Extracellular Vesicles in the Pathogenesis, Clinical Characterization, and Management of Dermatomyositis: A Narrative Review
Abstract
:1. Introduction
2. Role of Extracellular Vesicles in the Pathogenesis of Dermatomyositis
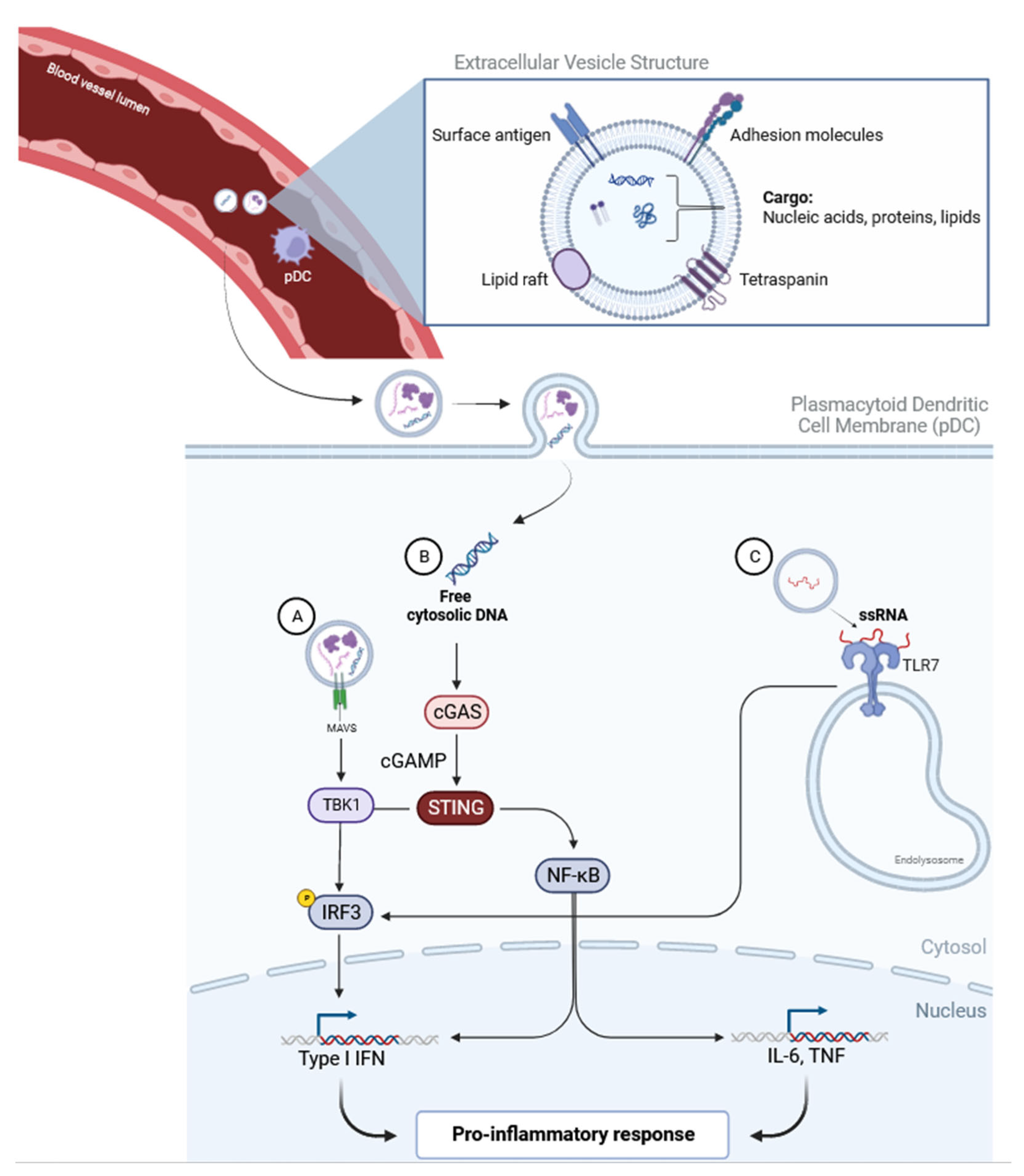
2.1. The Type 1 Interferon Pathway
2.2. The Complement Pathway
2.3. Vasculopathy, Muscle Damage, and the Proinflammatory Response
3. Characterization of Extracellular Vesicles in Dermatomyositis
4. EV-Associated Cargoes in DM
4.1. EV-Associated Protein Cargoes
4.2. EV-Associated Nucleic Acid Cargoes
4.2.1. EV-Associated MicroRNA (miRNA)
4.2.2. EV-Associated Long Non-Coding RNA (lncRNA)
4.2.3. EV-Associated Messenger RNA (mRNA)
4.2.4. EV-Associated dsDNA
5. The Clinical Implications of Extracellular Vesicles in Dermatomyositis
5.1. Extracellular Vesicles in Diagnosis and Prognosis of Dermatomyositis
5.1.1. Association between Extracellular Vesicles and Laboratory Parameters or Inflammatory Markers in Dermatomyositis
5.1.2. Association with Different Dermatomyositis Subsets
5.1.3. Association with Disease Activity in Dermatomyositis
5.1.4. Involvement of Extracellular Vesicles in Myositis due to Dermatomyositis
5.1.5. Extracellular Vesicles and Skin Inflammation in Dermatomyositis
5.1.6. Diagnostic Predictability of Extracellular Vesicles in Diagnosis and Prognosis of Dermatomyositis
5.2. Therapeutic Potential of Extracellular Vesicles in Dermatomyositis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, M.; Williams, K.J.; Werth, V.P. Microvesicles in autoimmune diseases. Adv. Clin. Chem. 2016, 77, 125–175. [Google Scholar] [PubMed]
- Vidal, M. Exosomes: Revisiting their role as “garbage bags”. Traffic 2019, 20, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Plaut, J.S.; Strzelecka-Kiliszek, A.; Bozycki, L.; Pikula, S.; Buchet, R.; Mebarek, S.; Chadli, M.; Bolean, M.; Simao, A.M.S.; Ciancaglini, P.; et al. Quantitative atomic force microscopy provides new insight into matrix vesicle mineralization. Arch. Biochem. Biophys. 2019, 667, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Andriantsitohaina, R.; Baharvand, H.; Bauer, N.N.; Baxter, A.A.; Beckham, C.; Bielska, E.; Boireau, W.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. 2021, 34, 1157–1167. [Google Scholar] [CrossRef]
- Tan, L.; Zhao, M.; Wu, H.; Zhang, Y.; Tong, X.; Gao, L.; Zhou, L.; Lu, Q.; Zeng, J. Downregulated Serum Exosomal miR-451a Expression Correlates With Renal Damage and Its Intercellular Communication Role in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 630112. [Google Scholar] [CrossRef]
- Uto, K.; Ueda, K.; Okano, T.; Akashi, K.; Takahashi, S.; Nakamachi, Y.; Imanishi, T.; Awano, H.; Morinobu, A.; Kawano, S.; et al. Identification of plexin D1 on circulating extracellular vesicles as a potential biomarker of polymyositis and dermatomyositis. Rheumatology 2022, 61, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wang, T.; Zhao, Q.; Hu, Q.; Chen, Y.; Li, H.; Liu, C.; Liu, D.; Hong, X. Proteomics Analysis of Plasma-Derived Exosomes Unveils the Aberrant Complement and Coagulation Cascades in Dermatomyositis/Polymyositis. J. Proteome Res. 2022, 22, 123. [Google Scholar] [CrossRef]
