Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery
Abstract
:1. Introduction
2. Chemical and Biological Properties of Xn
2.1. Structure and Chemistry of Xn
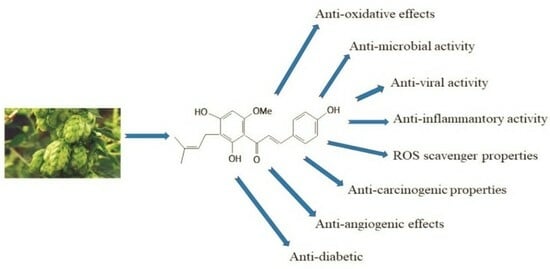
2.2. Biological Properties of Xn
2.2.1. Antibacterial Activity
2.2.2. Antifungal Activity
2.2.3. Antiviral Activity
2.2.4. Antimalarial Activity
2.2.5. Antiplatelet Activity
2.2.6. Safety of the Xn Use
2.2.7. Biotransformation, Pharmacokinetics and Clinical Applications of Xn
3. Biodegradable Polymers and Naturally Derived Compounds as the Carriers for Xn Delivery
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-PN | 6-prenylnaringenin |
| 8-PN | (±)-8-prenylnaringenin |
| A-2780 cells | Ovarian cancer cells |
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid |
| AE | Association Efficiency |
| AFM | Atomic Force Spectroscopy |
| AMPK | AMP-activated protein kinase |
| ASDs | Amorphous solid dispersions |
| b.w. | Body weight |
| B16F10 cells | Malignant cutaneous melanoma cells |
| BSEP | Bile salt export pump |
| BVDV | Bovine viral diarrhoea virus |
| Caco-2 cells | Human colorectal adenocarcinoma cells |
| CCD | Central Composite Design |
| CD | Crohn’s disease |
| CMV | Herpesviruses cytomegalovirus |
| CNP | Nanochitosan |
| COVID-19 | Coronavirus disease 2019 |
| CPE | Cytopathic effects |
| CRP | C-reactive protein |
| CS | Chitosan |
| DDSs | Drug Delivery Systems |
| DMX | Desmethylxanthohumol |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSC | Differential Scanning Calorimetry |
| EE | Entrapment Efficiency |
| FTIR | Fourier Transform Infrared Spectroscopy |
| FXR | Farnesoid X receptor |
| GMS | Glyceryl monostearate |
| HaCaT | Human keratinocytes |
| HA-g-PLLA | Hydroxyapatite-grafted poly(L-lactic acid) |
| HCE-T cells | Human corneal epithelial cells |
| HEM-DP | Human melanocytes from darkly pigmented skin |
| HRV | Human rhinovirus |
| HSV-1 | Herpes simplex virus type 1 |
| HSV-2 | Herpes simplex virus type 2 |
| HT-29 cells | Human colon cancer cells |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| IC50 | Half-maximal inhibitory concentrations |
| ICU | Intensive care unit |
| IL-12 | Interleukin-12 |
| IX | Isoxanthohumol |
| LPS | Lipopolysaccharide |
| MCF-7 cells | Human breast cancer cells |
| MCT | Medium-chain triglyceride |
| ME | Microemulsion |
| MIC | Minimal inhibitory concentration |
| MMPs | Matrix metalloproteinases |
| Mn | Average molecular weight |
| Mpro | Main protease |
| MRC-5 | Human lung fibroblast cells |
| NFκB | Nuclear factor-kappa B |
| NLR | Neutrophil-to-lymphocyte ratio |
| NPs | Nanoparticles |
| NRF2 | Nuclear factor erythroid 2 |
| OLN-93 | Oligodendroglia-derived cells |
| PAMs | Primary alveolar macrophages |
| PBMCs | Peripheral blood mononuclear cells |
| PC12 cells | Pheochromocytoma cells |
| PCL | Poly(ε-caprolactone) |
| PCR-DGGE | Polymerase chain reaction denaturing gradient gel electrophoresis |
| PDDSs | Polymer Drug Delivery Systems |
| PEO | Polyethylene oxide |
| PGA | Poly(glycolic acid) |
| PK | Pharmacokinetics |
| PLA | Poly(lactic acid) |
| PLACL | Poly(lactic acid-co-ε-caprolactone) |
| PLCγ2 | Phosphorylation of phospholipase Cγ2 |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLR | Platelet-to-lymphocyte ratio |
| PPO | Polypropylene oxide |
| PRRSV | Porcine reproductive and respiratory syndrome viruses |
| PZ | Serpin-like protein Z |
| RAW264.7 cells | Macrophagic mouse cells |
| RES | Resveratrol |
| ROS | Reactive Oxygen Species |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Sprague Dawley |
