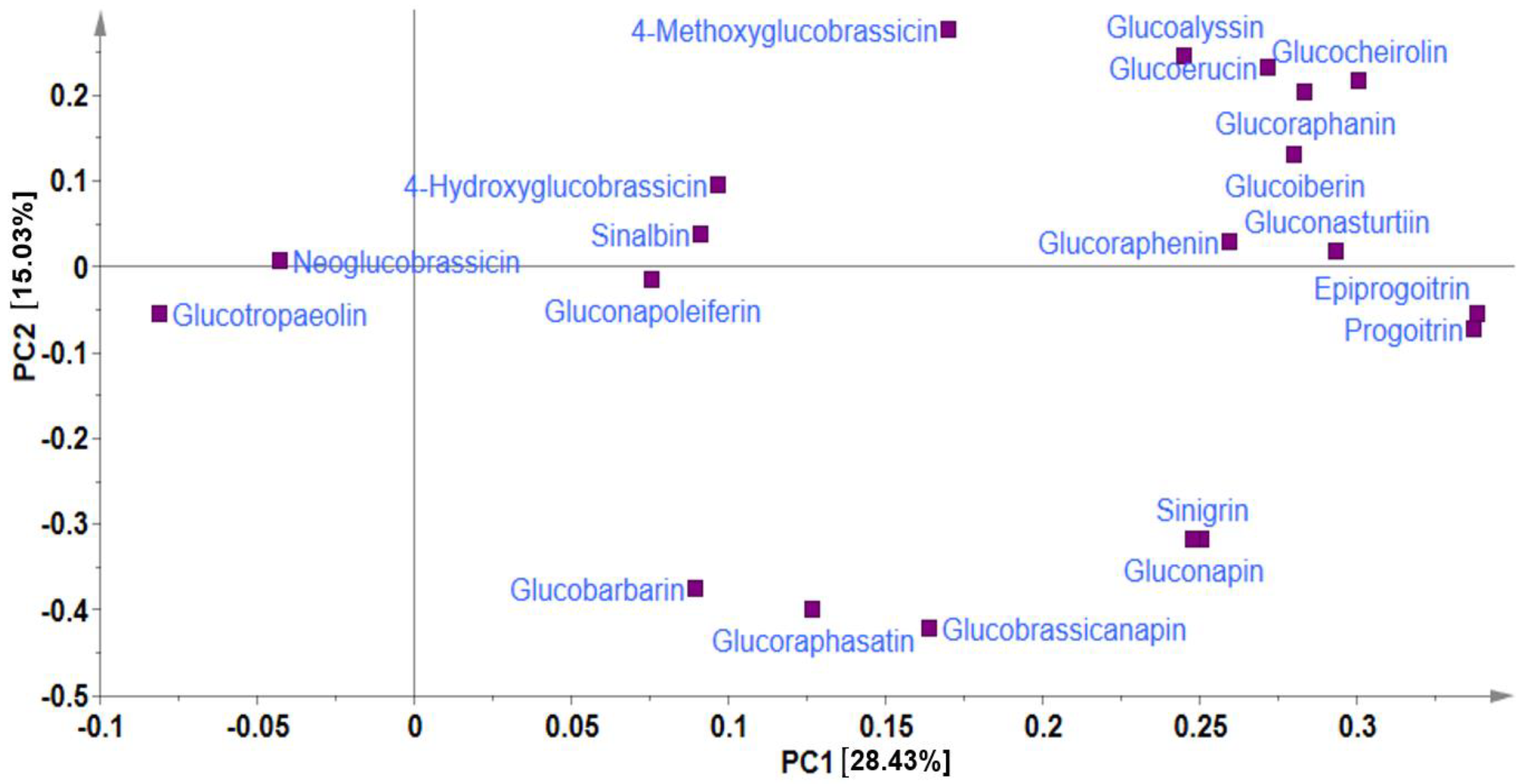
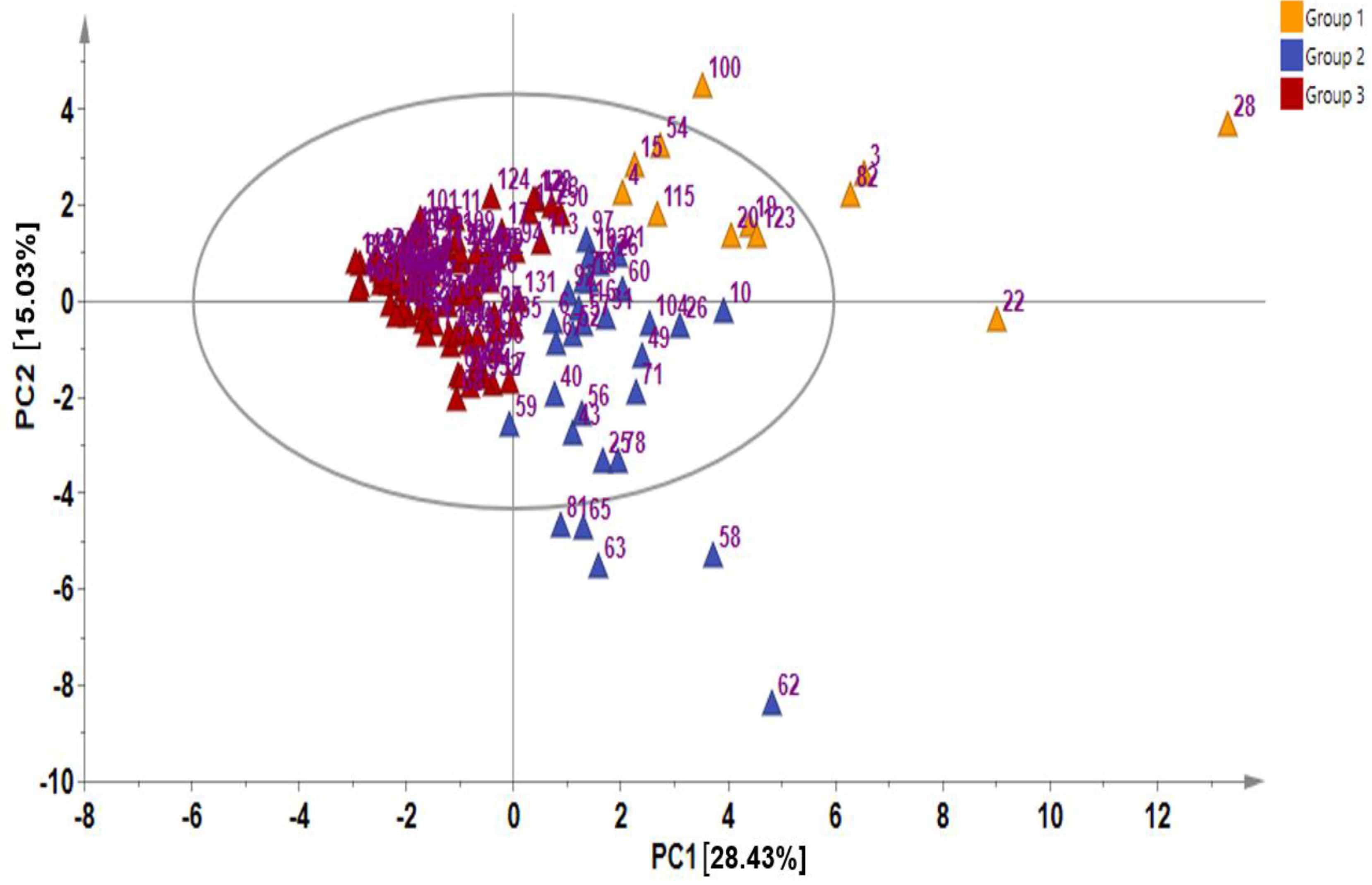
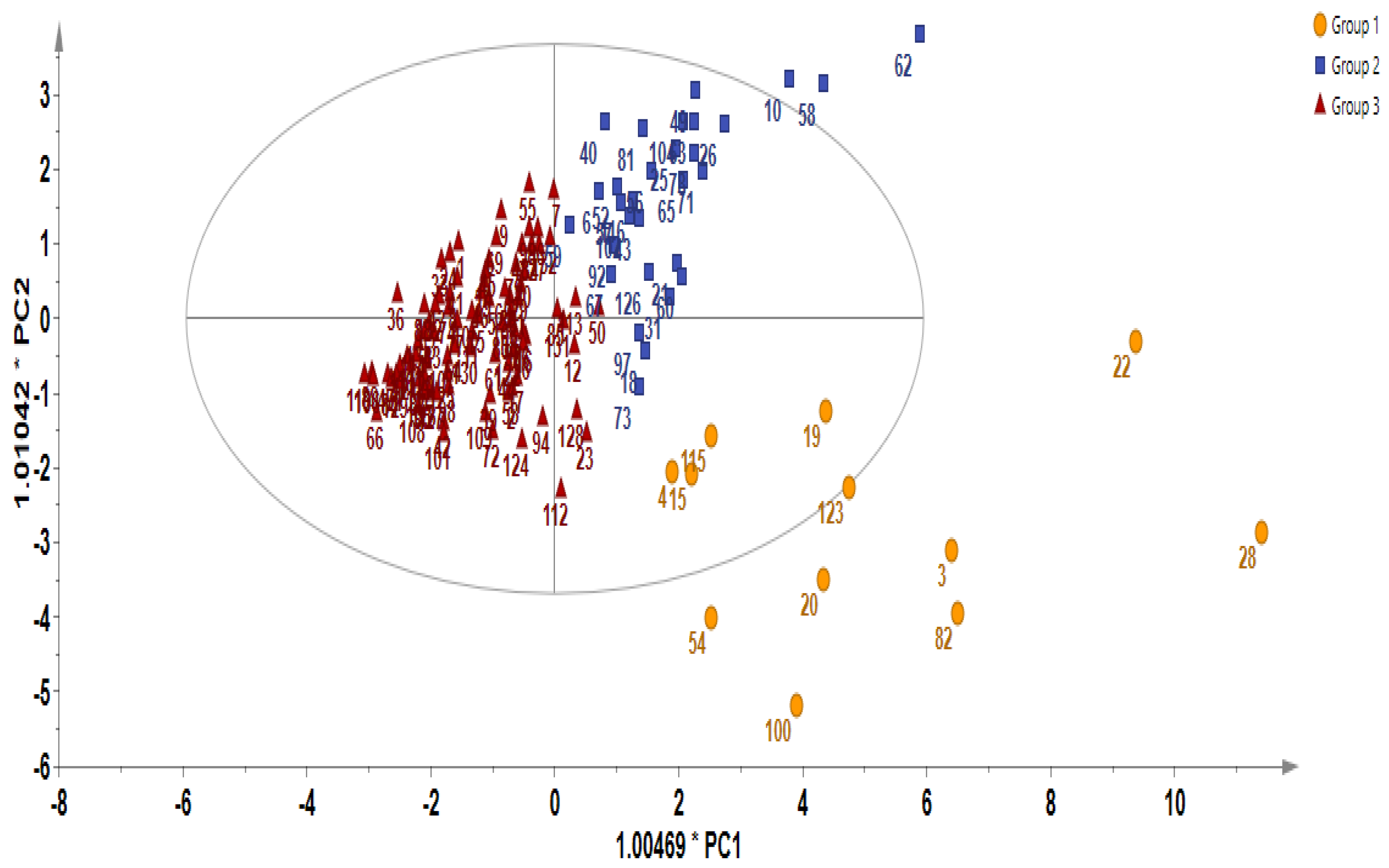
2.2. Multivariate Analysis
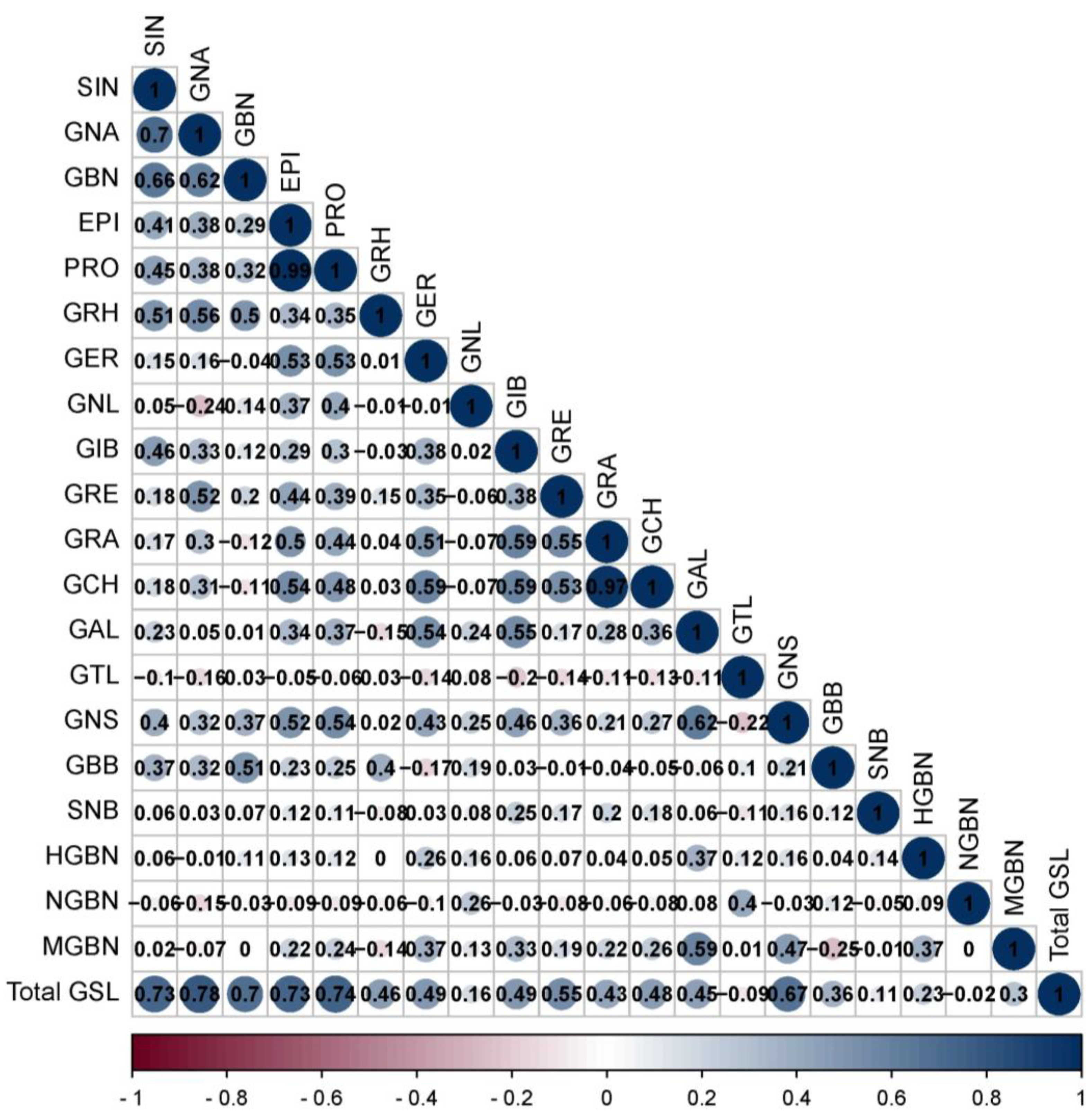
2.3. Correlation Analysis of Glucosinolates
3. Materials and Methods
3.1. Chemicals and Reagents
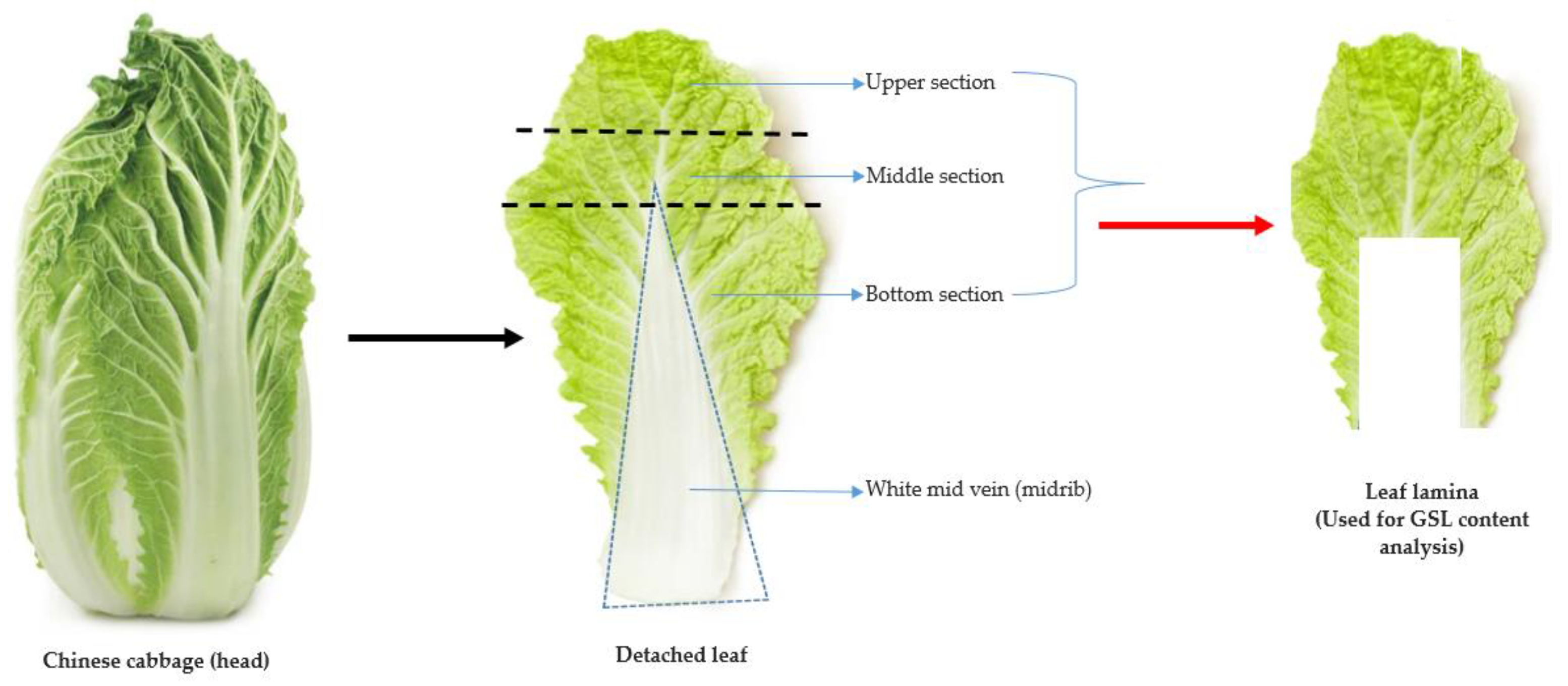
3.2. Plant Materials, Experimental Condition and Sampling
3.3. Sample Pretreatment and Extraction
3.4. The UPLC-MS/MS Analysis Condition and Identification of GSLs
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, V.; Devkar, S.; Gharat, K.; Kasbe, S.; Matharoo, D.K.; Pendse, S.; Bhosale, A.; Bhargava, A. Screening of phytochemicals as potential inhibitors of breast cancer using structure based multitargeted molecular docking analysis. Phytomed. Plus 2022, 2, 100227. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zeng, W.; Wang, J.; Zhang, F.; Sun, B.; Wang, Q. Improvement of glucosinolates by metabolic engineering in Brassica crops. Abiotech 2021, 2, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Chu, S.M.; Kim, S.J.; Lee, D.J.; Lee, S.Y.; Lim, S.H.; Ha, S.-H.; Kweon, S.J.; Cho, H.S. Variation of glucosinolates in vegetable crops of Brassica rapa L. ssp. pekinensis. Food Chem. 2010, 119, 423–428. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Ochar, K.; Hwang, A.; Lee, Y.-J.; Kang, H.J. Variability of Glucosinolates in Pak Choy (Brassica rapa subsp. chinensis) Germplasm. Plants 2023, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, G.