Neuropharmacological Evidence Implicating Drug-Induced Glutamate Receptor Dysfunction in Affective and Cognitive Sequelae of Subchronic Methamphetamine Self-Administration in Mice
Abstract
:1. Introduction
2. Results
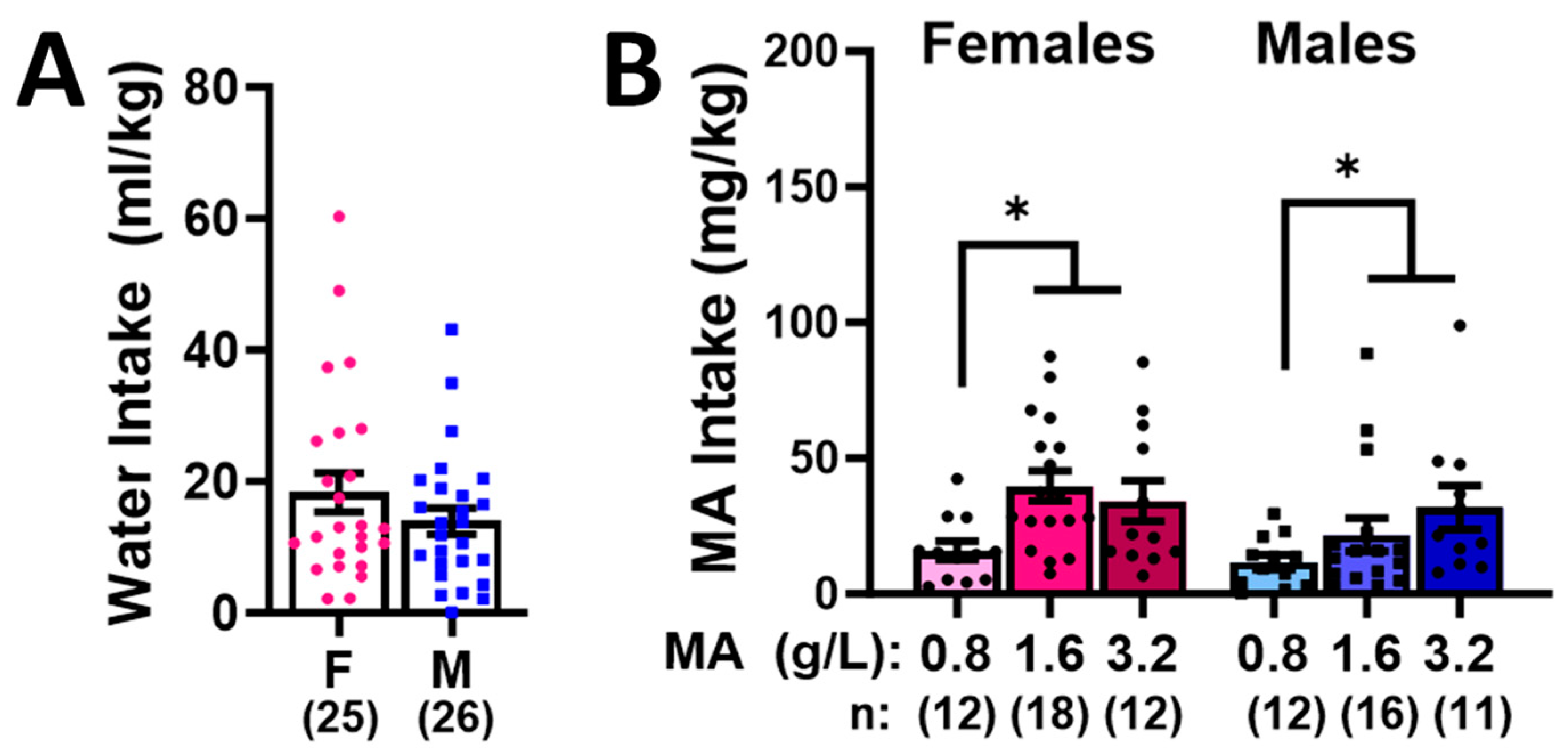
2.1. Sex Differences in High-Concentration Oral MA Intake
2.2. Behavioral Test Battery
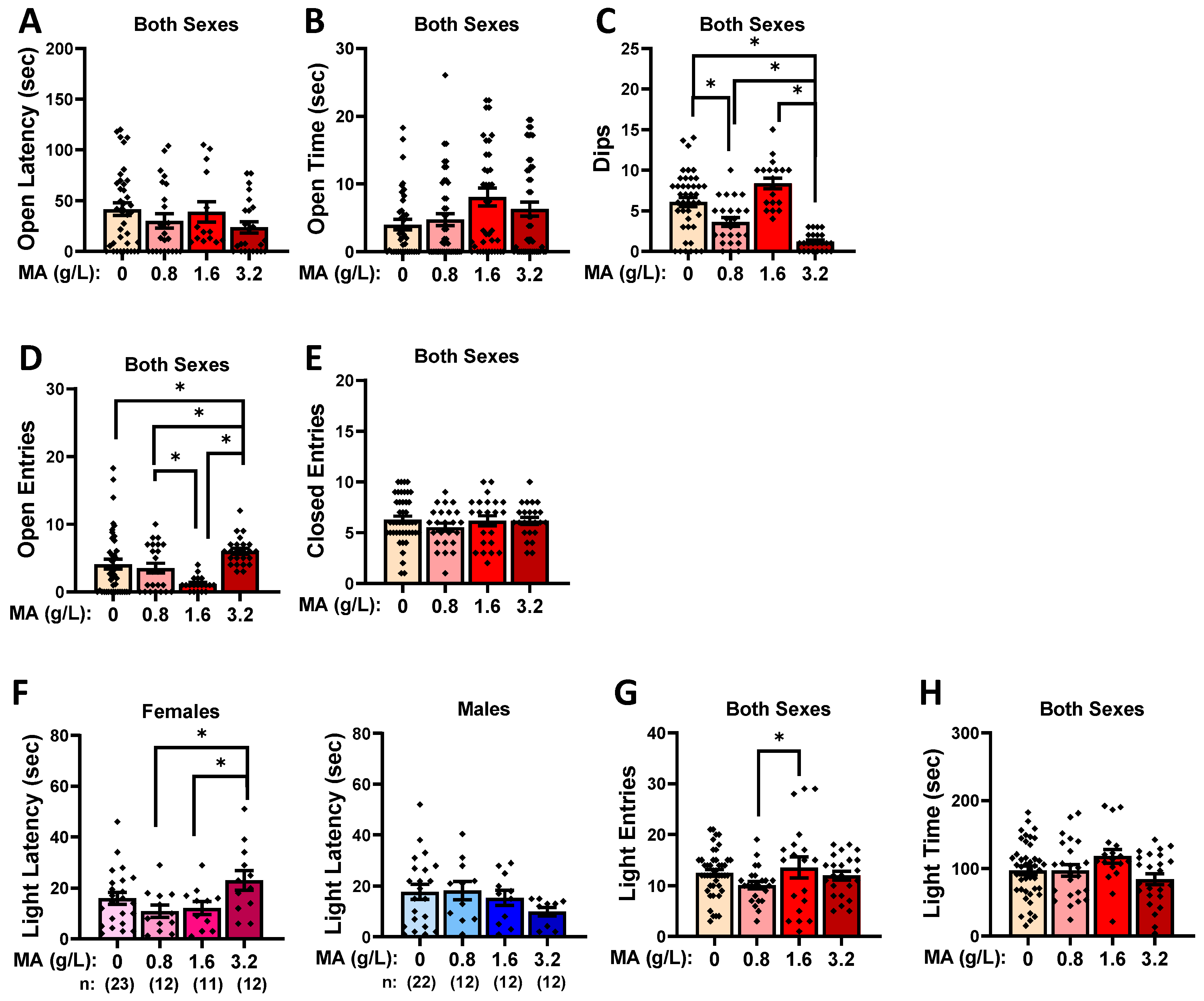
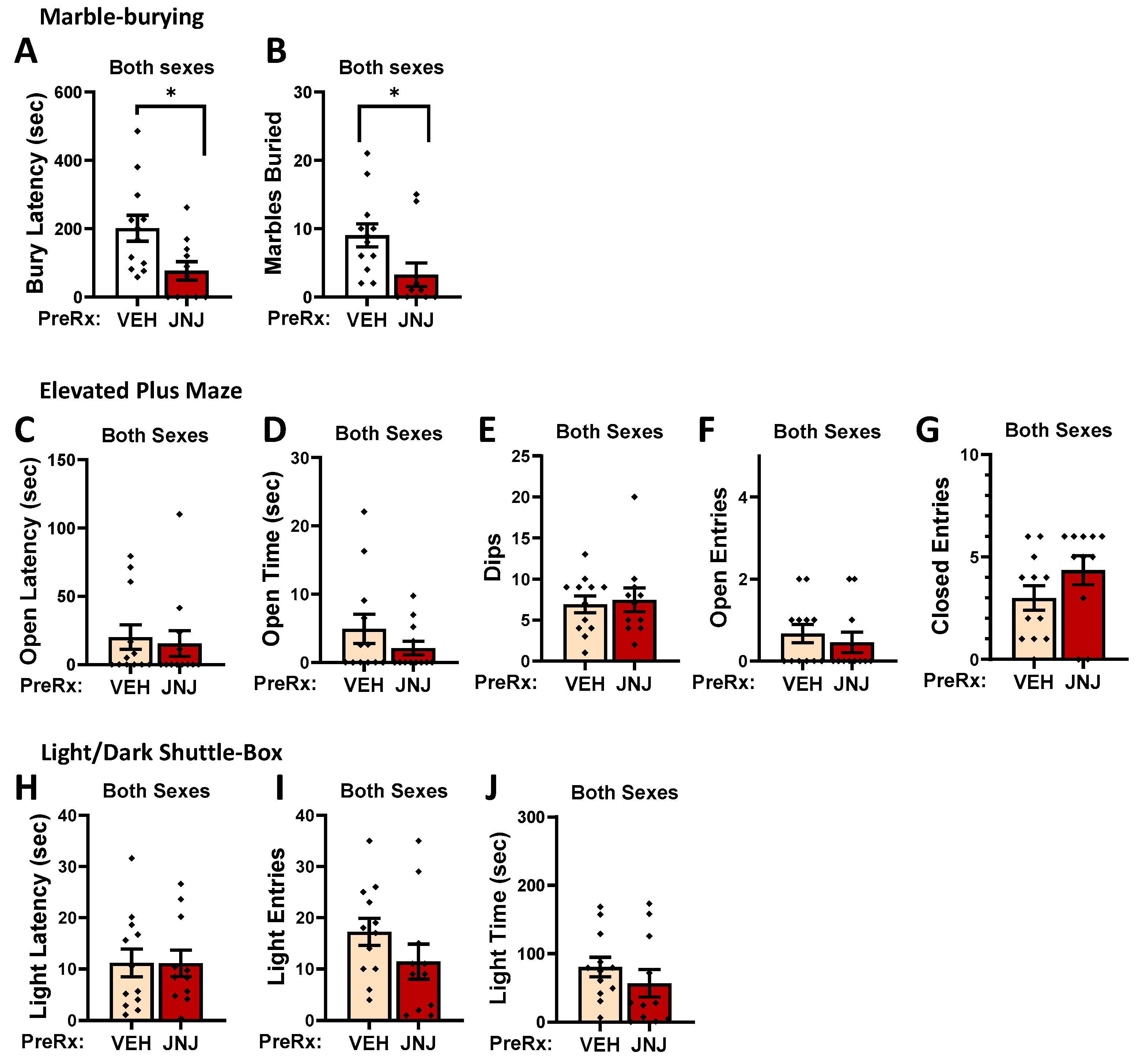
2.2.1. Marble Burying
2.2.2. Novel-Object Reactivity
2.2.3. Elevated Plus Maze
2.2.4. Light–Dark Shuttle Box
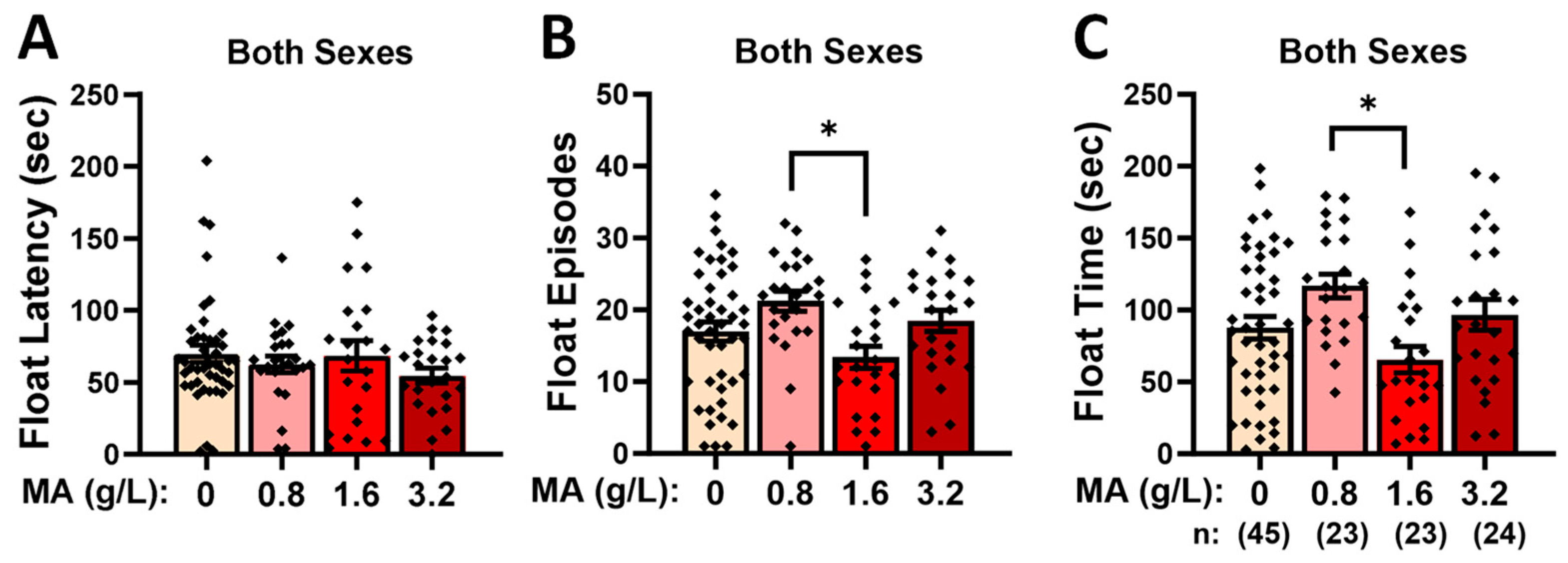
2.2.5. Forced-Swim Test
2.2.6. Acoustic Startle and Prepulse Inhibition
2.3. Morris Water Maze
2.3.1. Flag Test