| SLNs | Solid lipid nanoparticles |
| SLs | Sophorolipids |
| SR | Sarcoplasmic Reticulum |
| S-SNEDDS | Solid Self-Nanoemulsifying Drug Delivery System |
| T2DM | Type 2 diabetes mellitus |
| TI | Therapeutic index |
| UC | Ulcerative colitis |
| US FDA | US Food and Drug Administration |
| XG | Xanthogalenol |
| Xn | Xanthohumol |
| XnC | Xanthohumol C |
| X-ray Diffraction | X-ray Diffraction |
| ζ | Zeta potential |
References
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Moir, M. Hops—A Millennium Review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for Human Malignancies: Chemistry, Pharmacokinetics and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef]
- Olšovská, J.; Boštíková, V.; Dušek, M.; Jandovská, V.; Bogdanová, K.; Čermák, P.; Boštík, P.; Mikyska, A.; Kolář, M. Humulus lupulus L. (Hops)—A valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett. 2016, 85, 19–30. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Stevens, J.F.; Miranda, C.L.; Buhler, D.R.; Deinzer, M.L. Chemistry and Biology of Hop Flavonoids. J. Am. Soc. Brew. Chem. 1998, 56, 136–145. [Google Scholar] [CrossRef]
- Eri, S.; Khoo, B.K.; Lech, J.; Hartman, T.G. Direct Thermal Desorption−Gas Chromatography and Gas Chromatography−Mass Spectrometry Profiling of Hop (Humulus lupulus L.) Essential Oils in Support of Varietal Characterization. J. Agric. Food Chem. 2000, 48, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4′-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775. [Google Scholar] [CrossRef]
- Guo, J.; Nikolic, D.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Identification of human hepatic cytochrome p450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159. [Google Scholar] [CrossRef]
- Chadwick, L.; Pauli, G.; Farnsworth, N. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef]
- Verzele, M.; Stockx, J.; Fontijn, F.; Anteunis, M. Xanthohumol, a New Natural Chalkone. Bull. Des Sociétés Chim. Belg. 1957, 66, 452–475. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence methods for analysis of beer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2008; pp. 963–976. ISBN 9780123738912. [Google Scholar]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. J. Control. Release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.S.; Peppas, N.A. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Heller, J. Controlled release of biologically active compounds from bioerodible polymers. Biomaterials 1980, 1, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Khosla, L. Bioabsorbable Polymers for Implantable Therapeutic Systems. Drug Dev. Ind. Pharm. 1998, 24, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Pafiti, K.S.; Vlasiou, M.C. Evaluation of xanthohumol as a potent drug from nature: Synthesis, isolation and anticancer activity. SCIREA J. Chem. 2019, 4, 1–19. [Google Scholar]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef]
- Arczewska, M.; Kamiński, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Sławińska-Brych, A.; Gagoś, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 213–222. [Google Scholar] [CrossRef]
- Luo, J.; Pan, Q.; Chen, Y.; Huang, W.; Chen, Q.; Zhao, T.; Guo, Z.; Liu, Y.; Lu, B. Storage stability and degradation mechanism of xanthohumol in Humulus lupulus L. and beer. Food Chem. 2023, 437, 137778. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Della-Fera, M.A.; Rayalam, S.; Baile, C.A. Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis 2007, 12, 1953–1963. [Google Scholar] [CrossRef]