- Wu, W.; Song, S.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef]
- Parks, C.G.; Wilkerson, J.; Rose, K.M.; Faiq, A.; Noroozi Farhadi, P.; Long, C.S.; Bayat, N.; Brunner, H.I.; Goldberg, B.; McGrath, J.A.; et al. Association of Ultraviolet Radiation Exposure with Dermatomyositis in a National Myositis Patient Registry. Arthritis Care Res. 2020, 72, 1636–1644. [Google Scholar] [CrossRef]
- Schiffenbauer, A.; Faghihi-Kashani, S.; O’hanlon, T.P.; Flegel, W.A.; Adams, S.D.; Targoff, I.N.; Oddis, C.V.; Ytterberg, S.R.; Aggarwal, R.; Christopher-Stine, L.; et al. The Impact of Cigarette Smoking on the Clinical and Serological Phenotypes of Polymyositis and Dermatomyositis. Semin. Arthritis Rheum. 2018, 48, 504–512. [Google Scholar] [CrossRef]
- Bax, C.E.; Maddukuri, S.; Ravishankar, A.; Pappas-Taffer, L.; Werth, V.P. Environmental Triggers of Dermatomyositis: A Narrative Review. Ann. Transl. Med. 2021, 9, 434. [Google Scholar] [CrossRef]
- Smith, E.S.; Hallman, J.R.; Deluca, A.M.; Goldenberg, G.; Jorizzo, J.L.; Sangueza, O.P. Dermatomyositis: A Clinicopathological Study of 40 Patients. Am. J. Dermatopathol. 2009, 31, 61–67. [Google Scholar] [CrossRef]
- Li, Y.; Bax, C.; Patel, J.; Vazquez, T.; Ravishankar, A.; Bashir, M.M.; Grinnell, M.; Diaz, D.; Werth, V.P. Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis. Theranostics 2021, 11, 7144–7158. [Google Scholar] [CrossRef]
- Li, L.; Zuo, X.; Liu, D.; Luo, H.; Zhang, H.; Peng, Q.; Wang, G.; Zhu, H. Plasma exosomal RNAs have potential as both clinical biomarkers and therapeutic targets of dermatomyositis. Rheumatology 2022, 61, 2672–2681. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Xu, D.; Bai, J.; Wang, Q.; Zeng, X. Plasma-Derived Exosomal hsa-miR-4488 and hsa-miR-1228-5p: Novel Biomarkers for Dermatomyositis-Associated Interstitial Lung Disease with Anti-Melanoma Differentiation-Associated Protein 5 Antibody-Positive Subset. BioMed Res. Int. 2021, 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, C.; Hong, Y.; Krol, P.; Al Obaidi, M.; Pilkington, C.; Wedderburn, L.; Brogan, P.A.; Eleftheriou, D. The Vasculopathy of Juvenile Dermatomyositis: Endothelial Injury, Hypercoagulability, and Increased Arterial Stiffness. Arthritis Rheumatol. 2021, 73, 1253–1266. [Google Scholar] [CrossRef]
- Jiang, K.; Karasawa, R.; Hu, Z.; Chen, Y.; Holmes, L.; O’Neil, K.M.; Jarvis, J.N. Plasma exosomes from children with juvenile dermatomyositis are taken up by human aortic endothelial cells and are associated with altered gene expression in those cells. Pediatr. Rheumatol. 2019, 17, 41. [Google Scholar] [CrossRef]
- Baka, Z.; Senolt, L.; Vencovsky, J.; Mann, H.; Simon, P.S.; Kittel, Á.; Buzás, E.; Nagy, G. Increased serum concentration of immune cell derived microparticles in polymyositis/dermatomyositis. Immunol. Lett. 2010, 128, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, T.; Hamaguchi, H.; Higuchi, M.; Kanda, F. Measurement of platelet-derived microparticle levels using an enzyme-linked immunosorbent assay in polymyositis and dermatomyositis patients. Muscle Nerve 2009, 39, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zuo, X.; Liu, D.; Luo, H.; Zhu, H. The Functional Roles of RNAs Cargoes Released by Neutrophil-Derived Exosomes in Dermatomyositis. Front. Pharmacol. 2021, 12, 1656. [Google Scholar] [CrossRef]