2.3.2. Acquisition
2.3.3. Probe Test
2.3.4. Reversal Learning
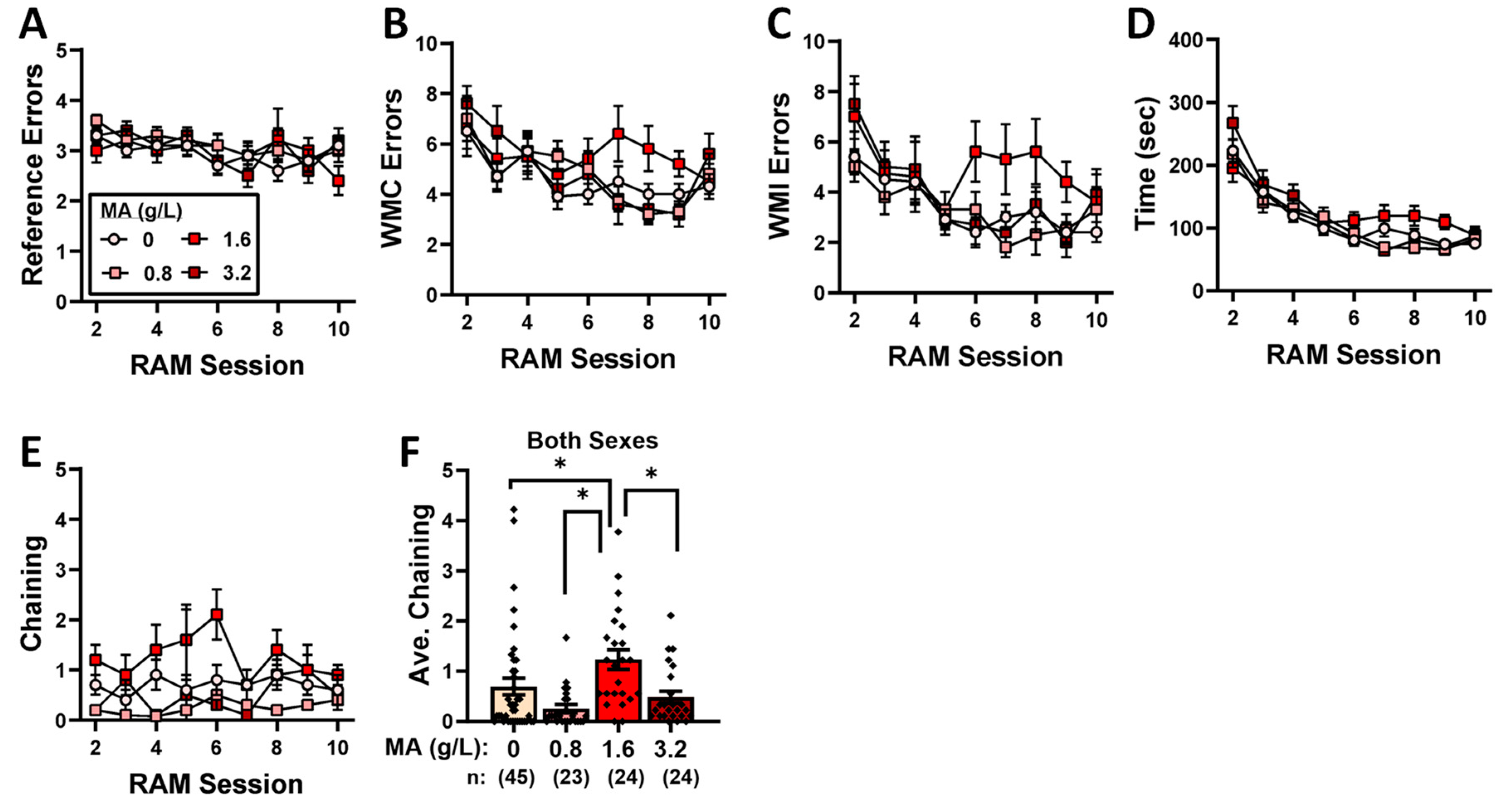
2.4. Radial-Arm Maze
2.5. Immunoblotting
2.5.1. Glutamate Receptor-Related Proteins in the vPFC
2.5.2. Downstream Effectors in the vPFC
2.5.3. Glutamate Receptor-Related Proteins in the dPFC
2.5.4. Downstream Effectors in the dPFC
2.5.5. Glutamate Receptor-Related Proteins in the Hippocampus
2.5.6. Downstream Effectors in the Hippocampus
2.5.7. Glutamate-Related Proteins within the Amygdala
2.5.8. Downstream Effectors in Amygdala
2.6. Behavioral Pharmacological Studies
2.6.1. Effects of Systemic Inhibition of mGlu1 on Measures of Negative Affect
2.6.2. Effects of NMDA Receptor Manipulation on Reversal Learning in Morris Water Maze