- Kiyofuji, A.; Yui, K.; Takahashi, K.; Osada, K. Effects of Xanthohumol-Rich Hop Extract on the Differentiation of Preadipocytes. J. Oleo Sci. 2014, 63, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodrigues, I.; Guardão, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhães, J.; Ferreira-De-Almeida, M.; Negrão, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef]
- Nozawa, H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-Ay mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Depigmenting effect of Xanthohumol from hop extract in MNT-1 human melanoma cells and normal human melanocytes. Biochem. Biophys. Rep. 2021, 26, 100955. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.-J.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Abstracts: Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. Int. J. Cosmet. Sci. 2010, 32, 395–396. [Google Scholar] [CrossRef]
- Cho, Y.-C.; You, S.-K.; Kim, H.J.; Cho, C.-W.; Lee, I.-S.; Kang, B.Y. Xanthohumol inhibits IL-12 production and reduces chronic allergic contact dermatitis. Int. Immunopharmacol. 2010, 10, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef]
- Lin, M.; Xiang, D.; Chen, X.; Huo, H. Role of Characteristic Components of Humulus lupulus in Promoting Human Health. J. Agric. Food Chem. 2019, 67, 8291–8302. [Google Scholar] [CrossRef]
- Nookandeh, A.; Frank, N.; Steiner, F.; Ellinger, R.; Schneider, B.; Gerhäuser, C.; Becker, H. Xanthohumol metabolites in faeces of rats. Phytochemistry 2004, 65, 561–570. [Google Scholar] [CrossRef]
- Hanske, L.; Hussong, R.; Frank, N.; Gerhäuser, C.; Blaut, M.; Braune, A. Xanthohumol does not affect the composition of rat intestinal microbiota. Mol. Nutr. Food Res. 2005, 49, 868–873. [Google Scholar] [CrossRef]
- Sleha, R.; Radochova, V.; Mikyska, A.; Houska, M.; Bolehovska, R.; Janovska, S.; Pejchal, J.; Muckova, L.; Cermak, P.; Bostik, P. Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics 2021, 10, 392. [Google Scholar] [CrossRef]
- Awouafack, M.D.; Lee, Y.-E.; Morita, H. Xanthohumol: Recent advances on resources, biosynthesis, bioavailability and pharmacology. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–23. ISBN 978-3-030-94753-8. [Google Scholar]
- Buckwold, V.; Wilson, R.J.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.; Buckheit, R.; Wei, J.; Wenzelmathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Z.-H.; Liu, J.-K.; Zheng, Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, J.; Jiang, C.; Song, Z.; Zhao, Y.; Nauwynck, H.; Jiang, P. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Veter Microbiol. 2019, 238, 108431. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zang, R.; Ma, Y.; Wang, Z.; Li, L.; Ding, S.; Zhang, R.; Wei, Z.; Yang, J.; Wang, X. Xanthohumol Is a Potent Pan-Inhibitor of Coronaviruses Targeting Main Protease. Int. J. Mol. Sci. 2021, 22, 12134. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Gagos, M.; Siwicka-Gieroba, D.; Piechota, M.; Siwiec, J.; Bielacz, M.; Kotfis, K.; Stepulak, A.; Grzycka-Kowalczyk, L.; Jaroszynski, A.; et al. Humulus lupus extract rich in xanthohumol improves the clinical course in critically ill COVID-19 patients. Biomed. Pharmacother. 2022, 158, 114082. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and Health Benefits of Xanthohumol, a Natural Product Derived from Hops and Beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Herath, W.; Ferreira, D.; Khan, S.I.; Khan, I.A. Identification and biological activity of microbial metabolites of xanthohu-mol. Chem. Pharm. Bull. 2003, 51, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Schini-Kerth, V.B.; Étienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular Protection by Natural Product-Derived Polyphenols:In VitroandIn VivoEvidence. Planta Medica 2011, 77, 1161–1167. [Google Scholar] [CrossRef]