-A.; Subramanian, P.; Hahn, B.-S. Quantification and diversity analyses of major glucosinolates in conserved chinese cabbage (Brassica rapa L. ssp. pekinensis) germplasms. Foods 2023, 12, 1243. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Blekkenhorst, L.C. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: Investigating the preclinical and clinical evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Prieto, M.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [PubMed]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Gupta, M.; Banga, S.S.; Heslop-Harrison, J. Identification of chromosomes and chromosome rearrangements in crop brassicas and Raphanus sativus: A cytogenetic toolkit using synthesized massive oligonucleotide libraries. Front. Plant Sci. 2020, 11, 598039. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Woody, S.; Greenham, K.; VanBuren, R.; Colle, M.; Edger, P.P.; Sartor, R.; Zheng, Y.; Levendoski, N.; Lim, J. Genetic and genomic resources to study natural variation in Brassica rapa. Plant Direct. 2020, 4, e00285. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-E.; Yoo, S.-A.; Seo, S.-H.; Lee, K.-I.; Na, C.-S.; Son, H.-S. GC–MS based metabolomics approach of Kimchi for the understanding of Lactobacillus plantarum fermentation characteristics. LWT-Food Sci. Technol. 2016, 68, 313–321. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Kim, Y.S.; Wang, T.T.; Smith, A.D. Indole-3-carbinol inhibits Citrobacter rodentium infection through multiple pathways including reduction of bacterial adhesion and enhancement of cytotoxic T cell activity. Nutrients 2020, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, H.; Childs, H.; Wu, Y.; Yu, L.; Pehrsson, P.R. Glucosinolates in Brassica vegetables: Characterization and factors that influence distribution, content, and intake. Annu. Rev. Food Sci. Technol. 2021, 12, 485–511. [Google Scholar] [CrossRef] [PubMed]
- Casajús, V.; Demkura, P.; Civello, P.; Lobato, M.G.; Martínez, G. Harvesting at different time-points of day affects glucosinolate metabolism during postharvest storage of broccoli. Food Res. Int. 2020, 136, 109529. [Google Scholar] [CrossRef]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT-Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Liang, X.; Lee, H.W.; Li, Z.; Lu, Y.; Zou, L.; Ong, C.N. Simultaneous quantification of 22 glucosinolates in 12 Brassicaceae vegetables by hydrophilic interaction chromatography–tandem mass spectrometry. ACS Omega 2018, 3, 15546–15553. [Google Scholar] [CrossRef] [PubMed]