3. Discussion
3.1. B6J Mice Consume Very Large Amounts of MA under Limited-Access Operant-Conditioning Procedures
3.2. A Brief History of Binge-Like MA Intake Produces Some Signs of Negative Affect in Early Withdrawal
3.3. Withdrawal from a Brief History of Oral MA Induces a Spatial Reversal-Learning Impairment in Female Mice
3.4. Withdrawal from a Brief History of Oral MA Induces Several Sex-Selective Changes in Protein Expression within Corticolimbic Structures
3.5. Behavioral Relevance of MA-Induced Changes in mGlu1 and NMDA Receptor Expression
3.6. Conclusions
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. Operant Conditioning for Oral MA Reinforcement
4.4. Behaivoral Test Battery
4.4.1. Light–Dark Shuttle Box
4.4.2. Novel-Object Reactivity
4.4.3. Elevated Plus Maze
4.4.4. Marble Burying
4.4.5. Acoustic Startle and PPI
4.4.6. Porsolt Forced-Swim Test
4.5. Morris Water Maze
4.6. Radial Water Maze
4.7. Immunoblotting
4.8. Behavioral Pharmacological Studies
4.8.1. NMDA Receptor Function and Reversal Learning
4.8.2. Group 1 mGlu Receptor Function and Negative Affect
4.9. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNODC. World Drug Report 2023 (United Nations Publication, 2023). WHO World Drug Report 2023. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr-2023_Special_Points.html (accessed on 17 August 2023).