- Luzak, B.; Kassassir, H.; Rój, E.; Stanczyk, L.; Watala, C.; Golanski, J. Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity. Arch. Physiol. Biochem. 2016, 123, 54–60. [Google Scholar] [CrossRef]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.-L.; Zhang, R.; et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free. Radic. Biol. Med. 2017, 108, 247–257. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Hsieh, K.-H.; Lu, W.-J.; Chou, H.-C.; Chou, D.-S.; Lien, L.-M.; Sheu, J.-R.; Lin, K.-H. Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), Prevents Platelet Activation in Human Platelets. Evid. Based Complement. Altern. Med. 2012, 2012, 852362. [Google Scholar] [CrossRef]
- Arnaiz-Cot, J.J.; Cleemann, L.; Morad, M. Xanthohumol Modulates Calcium Signaling in Rat Ventricular Myocytes: Possible Antiarrhythmic Properties. J. Pharmacol. Exp. Ther. 2016, 360, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Prog. Biophys. Mol. Biol. 2022, 172, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M.; Vang, O. (Eds.) Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-39854-5. [Google Scholar]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Logan, I.E.; Miranda, C.L.; Lowry, M.B.; Maier, C.S.; Stevens, J.F.; Gombart, A.F. Antiproliferative and Cytotoxic Activity of Xanthohumol and Its Non-Estrogenic Derivatives in Colon and Hepatocellular Carcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 1203. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; van Steendam, K.; Vanhoecke, B.; Deforce, D.; Bracke, M.; Heyerick, A. Prenylated chalcone xanthohumol associates with histones in breast cancer cells–a novel target identified by a monoclonal antibody. Mol. Nutr. Food Res. 2012, 56, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107. [Google Scholar] [PubMed]
- Hussong, R.; Frank, N.; Knauft, J.; Ittrich, C.; Owen, R.; Becker, H.; Gerhäuser, C. A safety study of oral xanthohumol administration and its influence on fertility in Sprague Dawley rats. Mol. Nutr. Food Res. 2005, 49, 861–867. [Google Scholar] [CrossRef]
- Avula, B.; Ganzera, M.; Warnick, J.E.; Feltenstein, M.W.; Sufka, K.J.; Khan, I.A. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004, 42, 378–382. [Google Scholar] [CrossRef]
- Pang, Y.; Nikolic, D.; Zhu, D.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; van Breemen, R.B. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007, 51, 872–879. [Google Scholar] [CrossRef]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2011, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2013, 58, 248–255. [Google Scholar] [CrossRef]
- Jung, F.; Staltner, R.; Baumann, A.; Burger, K.; Halilbasic, E.; Hellerbrand, C.; Bergheim, I. A Xanthohumol-Rich Hop Extract Diminishes Endotoxin-Induced Activation of TLR4 Signaling in Human Peripheral Blood Mononuclear Cells: A Study in Healthy Women. Int. J. Mol. Sci. 2022, 23, 12702. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.; Langley, B.O.; Ryan, J.J.; Phipps, J.; Hanes, D.A.; Stack, E.; Jansson, J.K.; Metz, T.O.; Stevens, J.F. Xanthohumol microbiome and signature in healthy adults (the XMaS trial): A phase I triple-masked, placebo-controlled clinical trial. Trials 2020, 21, 835. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.O.; Ryan, J.J.; Phipps, J.; Buttolph, L.; Bray, B.; Aslan, J.E.; Metz, T.O.; Stevens, J.F.; Bradley, R. Xanthohumol microbiome and signature in adults with Crohn’s disease (the XMaS trial): A protocol for a phase II triple-masked, placebo-controlled clinical trial. Trials 2022, 23, 885. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Thapa, R.; Hariani, H.N.; Volyanyuk, M.; Ogle, S.D.; Orloff, K.A.; Ankireddy, S.; Lai, K.; Žiniauskaitė, A.; Stubbs, E.B.; et al. Poly(lactic-co-glycolic acid) Nanoparticles Encapsulating the Prenylated Flavonoid, Xanthohumol, Protect Corneal Epithelial Cells from Dry Eye Disease-Associated Oxidative Stress. Pharmaceutics 2021, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Macedo, A.S.; Lima, S.A.C.; Reis, S.; Soares, R.; Fonte, P. Evaluation of the Antitumour and Antiproliferative Effect of Xanthohumol-Loaded PLGA Nanoparticles on Melanoma. Materials 2021, 14, 6421. [Google Scholar] [CrossRef] [PubMed]
- Ronka, S.; Kowalczyk, A.; Baczyńska, D.; Żołnierczyk, A.K. Pluronics-Based Drug Delivery Systems for Flavonoids Anticancer Treatment. Gels 2023, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S.M.H. Co-encapsulation of lupulon and xanthohumol in lecithin-based nanoliposomes developed by sonication method. J. Food Process. Preserv. 2019, 43, e14075. [Google Scholar] [CrossRef]
- Hanmantrao, M.; Chaterjee, S.; Kumar, R.; Vishwas, S.; Harish, V.; Porwal, O.; Alrouji, M.; Alomeir, O.; Alhajlah, S.; Gulati, M.; et al. Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol. Pharmaceutics 2022, 14, 2384. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Mohd, S.; Govindaiah, P.; Babu, M.R.; Kumar, R.; Gulati, M.; Gowthamarajan, K.; Madhunapantula, S.V.; Chellappan, D.K.; et al. Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells. Pharmaceutics 2022, 14, 2403. [Google Scholar] [CrossRef]
- Harish, V.; Almalki, W.H.; Alshehri, A.; Alzahrani, A.; Gupta, M.M.; Alzarea, S.I.; Kazmi, I.; Gulati, M.; Tewari, D.; Gupta, G.; et al. Quality by Design (QbD) Based Method for Estimation of Xanthohumol in Bulk and Solid Lipid Nanoparticles and Validation. Molecules 2023, 28, 472. [Google Scholar] [CrossRef]
- Luo, J.; Yang, B.; Yang, X.; Ji, S.; Guo, Z.; Liu, Y.; Chen, Q.; Zhao, T.; Wang, Y.; Lu, B. Sophorolipid-based microemulsion delivery system: Multifaceted enhancement of physicochemical properties of xanthohumol. Food Chem. 2023, 413, 135631. [Google Scholar] [CrossRef]
- Leonida, M.D.; Belbekhouche, S.; Benzecry, A.; Peddineni, M.; Suria, A.; Carbonnier, B. Antibacterial hop extracts encapsulated in nanochitosan matrices. Int. J. Biol. Macromol. 2018, 120, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Johnpaul, I.A.; Hong, K.; Gao, H.; Song, Y.; Lv, C.; Ma, C. Protein Z-based promising carriers for enhancing solubility and bioaccessibility of Xanthohumol. Food Hydrocoll. 2022, 131, 107771. [Google Scholar] [CrossRef]
- Chatterjee, S.; Corrie, L.; Hanmantrao, M.; Vishwas, S.; Kumar, R.; Alotaibi, F.; Ansari, M.J.; Rehman, Z.U.; Porwal, O.; Khursheed, R.; et al. Quality by design-oriented formulation optimization and characterization of guar gum-pectin based oral colon targeted liquisolid formulation of xanthohumol. J. Drug Deliv. Sci. Technol. 2023, 82, 104350. [Google Scholar] [CrossRef]
- Qiao, T.; Jiang, S.; Song, P.; Song, X.; Liu, Q.; Wang, L.; Chen, X. Effect of blending HA-g-PLLA on xanthohumol-loaded PLGA fiber membrane. Colloids Surf. B Biointerfaces 2016, 146, 221–227. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Sun, Q.; Xia, W.; Zhou, Q.; Zhang, Z.; Song, X. Controlled release of resveratrol and xanthohumol via coaxial electrospinning fibers. J. Biomater. Sci. Polym. Ed. 2019, 31, 456–471. [Google Scholar] [CrossRef]