- Klopsch, R.; Witzel, K.; Artemyeva, A.; Ruppel, S.; Hanschen, F.S. Genotypic variation of glucosinolates and their breakdown products in leaves of Brassica rapa. J. Agric. Food Chem. 2018, 66, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Z.; Gerendás, J.; Zimmermann, N. Glucosinolates in Chinese Brassica campestris vegetables: Chinese cabbage, purple cai-tai, choysum, pakchoi, and turnip. HortScience 2008, 43, 571–574. [Google Scholar] [CrossRef]
- Chun, J.-H.; Kim, N.-H.; Seo, M.-S.; Jin, M.; Park, S.U.; Arasu, M.V.; Kim, S.-J.; Al-Dhabi, N.A. Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Saudi J. Biol. Sci. 2018, 25, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Nugroho, A.B.D.; Nugroho, D.; Kim, D.-H. Correlation analysis of glucosinolate profiles and GSL biosynthetic genes in radishes (Raphanus sativus L.). Hortic. Environ. Biotechnol. 2024, 65, 157–167. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates–gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Kim, S.-J.; Kim, G.-H. Identification and quantitative determination of glucosinolates in seeds and edible parts of Korean Chinese cabbage. Food Chem. 2011, 128, 1115–1120. [Google Scholar] [CrossRef]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A. The role of isothiocyanates as cancer chemo-preventive, chemo-therapeutic and anti-melanoma agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef]
- Baek, S.-A.; Jung, Y.-H.; Lim, S.