- Huard, C.; Gullà, S.V.; Bennett, D.V.; Coyle, A.J.; Vleugels, R.A.; Greenberg, S.A. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon β in dermatomyositis. Br. J. Dermatol. 2017, 176, 1224–1230. [Google Scholar] [CrossRef]
- Wong, D.; Kea, B.; Pesich, R.; Higgs, B.W.; Zhu, W.; Brown, P.; Yao, Y.; Fiorentino, D. Interferon and Biologic Signatures in Dermatomyositis Skin: Specificity and Heterogeneity across Diseases. PLoS ONE 2012, 7, e29161. [Google Scholar] [CrossRef]
- Gürtler, C.; Bowie, A.G. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013, 21, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.A.; Pinkus, J.L.; Pinkus, G.S.; Burleson, T.; Sanoudou, D.; Tawil, R.; Barohn, R.J.; Saperstein, D.S.; Briemberg, H.R.; Ericsson, M.; et al. Interferon-α/β–mediated innate immune mechanisms in dermatomyositis. Ann. Neurol. 2005, 57, 664–678. [Google Scholar] [CrossRef]
- Greenberg, S.A. Dermatomyositis and Type 1 Interferons. Curr. Rheumatol. Rep. 2010, 12, 198–203. [Google Scholar] [CrossRef]
- Salajegheh, M.; Kong, S.W.; Pinkus, J.L.; Walsh, R.J.; Liao, A.; Nazareno, R.; Amato, A.A.; Krastins, B.; Morehouse, C.; Higgs, B.W.; et al. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann. Neurol. 2010, 67, 53–63. [Google Scholar] [CrossRef]
- Assil, S.; Webster, B.; Dreux, M. Regulation of the Host Antiviral State by Intercellular Communications. Viruses 2015, 7, 4707–4733. [Google Scholar] [CrossRef] [PubMed]
- Dreux, M.; Garaigorta, U.; Boyd, B.; Décembre, E.; Chung, J.; Whitten-Bauer, C.; Wieland, S.; Chisari, F. Short-Range Exosomal Transfer of Viral RNA from Infected Cells to Plasmacytoid Dendritic Cells Triggers Innate Immunity. Cell Host Microbe 2012, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Li, Y.; Zeidi, M.; Bashir, M.M.; Werth, V.P.; Liu, M. Extracellular MAVS associates with microvesicles that can actively trigger IFNβ production. J. Investig. Dermatol. 2019, 139, S9. [Google Scholar] [CrossRef]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.G.L.; Moulding, D.; McDonnell, T.C.R.; Orford, M.; Wincup, C.; Ting, J.Y.J.; Otto, G.W.; Restuadi, R.; Kelberman, D.; Papadopoulou, C.; et al. Role of CD14+ monocyte-derived oxidised mitochondrial DNA in the inflammatory interferon type 1 signature in juvenile dermatomyositis. Ann. Rheum. Dis. 2023, 82, 658–669. [Google Scholar] [CrossRef]
- Adapted from “cGAS-STING DNA Detection”, by BioRender.com. 2023. Available online: https://app.biorender.com/biorender-templates (accessed on 10 December 2023).
- Adapted from “Extracellular Vesicles”, by BioRender.com. 2023. Available online: https://app.biorender.com/biorender-templates (accessed on 10 December 2023).