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry 2021, 78, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.; Srisurapanont, M.; Jittiwutikarn, J.; Laobhripatr, S.; Wongtan, T.; White, J.M. The nature, time course and severity of methamphetamine withdrawal. Addiction 2005, 100, 1320–1329. [Google Scholar] [CrossRef]
- Newton, T.F.; Kalechstein, A.D.; Duran, S.; Vansluis, N.; Ling, W. Methamphetamine abstinence syndrome: Preliminary findings. Am. J. Addict. 2004, 13, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.C.; Groman, S.M.; Morales, A.M.; London, E.D. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 2013, 38, 259–274. [Google Scholar] [CrossRef]
- Scott, J.C.; Woods, S.P.; Matt, G.E.; Meyer, R.A.; Heaton, R.K.; Atkinson, J.H.; Grant, I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol. Rev. 2007, 17, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.L.; Marvin, C.B.; Silver, R.; Smith, E.E. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology 2012, 37, 586–608. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Dean, A.C.; Cordova, X.; Monterosso, J.R.; London, E.D. Methamphetamine dependence and neuropsychological functioning: Evaluating change during early abstinence. J. Stud. Alcohol Drugs 2010, 71, 335–344. [Google Scholar] [CrossRef]
- Woods, S.P.; Rippeth, J.D.; Conover, E.; Gongvatana, A.; Gonzalez, R.; Carey, C.L.; Cherner, M.; Heaton, R.K.; Grant, I. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology 2005, 19, 35–43. [Google Scholar] [CrossRef]
- Kalechstein, A.D.; De La Garza, R., II.; Newton, T.F. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am. J. Addict. 2010, 19, 340–344. [Google Scholar] [CrossRef]
- Becker, J.B.; Hu, M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008, 29, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Dluzen, D.E.; Liu, B. Gender differences in methamphetamine use and responses: A review. Gend. Med. 2008, 5, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hser, Y.I.; Evans, E.; Huang, Y.C. Treatment outcomes among women and men methamphetamine abusers in California. J. Subst. Abus. Treat. 2005, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ikeda, N.; Kudo, K.; Ishida, T.; Terada, M.; Matoba, R. Methamphetamine-related sudden death with a concentration which was of a ‘toxic level’. Leg. Med. 2006, 8, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Schep, L.J.; Slaughter, R.J.; Beasley, D.M. The clinical toxicology of metamfetamine. Clin. Toxicol. 2010, 48, 675–694. [Google Scholar] [CrossRef] [PubMed]
- Honeywell, K.M.; Van Doren, E.; Szumlinski, K.K. Selective Inhibition of PDE4B Reduces Methamphetamine Reinforcement in Two C57BL/6 Substrains. Int. J. Mol. Sci. 2022, 23, 4872. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.T.; Yazdani, N.; Blum, B.C.; Beierle, J.A.; Lin, W.; Coelho, M.A.; Fultz, E.K.; Healy, A.F.; Shahin, J.R.; Kandola, A.K.; et al. A Mutation in Hnrnph1 That Decreases Methamphetamine-Induced Reinforcement, Reward, and Dopamine Release and Increases Synaptosomal hnRNP H and Mitochondrial Proteins. J. Neurosci. 2020, 40, 107–130. [Google Scholar] [CrossRef]
- Shabani, S.; Schmidt, B.; Ghimire, B.; Houlton, S.K.; Hellmuth, L.; Mojica, E.; Phillips, T.J. Depression-like symptoms of withdrawal in a genetic mouse model of binge methamphetamine intake. Genes Brain Behav. 2019, 18, e12533. [Google Scholar] [CrossRef]
- Shabani, S.; Houlton, S.K.; Hellmuth, L.; Mojica, E.; Mootz, J.R.; Zhu, Z.; Reed, C.; Phillips, T.J. A Mouse Model for Binge-Level Methamphetamine Use. Front. Neurosci. 2016, 10, 493. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Reed, C.; Burkhart-Kasch, S.; Li, N.; Cunningham, C.L.; Janowsky, A.; Franken, F.H.; Wiren, K.M.; Hashimoto, J.G.; Scibelli, A.C.; et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009, 8, 758–771. [Google Scholar] [CrossRef]
- Szumlinski, K.K.; Lominac, K.D.; Campbell, R.R.; Cohen, M.; Fultz, E.K.; Brown, C.N.; Miller, B.W.; Quadir, S.G.; Martin, D.; Thompson, A.B.; et al. Methamphetamine addiction vulnerability: The glutamate, the bad and the ugly. Biol. Psychiatry 2017, 81, 959–970. [Google Scholar] [CrossRef]
- Fultz, E.K.; Quadir, S.G.; Martin, D.; Flaherty, D.M.; Worley, P.F.; Kippin, T.E.; Szumlinski, K.K. ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse. Int. J. Mol. Sci. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Harmony, Z.R.; Alderson, E.M.; Garcia-Carachure, I.; Bituin, L.D.; Crawford, C.A. Effects of nicotine exposure on oral methamphetamine self-administration, extinction, and drug-primed reinstatement in adolescent male and female rats. Drug Alcohol Depend. 2020, 209, 107927. [Google Scholar] [CrossRef]
- Yates, J.R.; Berling, K.L.; Broderick, M.R.; Bako, R.E.; Dillon, S.L. Rats have low motivation to self-administer oral methamphetamine across increasing response requirements. Behav. Brain Res. 2023, 455, 114673. [Google Scholar] [CrossRef]
- Cho, A.K.; Melega, W.P.; Kuczenski, R.; Segal, D.S. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse 2001, 39, 161–166. [Google Scholar] [CrossRef]
- Riviére, G.J.; Gentry, W.B.; Owens, S.M. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. J. Pharmacol. Exp. Ther. 2000, 292, 1042–1047. [Google Scholar]