-H.; Park, S.U.; Kim, J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. subsp. pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef] [PubMed]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Biopharmaceutical understanding of excipient variability on drug apparent solubility based on drug physicochemical properties. Case study: Superdisintegrants. AAPS J. 2020, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Steuer, R.; Kurths, J.; Fiehn, O.; Weckwerth, W. Interpreting correlations in metabolomic networks. Biochem. Soc. Trans. 2003, 31, 1476–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Subramanian, P.; Hahn, B.-S. Glucosinolate diversity analysis in Choy Sum (Brassica rapa subsp. chinensis var. parachinensis) Germplasms for functional food breeding. Foods 2023, 12, 2400. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.D.; Kim, S.-H.; Ko, H.C.; Ro, N.; Subramanian, P.; Chung, Y.-J.; Lee, Y.-H.; Hahn, B.-S.; Rhee, J.-H. Leaf Mustard (Brassica juncea) Germplasm Resources Showed Diverse Characteristics in Agro-Morphological Traits and Glucosinolate Levels. Foods 2023, 12, 4374. [Google Scholar] [CrossRef]
- Rhee, J.-H.; Choi, S.; Lee, J.-E.; Hur, O.-S.; Ro, N.-Y.; Hwang, A.-J.; Ko, H.-C.; Chung, Y.-J.; Noh, J.-J.; Assefa, A.D. Glucosinolate content in Brassica genetic resources and their distribution pattern within and between inner, middle, and outer leaves. Plants 2020, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dong, X.; Choi, K.; Song, H.; Yi, H.; Hur, Y. Identification of source-sink tissues in the leaf of Chinese cabbage (Brassica rapa ssp. pekinensis) by carbohydrate content and transcriptomic analysis. Genes Genom. 2020, 42, 13–24. [Google Scholar] [CrossRef]