- Dalakas, M.C.; Alexopoulos, H.; Spaeth, P.J. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat. Rev. Neurol. 2020, 16, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Lahoria, R.; Selcen, D.; Engel, A.G. Microvascular alterations and the role of complement in dermatomyositis. Brain 2016, 139, 1891–1903. [Google Scholar] [CrossRef]
- Dalakas, M.C. Complement in autoimmune inflammatory myopathies, the role of myositis-associated antibodies, COVID-19 associations, and muscle amyloid deposits. Expert Rev. Clin. Immunol. 2022, 18, 413–423. [Google Scholar] [CrossRef]
- Buzas, E.I.; Toth, E.A.; Sodar, B.W.; Szabo-Taylor, K.E. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef]
- Pilzer, D.; Gasser, O.; Moskovich, O.; Schifferli, J.A.; Fishelson, Z. Emission of membrane vesicles: Roles in complement resistance, immunity and cancer. Semin. Immunopathol. 2005, 27, 375–387. [Google Scholar] [CrossRef]
- Figarella-Branger, D.; Schleinitz, N.; Boutière-Albanèse, B.; Camoin, L.; Bardin, N.; Guis, S.; Pouget, J.; Cognet, C.; Pellissier, J.; Dignat-George, F. Platelet-endothelial cell adhesion molecule-1 and CD146: Soluble levels and in situ expression of cellular adhesion molecules implicated in the cohesion of endothelial cells in idiopathic inflammatory myopathies. J. Rheumatol. 2006, 33, 1623–1630. [Google Scholar]
- Walsh, R.J.; Kong, S.W.; Yao, Y.; Jallal, B.; Kiener, P.A.; Pinkus, J.L.; Beggs, A.H.; Amato, A.A.; Greenberg, S.A. Type I interferon–inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007, 56, 3784–3792. [Google Scholar] [CrossRef]
- Rigolet, M.; Hou, C.; Baba Amer, Y.; Aouizerate, J.; Periou, B.; Gherardi, R.K.; Lafuste, P.; Authier, F.J. Distinct interferon signatures stratify inflammatory and dysimmune myopathies. RMD Open 2019, 5, e000811. [Google Scholar] [CrossRef]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of Tissue Factor into Developing Thrombi In Vivo Is Dependent upon Microparticle P-Selectin Glycoprotein Ligand 1 and Platelet P-Selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Hong, Y.; Klein, N.J.; Brogan, P.A. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J. Thromb. Haemost. 2011, 9, 1864–1867. [Google Scholar] [CrossRef]
- Kato, Y.; Park, J.; Takamatsu, H.; Konaka, H.; Aoki, W.; Aburaya, S.; Ueda, M.; Nishide, M.; Koyama, S.; Hayama, Y.; et al. Apoptosis-derived membrane vesicles drive the cGAS–STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 2018, 77, 1507–1515. [Google Scholar] [CrossRef]
- Abe, T.; Barber, G.N. Cytosolic-DNA-Mediated, STING-Dependent Proinflammatory Gene Induction Necessitates Canonical NF-κB Activation through TBK1. J. Virol. 2014, 88, 5328–5341. [Google Scholar] [CrossRef] [PubMed]
- Pedrol, E.; Grau, J.M.; Casademont, J.; Cid, M.C.; Nasanes, F.; Fernandez-Sola, J.; Urbano-Marquez, A. Idiopathic inflammatory myopathies. Immunohistochemical analysis of the major histocompatibility complex antigen expression, inflammatory infiltrate phenotype and activation cell markers. Clin. Neuropathol. 1995, 14, 179–184. [Google Scholar]