- Simon, S.L.; Richardson, K.; Dacey, J.; Glynn, S.; Domier, C.P.; Rawson, R.A.; Ling, W. A comparison of patterns of methamphetamine and cocaine use. J. Addict. Dis. 2002, 21, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.; Huber, A.; Brethen, P.; Obert, J.; Gulati, V.; Shoptaw, S.; Ling, W. Methamphetamine and cocaine users: Differences in characteristics and treatment retention. J. Psychoact. Drugs 2000, 32, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Coelho, M.A.; McGregor, H.A.; Solton, N.R.; Cohen, M.; Szumlinski, K.K. Adolescent Mice Are Resilient to Alcohol Withdrawal-Induced Anxiety and Changes in Indices of Glutamate Function within the Nucleus Accumbens. Front. Cell Neurosci. 2016, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Coehlo, M.A.; Solton, N.R.; Szumlinski, K.K. Negative Affect and Excessive Alcohol Intake Incubate during Protracted Withdrawal from Binge-Drinking in Adolescent, But Not Adult, Mice. Front. Psychol. 2017, 8, 1128. [Google Scholar] [CrossRef] [PubMed]
- Szumlinski, K.K.; Coelho, M.A.; Lee, K.M.; Tran, T.; Sern, K.R.; Bernal, A.; Kippin, T.E. DID it or DIDn’t it? Exploration of a failure to replicate binge-like alcohol-drinking in C57BL/6J mice. Pharmacol. Biochem. Behav. 2019, 178, 3–18. [Google Scholar] [CrossRef]
- Lee, K.M.; Coehlo, M.; McGregor, H.A.; Waltermire, R.S.; Szumlinski, K.K. Binge alcohol drinking elicits persistent negative affect in mice. Behav. Brain Res. 2015, 291, 385–398. [Google Scholar] [CrossRef]
- Ohmori, T.; Abekawa, T.; Koyama, T. The role of glutamate in behavioral and neurotoxic effects of methamphetamine. Neurochem. Int. 1996, 29, 301–307. [Google Scholar] [CrossRef]
- Hámor, P.U.; Knackstedt, L.A.; Schwendt, M. The role of metabotropic glutamate receptors in neurobehavioral effects associated with methamphetamine use. Int. Rev. Neurobiol. 2023, 168, 177–219. [Google Scholar] [CrossRef]
- Tata, D.A.; Yamamoto, B.K. Interactions between methamphetamine and environmental stress: Role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction 2007, 102 (Suppl. 1), 49–60. [Google Scholar] [CrossRef]
- Murray, C.H.; Loweth, J.A.; Milovanovic, M.; Stefanik, M.T.; Caccamise, A.J.; Dolubizno, H.; Funke, J.R.; Olive, M.F.; Wolf, M.E. AMPA receptor and metabotropic glutamate receptor 1 adaptations in the nucleus accumbens core during incubation of methamphetamine craving. Neuropsychopharmacology 2019, 44, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Lominac, K.D.; Sacramento, A.D.; Szumlinski, K.K.; Kippin, T.E. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 2012, 37, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Parsegian, A.; See, R.E. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology 2014, 39, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lominac, K.D.; Quadir, S.G.; Barrett, H.M.; McKenna, C.L.; Schwartz, L.M.; Ruiz, P.N.; Wroten, M.G.; Campbell, R.R.; Miller, B.W.; Holloway, J.J.; et al. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur. J. Neurosci. 2016, 43, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Schwendt, M.; Reichel, C.M.; See, R.E. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS ONE 2010, 7, e34299. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, A.F.; Loweth, J.A.; Christian, D.T.; Uejima, J.; Rabei, R.; Le, T.; Dolubizno, H.; Stefanik, M.T.; Murray, C.H.; Sakas, C.; et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol. Psychiatry 2016, 80, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.H.; Christian, D.T.; Milovanovic, M.; Loweth, J.A.; Hwang, E.K.; Caccamise, A.J.; Funke, J.R.; Wolf, M.E. mGlu5 function in the nucleus accumbens core during the incubation of methamphetamine craving. Neuropharmacology 2021, 186, 108452. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fibuch, E.E.; Mao, L. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007, 100, 1–11. [Google Scholar] [CrossRef]
- Haddad, J.J. N-methyl-D-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: A revolving neurochemical axis for therapeutic intervention? Prog. Neurobiol. 2005, 77, 252–282. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.M.; Bodepudi, A.; Chu, X.P.; Wang, J.Q. Group I Metabotropic Glutamate Receptors and Interacting Partners: An Update. Int. J. Mol. Sci. 2022, 23, 840. [Google Scholar] [CrossRef]
- Bayer, K.U.; Schulman, H. CaM Kinase: Still Inspiring at 40. Neuron 2019, 103, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Hell, J.W. Binding of CaMKII to the NMDA receptor is sufficient for long-term potentiation. Sci. Signal. 2023, 16, 9224. [Google Scholar] [CrossRef]
- Reichel, C.M.; Chan, C.H.; Ghee, S.M.; See, R.E. Sex differences in escalation of methamphetamine self-administration: Cognitive and motivational consequences in rats. Psychopharmacology 2012, 223, 371–380. [Google Scholar] [CrossRef]
- Cox, B.M.; Young, A.B.; See, R.E.; Reichel, C.M. Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 2013, 38, 2343–2353. [Google Scholar] [CrossRef]
- Roth, M.E.; Carroll, M.E. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology 2004, 172, 443–449. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Ladenheim, B.; McCoy, M.T.; Brannock, C.; Schroeder, J.; Cadet, J.L. Sex Differences in Escalated Methamphetamine Self-Administration and Altered Gene Expression Associated With Incubation of Methamphetamine Seeking. Int. J. Neuropsychopharmacol. 2019, 22, 710–723. [Google Scholar] [CrossRef]
- Funke, J.R.; Hwang, E.K.; Wunsch, A.M.; Baker, R.; Engeln, K.A.; Murray, C.H.; Milovanovic, M.; Caccamise, A.J.; Wolf, M.E. Persistent Neuroadaptations in the Nucleus Accumbens Core Accompany Incubation of Methamphetamine Craving in Male and Female Rats. eNeuro 2023, 10, ENEURO.0480-22.2023. [Google Scholar] [CrossRef]