| S/N | Trivia Name | Semi-Systematic | Molecular Formula | MW (g/mol) | Precursor Amino Acid | Class |
|---|---|---|---|---|---|---|
| 1 | Sinigrin | Prop-2-enyl | C10H17NO9S2 | 397.5 | Met | Aliphatic |
| 2 | Gluconapin | But-3-enyl | C11H19NO9S2 | 373.4 | Met | Aliphatic |
| 3 | Glucobrassicanapin | Pent-4-enyl | C12H21NO9S2 | 387.4 | Met | Aliphatic |
| 4 | Epiprogoitrin | (2S)-2-Hydroxybut-3-enyl | C11H18NO10S2 | 389.4 | Met | Aliphatic |
| 5 | Progoitrin | (2R)-2-Hydroxybut-3-enyl | C11H19NO10S2 | 389.4 | Met | Aliphatic |
| 6 | Glucoraphasatin | 4-Methylthio-3-butenyl | C12H21NO9S3 | 435.5 | Met | Aliphatic |
| 7 | Glucoerucin | 4-(Methylsulfanyl)butyl, 4-(Methylthio)butyl | C12H23NO9S3 | 421.5 | Met | Aliphatic |
| 8 | Gluconapoleiferin | (2S)-2-Hydroxypent-4-enyl | C12H20NO10S2 | 403.4 | Met | Aliphatic |
| 9 | Glucoiberin | (RS)-3-(Methylsulfinyl)propyl | C11H21NO10S3 | 423.5 | Met | Aliphatic |
| 10 | Glucoraphenin | (RS,3E)-4-(Methylsulfinyl)but-3-enyl | C12H21NO10S3 | 435.5 | Met | Aliphatic |
| 11 | Glucoraphanin | (RS)-4-(Methylsulfinyl)butyl | C12H23NO10S3 | 437.5 | Met | Aliphatic |
| 12 | Glucocheirolin | 3-(Methylsulfonyl)propyl | C11H21NO11S3 | 477.6 | Met | Aliphatic |
| 13 | Glucoalyssin | (RS)-5-(Methylsulfinyl)pentyl | C13H25NO10S3 | 451.5 | Met | Aliphatic |