- Arahata, K.; Engel, A.G. Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann. Neurol. 1984, 16, 193–208. [Google Scholar] [CrossRef]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, 640. [Google Scholar] [CrossRef] [PubMed]
- Holl, E.K.; Roney, K.E.; Allen, I.C.; Steinbach, E.; Arthur, J.C.; Buntzman, A.; Plevy, S.; Frelinger, J.; Ting, J.P. Plexin-B2 and Plexin-D1 in Dendritic Cells: Expression and IL-12/IL-23p40 Production. PLoS ONE 2012, 7, e43333. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Rafael-Vidal, C.; Malvar-Fernandez, B.; Lopes, A.P.; Pego-Reigosa, J.M.; Radstake, T.R.D.J.; Garcia, S. Semaphorin4A-Plexin D1 Axis Induces Th2 and Th17 While Represses Th1 Skewing in an Autocrine Manner. Int. J. Mol. Sci. 2020, 21, 6965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, R.; Geraci, N.; Shrestha, S.; Gordish-Dressman, H.; Pachman, L.M. Duration of chronic inflammation alters gene expression in muscle from untreated girls with juvenile dermatomyositis. BMC Immunol. 2008, 9, 43. [Google Scholar] [CrossRef]
- Mazzotta, C.; Romano, E.; Bruni, C.; Manetti, M.; Lepri, G.; Bellando-Randone, S.; Blagojevic, J.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Guiducci, S. Plexin-D1/Semaphorin 3E pathway may contribute to dysregulation of vascular tone control and defective angiogenesis in systemic sclerosis. Arthritis Res. Ther. 2015, 17, 221. [Google Scholar] [CrossRef]
- Miranda, K.C.; Bond, D.T.; McKee, M.; Skog, J.; Păunescu, T.G.; Da Silva, N.; Brown, D.; Russo, L.M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010, 78, 191–199. [Google Scholar] [CrossRef]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, B.; Huang, Y.; Lan, X.; Liu, W.; Chen, H. Exosomal RNAs: Novel Potential Biomarkers for Diseases—A Review. Int. J. Mol. Sci. 2022, 23, 2461. [Google Scholar] [CrossRef]
- Misunova, M.; Salinas-Riester, G.; Luthin, S.; Pommerenke, C.; Husakova, M.; Zavada, J.; Klein, M.; Plestilova, L.; Svitalkova, T.; Cepek, P.; et al. Microarray analysis of circulating micro RNAs in the serum of patients with polymyositis and dermatomyositis reveals a distinct disease expression profile and is associated with disease activity. Clin. Exp. Rheumatol. 2016, 34, 17–24. [Google Scholar]
- Peng, Q.; Zhang, Y.; Yang, H.; Shu, X.; Lu, X.; Wang, G. Transcriptomic profiling of long non-coding RNAs in dermatomyositis by microarray analysis. Sci. Rep. 2016, 6, 32818. [Google Scholar] [CrossRef]
- Yakubovich, E.I.; Polischouk, A.G.; Evtushenko, V.I. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. Mosc. Suppl. Ser. A 2022, 16, 115–126. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nature 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Li, K.; Wong, D.K.; Luk, F.S.; Kim, R.Y.; Raffai, R.L. Isolation of Plasma Lipoproteins as a Source of Extracellular RNA. In Extracellular RNA; Springer: New York, NY, USA, 2018; Volume 1740, pp. 139–153. [Google Scholar]
- Alinovskaya, L.I.; Sedykh, S.E.; Ivanisenko, N.V.; Soboleva, S.E.; Nevinsky, G.A. How human serum albumin recognizes DNA and RNA. Biol. Chem. 2018, 399, 347–360. [Google Scholar] [CrossRef]