- Sánchez-Aguinagalde, O.; Meaurio, E.; Lejardi, A.; Sarasua, J.-R. Amorphous solid dispersions in poly(ε-caprolactone)/xanthohumol bioactive blends: Physicochemical and mechanical characterization. J. Mater. Chem. B 2021, 9, 4219–4229. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Heilmann, J. Synthesis, Cytotoxicity, and Antioxidative Activity of Minor Prenylated Chalcones from Humulus lupulus. J. Nat. Prod. 2008, 71, 1237–1241. [Google Scholar] [CrossRef]
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Médard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B 2018, 1095, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.-H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Oberbauer, E.; Urmann, C.; Steffenhagen, C.; Bieler, L.; Brunner, D.; Furtner, T.; Humpel, C.; Bäumer, B.; Bandtlow, C.; Couillard-Despres, S.; et al. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J. Nutr. Biochem. 2013, 24, 1953–1962. [Google Scholar] [CrossRef]
- Kirchinger, M.; Bieler, L.; Tevini, J.; Vogl, M.; Haschke-Becher, E.; Felder, T.K.; Couillard-Després, S.; Riepl, H.; Urmann, C. Development and Characterization of the Neuroregenerative Xanthohumol C/Hydroxypropyl-β-cyclodextrin Complex Suitable for Parenteral Administration. Planta Medica 2019, 85, 1233–1241. [Google Scholar] [CrossRef]


| No | Xanthohumol (Xn) Formulation | Material Used | Particle Size [nm] | Entrapment Efficiency (EE) [%] | Zeta Potential (ζ) [mV] | Model Used | Outcomes | Ref |
|---|---|---|---|---|---|---|---|---|
| 1. | Nanoparticles (NPs) | PLGA | 191.0 ± 0.8 | 13.1 ± 0.06 | −24.8 ± 0.2 | Human corneal epithelial (HCE-T) cells, the mouse desiccating stress/scopolamine model and corneal epithelial cells. | Pure Xn prevented tert-butyl hydroperoxide-induced loss of cell viability in HCE-T cells in a dose-dependent manner and significantly increase in expression of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). Xn-loaded PLGA NPs were cytoprotective against oxidative stress in vitro, significantly reduced ocular surface damage and oxidative stress associated DNA damage in corneal epithelial cells in the mouse desiccating stress/scopolamine model for dry eye disease in vivo. | [74] |
| 2. | Nanoparticles (NPs) | PLGA | 312 ± 49 | 88.7 ± 4.3 | −18.2 ± 1.4 | B16F10, malignant cutaneous melanoma, and RAW264.7, macrophagic, mouse cell lines | Similar viability cytoxicity between pure Xn and Xn-loaded PLGA NPs at 48 h with the IC50 at 10 μM. Similar antimigration effects for pure Xn and Xn-loaded PLGA NPs. The M1 antitumour phenotype was stimulated on macrophages. The ultimate anti-melanoma effect emerges from an association between the viability, migration, and macrophagic phenotype modulation. | [75] |
| 3. | Nanomicelles | Pluronic P123, F127 (polyethylene oxide (PEO) and polypropylene oxide (PPO) (PEO-PPO-PEO block copolymer) | 30.4 ± 0.1–30.5 ± 0.2 (varied in Xn to Pluronic ratio) | 93.5–100.0 (varied in Xn to Pluronic ratio) | −4.26–+4.26 (varied in Xn to Pluronic ratio) | Human colon cancer cell (HT-29 cells) | An enhanced in vitro cytotoxic activity was not achieved (it was associated with a lower polymer content in the tested formulations). | [76] |
| 4. | Nanoliposomes | Lecithin | 149.5–394.4 | 67.81 | −19.9–−38.2 | Antioxidant activities determined by DPPH method | The IC50 value for antioxidant activity was calculated to be 54.90 and 60.38 μg/mL for free Xn and nanoliposomal Xn. | [77] |