| 14 | Glucotropaeolin | Benzyl | C14H19NO9S2 | 409.4 | Phe | Aromatic |
| 15 | Gluconasturtiin | 2-Phenylethyl, Phenethyl | C15H21NO9S2 | 423.5 | Phe | Aromatic |
| 16 | Glucobarbarin | (2S)-2-hydroxy-2-phenylethyl | C15H21NO10S2 | 477.6 | Phe | Aromatic |
| 17 | Sinalbin | 4-Hydroxybenzyl | C30H42N2O15S2 | 425.4 | Phe/Tyr | Aromatic |
| 18 | 4-Hydroxyglucobrassicin | 4-Hydroxy-3-indolylmethyl | C16H20N2O10S2 | 464.5 | Trp | Indole |
| 19 | Neoglucobrassicin | 1-Methoxyindol-3-ylmethyl, N-Methoxyindol-3-ylmethyl | C17H22N2O10S2 | 478.5 | Trp | Indole |
| 20 | 4-Methoxyglucobrassicin | 4-Methoxyindol-3-ylmethyl | C17H22N2O10S2 | 478.5 | Trp | Indole |
| Glucosinolate | Mean | Median | Range |
|---|---|---|---|
| Aliphatic glucosinolates (μmol/kg DW) | |||
| Sinigrin | 3.41 | 2.09 | 0.27–23.23 |
| Gluconapin | 1258.46 | 642.91 | 18.07–7397.62 |
| Glucobrassicanapin | 1558.49 | 1265.92 | 13.43–6460.73 |
| Epiprogoitrin | 425.26 | 301.79 | 10.35–2412.79 |
| Progoitrin | 457.72 | 356.75 | 11.33–2026.53 |
| Glucoraphasatin | 0.28 | 0.17 | 0.00–2.78 |
| Glucoerucin | 185.44 | 14.29 | 0.03–3918.15 |
| Gluconapoleiferin | 222.17 | 162.15 | 1.61–931.81 |
| Glucoiberin | 0.29 | 0.19 | 0.00–2.07 |
| Glucoraphenin | 0.44 | 0.23 | 0.00–3.46 |
| Glucoraphanin | 165.98 | 61.86 | 1.14–4242.08 |
| Glucocheirolin | 4.34 | 1.64 | 0.07–87.44 |
| Glucoalyssin | 426.48 | 256.79 | 5.10–2147.02 |
| Aromatic glucosinolates (μmol/kg DW) | |||