- Vita, G.M.; De Simone, G.; De Marinis, E.; Nervi, C.; Ascenzi, P.; di Masi, A. Serum albumin and nucleic acids biodistribution: From molecular aspects to biotechnological applications. IUBMB Life 2022, 74, 866–879. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat. Protoc. 2023, 18, 1462–1487. [Google Scholar] [CrossRef]
- Noguchi, S.; Tozawa, S.; Sakurai, T.; Ohkuchi, A.; Takahashi, H.; Fujiwara, H.; Takizawa, T. BeWo exomeres are enriched for bioactive extracellular placenta-specific C19MC miRNAs. J. Reprod. Immunol. 2024, 161, 104187. [Google Scholar] [CrossRef]
- Cocozza, F.; Martin-Jaular, L.; Lippens, L.; Di Cicco, A.; Arribas, Y.A.; Ansart, N.; Dingli, F.; Richard, M.; Merle, L.; Jouve San Roman, M.; et al. Extracellular vesicles and co-isolated endogenous retroviruses from murine cancer cells differentially affect dendritic cells. EMBO J. 2023, 42, e113590. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernández-Delgado, I.; Latorre-Pellicer, A.; Acín-Pérez, R.; Martín-Cófreces, N.B.; Jaso-Tamame, Á.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M. Structure of von Willebrand factor and its function in platelet adhesion and thrombus formation. Best Pract. Res. Clin. Haematol. 2001, 14, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.A.; Galbusera, M.; Ruggeri, Z.M. Heterogeneity of plasma von Willebrand factor multimers resulting from proteolysis of the constituent subunit. J. Clin. Investig. 1991, 88, 774–782. [Google Scholar] [CrossRef]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Tanaka, S.; Ceribelli, A.; Calise, S.J.; Chan, E.K.L. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin. Rev. Allerg. Immunol. 2017, 52, 1–19. [Google Scholar] [CrossRef]
- Betteridge, Z.; McHugh, N. Myositis-specific autoantibodies: An important tool to support diagnosis of myositis. J. Intern. Med. 2016, 280, 8–23. [Google Scholar] [CrossRef]
- Friedman, A.W.; Targoff, I.N.; Arnett, F.C. Interstitial lung disease with autoantibodies againstaminoacyl-tRNA synthetases in the absence of clinically apparent myositis. Semin. Arthritis Rheum. 1996, 26, 459–467. [Google Scholar] [CrossRef]
- Clawson, K.; Oddis, C.V. Adult respiratory distress syndrome in polymyositis patients with the anti–jo-1 antibody. Arthritis Rheum. 1995, 38, 1519–1523. [Google Scholar] [CrossRef]
- Sato, S.; Hoshino, K.; Satoh, T.; Fujita, T.; Kawakami, Y.; Fujita, T.; Kuwana, M. RNA helicase encoded by melanoma differentiation–associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 2193–2200. [Google Scholar] [CrossRef]
- Hoshino, K.; Muro, Y.; Sugiura, K.; Tomita, Y.; Nakashima, R.; Mimori, T. Anti-MDA5 and anti-TIF1-γ antibodies have clinical significance for patients with dermatomyositis. Rheumatology 2010, 49, 1726–1733. [Google Scholar] [CrossRef]
- Chen, F.; Wang, D.; Shu, X.; Nakashima, R.; Wang, G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol. Int. 2012, 32, 3909–3915. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wright, H.T.; Janciauskiene, S. A β 1–42, α 1-antichymotrypsin, and their mixture. Cell. Mol. Life Sci. CMLS 2002, 59, 1734. [Google Scholar] [CrossRef]