- Jones, E.S.; Rayner, B.L. Hypertension, end-stage renal disease and mesangiocapillary glomerulonephritis in methamphetamine users. S. Afr. Med. J. 2015, 105, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.S.; Garfein, R.S.; Semple, S.J.; Strathidee, S.A.; Zians, J.K.; Patterson, T.L. Binge use and sex and drug use behaviors among HIV(−), heterosexual methamphetamine users in San Diego. Subst. Use Misuse 2010, 45, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Mancino, M.J.; Gentry, B.W.; Feldman, Z.; Mendelson, J.; Oliveto, A. Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: A pilot field study. Am. J. Drug Alcohol Abuse 2011, 37, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Zorick, T.; Nestor, L.; Miotto, K.; Sugar, C.; Hellemann, G.; Scanlon, G.; Rawson, R.; London, E.D. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 2010, 105, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Dang, D.K.; Tran, T.V.; Tran, H.Q.; Jeong, J.H.; Nah, S.Y.; Jang, C.G.; Yamada, K.; Nabeshima, T.; Kim, H.C. Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors. Arch. Pharm. Res. 2017, 40, 403–428. [Google Scholar] [CrossRef]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Cryan, J.F.; Hoyer, D.; Markou, A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol. Psychiatry 2003, 54, 49–58. [Google Scholar] [CrossRef]
- Jang, C.G.; Whitfield, T.; Schulteis, G.; Koob, G.F.; Wee, S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology 2013, 225, 753–763. [Google Scholar] [CrossRef]
- Guerin, A.A.; Bonomo, Y.; Lawrence, A.J.; Baune, B.T.; Nestler, E.J.; Rossell, S.L.; Kim, J.H. Cognition and Related Neural Findings on Methamphetamine Use Disorder: Insights and Treatment Implications From Schizophrenia Research. Front. Psychiatry 2019, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, A.; See, R.E.; Reichel, C.M. Chronic methamphetamine self-administration disrupts cortical control of cognition. Neurosci. Biobehav. Rev. 2016, 69, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Takuma, K.; Dohniwa, M.; Ibi, D.; Mizoguchi, H.; Kamei, H.; Nabeshima, T.; Yamada, K. Repeated methamphetamine treatment impairs spatial working memory in rats: Reversal by clozapine but not haloperidol. Psychopharmacology 2007, 194, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Camarasa, J.; Rodrigo, T.; Pubill, D.; Escubedo, E. Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol. Res. 2010, 62, 450–456. [Google Scholar] [CrossRef]
- Belcher, A.M.; Feinstein, E.M.; O’Dell, S.J.; Marshall, J.F. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharmacology 2007, 33, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Schutová, B.; Hrubá, L.; Pometlová, M.; Deykun, K.; Slamberová, R. Impact of methamphetamine administered prenatally and in adulthood on cognitive functions of male rats tested in Morris water maze. Prague Med. Rep. 2008, 109, 62–70. [Google Scholar]
- Etaee, F.; Rezvani-Kamran, A.; Komaki, S.; Asadbegi, M.; Faraji, N.; Raoufi, S.; Taheri, M.; Kourosh-Arami, M.; Komaki, A. Effects of Buprenorphine on the Memory and Learning Deficit Induced by Methamphetamine Administration in Male Rats. Front. Behav. Neurosci. 2021, 15, 748563. [Google Scholar] [CrossRef]
- Ghazvini, H.; Khaksari, M.; Esmaeilpour, K.; Shabani, M.; Asadi-Shekaari, M.; Khodamoradi, M.; Sheibani, V. Effects of treatment with estrogen and progesterone on the methamphetamine-induced cognitive impairment in ovariectomized rats. Neurosci. Lett. 2016, 619, 60–67. [Google Scholar] [CrossRef]
- Cheng, R.-K.; Etchegaray, M.; Meck, W.H. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007, 1186, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Belcher, A.M.; Scott, L.; Cazares, V.A.; Chen, J.; O’Dell, S.J.; Malvaez, M.; Wu, T.; Marshall, J.F. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: Possible involvement of striatal dopamine. Neuropsychopharmacology 2010, 35, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Kosheleff, A.R.; Rodriguez, D.; O’Dell, S.J.; Marshall, J.F.; Izquierdo, A. Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology 2012, 224, 459–467. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Minamoto, T.; Odell, J.R.; Mayhorn, J.; White, W. Brief exposure to methamphetamine (METH) and phencyclidine (PCP) during late development leads to long-term learning deficits in rats. Brain Res. 2009, 1266, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, L.; Khaksari, M.; Rafaiee, R.; Kalalian Moghaddam, H. Neuroprotective Effects of Berberine Hydrochloride on Methamphetamine-induced Cognitive Dysfunction: Immunohistochemical and Behavioral Studies in Rats. Basic Clin. Neurosci. 2022, 13, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Schweppe, C.A.; Burzynski, C.; Jayanthi, S.; Ladenheim, B.; Cadet, J.L.; Gardner, E.L.; Xi, Z.X.; van Praag, H.; Newman, A.H.; Keck, T.M. Neurochemical and behavioral comparisons of contingent and non-contingent methamphetamine exposure following binge or yoked long-access self-administration paradigms. Psychopharmacology 2020, 237, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M.; Cope, Z.A.; Parsegian, A.; Floresco, S.B.; Aston-Jones, G.; See, R.E. Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology 2016, 233, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Recinto, P.; Samant, A.R.; Chavez, G.; Kim, A.; Yuan, C.J.; Soleiman, M.; Grant, Y.; Edwards, S.; Wee, S.; Koob, G.F.; et al. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 2012, 37, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Persons, A.L.; Desai Bradaric, B.; Kelly, L.P.; Kousik, S.M.; Graves, S.M.; Yamamoto, B.K.; Napier, T.C. Gut and brain profiles that resemble pre-motor and early-stage Parkinson’s disease in methamphetamine self-administering rats. Drug Alcohol Depend. 2021, 225, 108746. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Kim, R.; Delis, F.; Ananth, M.; Chachati, G.; Rocco, M.J.; Masad, I.; Muniz, J.A.; Grant, S.C.; Gold, M.S.; et al. Chronic Methamphetamine Effects on Brain Structure and Function in Rats. PLoS ONE 2016, 8, e0155457. [Google Scholar] [CrossRef]