| 5. | Solid self-nanoemulsifying drug delivery system (S-SNEDDS) | Liquid (L)-SNEDDS composed of Labrafac PG, Tween 80 and Transcutol P was adsorbed onto the surface of guar gum and pectin to form S-SNEDDS | 118.96 ± 5.94 | 94.20 ± 4.71 | −22.78 ± 1.13 | Human colorectal adenocarcinoma (Caco-2) cell lines | 43.90 ± 3.98% and 31.98 ± 3.10% cells were found viable at the end of 12 and 24 h. Increase in cytotoxicity of Xn-loaded S-SNEDDS was about 1.42-fold and 1.51-fold at the end of 12 h and 24 h as that of cytotoxicity of raw Xn. | [78] |
| 6. | Solid lipid nanoparticles (SLNs) | Compritol E ATO (CE), precirol ATO5, Lipoid E 80SN (LE-80), lipoid S75, phospholipon 90H, pluronic F-68, tween 80, glyceryl monostearate (GMS), carnauba wax, palmitic and stearic acids | 108.60 ± 3.21 | 80.20 ± 2.95 | −12.7 | Prostate cancer cell lines (PC-3); in vivo pharmacokinetic study; cell permeability studies | The cell inhibition percentage was noted in a dose- and time-dependent manner; a significant (p < 0.05) reduction in inhibitory activity was observed in the case of Xn-SLNs as that of naive Xn in the first 12 h; at 5 h, the release of Xn from Xn-SLNs was found to be 6.2 ± 0.98 nmol, whereas naive XH showed only 1.34 ± 0.087 nmol permeation (the permeation of Xn from Xn-SLNs was about 4.62-fold higher than naive Xn and 0.31-fold-lower drug excretion than naive Xn; the enhancement in the bioavailability of the Xn was confirmed from an increase in Cmax (1.07-fold), AUC0-t (4.70-fold), t1/2 (6.47-fold), and MRT (6.13-fold) after loading into SLNs; the relative bioavailability of Xn-SLNs and naive Xn was found to be 4791% and 20.80%. | [79,80] |
| 7. | Microemulsion (ME) delivery system based on biosurfactant sophorolipids (SLs) | Tween 20/40/60/80, Span 20/80, isoamyl acetate (IA), SLs (lactonic), medium-chain triglyceride (MCT) | 15.79–17.62 | 96.52 ± 0.23–97.51 ± 0.89 | - | The in vitro digestion model | The antioxidant properties of Xn-SL-MEs were reflected in the IC50 values, with the decreases of 57.76% (DPPH), 83.94% (ABTS), and 34.34% (⋅OH); increase the solubility of Xn by about 4000 times, and its half-life during storage was extended to over 150 days; in vitro models revealed that the release profile of Xn followed non-Fickian diffusion, and the ME structure markedly strengthened its digestive stability and bioaccessibility. | [81] |
| 8. | Nanochitosan (CNP) matrices encapsulated hop extracts | Chitosan (CS) | 28.1–49.4 (varied in different synthesis conditions) | 81.00–97.16 (varied in different molar weight of chitosan) | −17.6–47.3 (varied in different synthesis conditions) | S. aureus, S. epidermis, P. aeruginosa, P. putida, C. albicans, C. lipolytica | The decrease in MIC values (by factors from 1.25 to 6) was higher for the Gram-negative and for the Candida species; increased antimicrobial effect against a broad spectrum of species and remarkable synergistic interactions between chitosan and hops. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oledzka, E. Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery. Int. J. Mol. Sci. 2024, 25, 3398. https://doi.org/10.3390/ijms25063398
Oledzka E. Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery. International Journal of Molecular Sciences. 2024; 25(6):3398. https://doi.org/10.3390/ijms25063398
Chicago/Turabian StyleOledzka, Ewa. 2024. "Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery" International Journal of Molecular Sciences 25, no. 6: 3398. https://doi.org/10.3390/ijms25063398