| Glucotropaeolin | 1.56 | 1.24 | 0.11–4.91 |
| Gluconasturtiin | 283.81 | 235.01 | 7.94–1604.40 |
| Glucobarbarin | 108.91 | 93.65 | 17.74–383.92 |
| Sinalbin | 0.08 | 0.03 | 0.00–0.31 |
| Indole glucosinolates (μmol/kg DW) | |||
| 4-Hydroxyglucobrassicin | 61.31 | 27.81 | 3.72–796.51 |
| Neoglucobrassicin | 253.55 | 190/45 | 27.54–1385.02 |
| 4-Methoxyglucobrassicin | 451.47 | 353.89 | 64.11–2016.69 |
| Total glucosinolate | 5869.44 | 4878.99 | 626.53–21,199.02 |
| Total aliphatic glucosinolate | 4708.75 | 6541.95 | 149.96–17,972.00 |
| Total aromatic glucosinolate | 394.36 | 12201.62 | 35.46–1731.84 |
| Total indole glucosinolate | 766.32 | 23493.65 | 188.14–3261.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Ochar, K.; Iwar, K.; Lee, Y.-J.; Kang, H.J.; Na, Y.-W. Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS. Int. J. Mol. Sci. 2024, 25, 4829. https://doi.org/10.3390/ijms25094829
Kim S-H, Ochar K, Iwar K, Lee Y-J, Kang HJ, Na Y-W. Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS. International Journal of Molecular Sciences. 2024; 25(9):4829. https://doi.org/10.3390/ijms25094829
Chicago/Turabian StyleKim, Seong-Hoon, Kingsley Ochar, Kanivalan Iwar, Yoon-Jung Lee, Hae Ju Kang, and Young-Wang Na. 2024. "Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS" International Journal of Molecular Sciences 25, no. 9: 4829. https://doi.org/10.3390/ijms25094829