- Bodmer, J.L.; Schnebli, H.P. Plasma proteinase inhibitors. Schweiz. Med. Wochenschr. 1984, 114, 1359–1363. [Google Scholar]
- Turnier, J.L.; Brunner, H.I.; Bennett, M.; Aleed, A.; Gulati, G.; Haffey, W.D.; Thornton, S.; Wagner, M.; Devarajan, P.; Witte, D.; et al. Discovery of SERPINA3 as a candidate urinary biomarker of lupus nephritis activity. Rheumatology 2019, 58, 321–330. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.; Su, L.; Huang, A. Association of MASP2 levels and MASP2 gene polymorphisms with systemic lupus erythematosus. J. Cell. Mol. Med. 2020, 24, 10432–10443. [Google Scholar] [CrossRef]
- Asanuma, Y.; Nozawa, K.; Matsushita, M.; Kusaoi, M.; Abe, Y.; Yamaji, K.; Tamura, N. Critical role of lectin pathway mediated by MBL-associated serine proteases in complement activation for the pathogenesis in systemic lupus erythematosus. Heliyon 2023, 9, e19072. [Google Scholar] [CrossRef]
- Anyanwu, C.O.; Fiorentino, D.F.; Chung, L.; Dzuong, C.; Wang, Y.; Okawa, J.; Carr, K.; Propert, K.J.; Werth, V.P. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: Characterizing disease severity and assessing responsiveness to clinical change. Br. J. Dermatol. 2015, 173, 969–974. [Google Scholar] [CrossRef]
- Liu, M.; Werth, V.P.; Williams, K.J. Blood plasma versus serum: Which is right for sampling circulating membrane microvesicles in human subjects? Ann. Rheum. Dis. 2020, 79, e73. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef]
| Laboratory Parameter | EV Component | Strength of Correlation (r) | References |
|---|---|---|---|
| CRP | C1QB | +0.648 | Meng et al., 2022 [8] |
| C1QC | +0.682 | ||
| SAA1 | +0.546 | ||
| ESR | C1QB | +0.611 | |
| C1QC | +0.628 | ||
| SERPINA3 | +0.657 | ||
| SAA1 | +0.523 | ||
| Platelet count | C1QB | +0.035 | |
| C1QC | +0.523 | ||
| Plexin D1 | +0.408 | Uto et al., 2022 [7] | |
| Ferritin | VWF | +0.673 | Meng et al., 2022 [8] |
| ANA | ANGPTL6 | +0.693 | |
| VWF | +0.740 | ||
| COLEC11 | +0.668 | ||
| Aldolase | Plexin D1 | +0.481 | Uto et al., 2022 [7] |
| WBCs count | Plexin D1 | +0.381 | |
| Neutrophils count | Plexin D1 | +0.450 | |
| AST | hsa-miR-125a-3p | + | Li et al., 2022 [15] |
| hsa-miR-3614 | + | ||
| ALT | hsa-miR-125a-3p | + | |
| LDH | hsa-miR-125a-3p | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricco, C.; Eldaboush, A.; Liu, M.-L.; Werth, V.P. Extracellular Vesicles in the Pathogenesis, Clinical Characterization, and Management of Dermatomyositis: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1967. https://doi.org/10.3390/ijms25041967
Ricco C, Eldaboush A, Liu M-L, Werth VP. Extracellular Vesicles in the Pathogenesis, Clinical Characterization, and Management of Dermatomyositis: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(4):1967. https://doi.org/10.3390/ijms25041967
Chicago/Turabian StyleRicco, Cristina, Ahmed Eldaboush, Ming-Lin Liu, and Victoria P. Werth. 2024. "Extracellular Vesicles in the Pathogenesis, Clinical Characterization, and Management of Dermatomyositis: A Narrative Review" International Journal of Molecular Sciences 25, no. 4: 1967. https://doi.org/10.3390/ijms25041967