- Moenk, M.D.; Matuszewich, L. Juvenile but not adult methamphetamine exposure improves performance in the Morris Water Maze in male rats. Int. J. Dev. Neurosci. 2012, 30, 325–331. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Reed, T.M.; Morford, L.L.; Fukumura, M.; Wood, S.L.; Brown, C.A.; Skelton, M.R.; McCrea, A.E.; Rock, S.L.; Williams, M.T. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60). Neurotoxicol. Teratol. 2005, 27, 117–134. [Google Scholar] [CrossRef]
- Liu, B.; Dluzen, D. New research on methamphetamine abuse (gender differences in methamphetamine effects: Review of animal and human studies). In Drug and Alcohol Abuse Research Focus; Walcott, T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 1–24. [Google Scholar]
- Bourque, M.; Liu, B.; Dluzen, D.E.; Di Paolo, T. Sex differences in methamphetamine toxicity in mice: Effect on brain dopamine signaling pathways. Psychoneuroendocrinology 2011, 36, 955–969. [Google Scholar] [CrossRef]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Sex and temporally-dependent effects of methamphetamine toxicity on dopamine markers and signaling pathways. Neuropharmacology 2012, 62, 2363–2372. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Cadet, J.L. Sex differences in methamphetamine use disorder perused from pre-clinical and clinical studies: Potential therapeutic impacts. Neurosci. Biobehav. Rev. 2022, 137, 104674. [Google Scholar] [CrossRef]
- Pena-Bravo, J.I.; Penrod, R.; Reichel, C.M.; Lavin, A. Methamphetamine Self-Administration Elicits Sex-Related Changes in Postsynaptic Glutamate Transmission in the Prefrontal Cortex. eNeuro 2019, 28, ENEURO.0401-18.2018. [Google Scholar] [CrossRef]
- Mishra, D.; Pena-Bravo, J.I.; Leong, K.C.; Lavin, A.; Reichel, C.M. Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct. Funct. 2017, 222, 2031–2039. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Jayanthi, S.; Cadet, J.L. Sex- and brain region-specific changes in gene expression in male and female rats as consequences of methamphetamine self-administration and abstinence. Neuroscience 2021, 452, 265–279. [Google Scholar] [CrossRef]
- Johansen, A.; McFadden, L.M. The neurochemical consequences of methamphetamine self-administration in male and female rats. Drug Alcohol Depend. 2017, 178, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Jacobskind, J.S.; Rosinger, Z.J.; Gonzalez, T.; Zuloaga, K.L.; Zuloaga, D.G. Chronic methamphetamine exposure attenuates neural activation in hypothalamic-pituitary-adrenal axis-associated brain regions in a sex-specific manner. Neuroscience 2018, 380, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Klambatsen, A.; Nygard, S.K.; Chang, A.J.; Quinones, V.; Jenab, S. Sex differences in memory and intracellular signaling after methamphetamine binge treatment. Brain Res. 2019, 1711, 16–22. [Google Scholar] [CrossRef]
- Pittenger, S.T.; Chou, S.; Murawski, N.J.; Barrett, S.T.; Loh, O.; Duque, J.F.; Li, M.; Bevins, R.A. Female rats display higher methamphetamine-primed reinstatement and c-Fos immunoreactivity than male rats. Pharm. Biochem. Behav. 2021, 201, 173089. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Conn, P.J. Targeting metabotropic glutamate receptors for the treatment of depression and other stress-related disorders. Neuropharmacology 2021, 15, 108687. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska, J.; Bochenski, M. The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur. J. Pharmacol. 2008, 598, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Southcott, S.; Sacco, C.; Wagner, A.D.; Ghose, S. Glutamate dysfunction in hippocampus: Relevance of dentate gyrus and CA3 signaling. Schizophr. Bull. 2012, 38, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Kubo, T.; Shibanoki, S.; Matsumoto, A.; Hata, H.; Asai, S. Hippocampal degeneration inducing impairment of learning in rats: Model of dementia? Behav. Brain Res. 1997, 83, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Brigman, J.L.; Radke, A.K.; Rudebeck, P.H.; Holmes, A. The neural basis of reversal learning: An updated perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Clark, L.; Owen, A.M.; Robbins, T.W. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 2002, 22, 4563–4567. [Google Scholar] [CrossRef] [PubMed]
- Hlinák, Z.; Krejcí, I. N-methyl-D-aspartate prevented memory deficits induced by MK-801 in mice. Physiol. Res. 2003, 52, 809–812. [Google Scholar] [CrossRef]
- Heale, V.; Harley, C. MK-801 and AP5 impair acquisition, but not retention, of the Morris milk maze. Pharmacol. Biochem. Behav. 1990, 36, 145–149. [Google Scholar] [CrossRef]
- McLamb, R.L.; Williams, L.R.; Nanry, K.P.; Wilson, W.A.; Tilson, H.A. MK-801 impedes the acquisition of a spatial memory task in rats. Pharmacol. Biochem. Behav. 1990, 37, 41–45. [Google Scholar] [CrossRef]
- Filliat, P.; Blanchet, G. Effects of TCP on spatial memory: Comparison with MK-801. Pharmacol. Biochem. Behav. 1995, 51, 429–434. [Google Scholar] [CrossRef]
- Ylinen, A.; Pitkänen, M.; Sirviö, J.; Hartikainen, T.; Sivenius, J.; Koivisto, E.; Riekkinen, P.J., Sr. The effects of NMDA receptor antagonists at anticonvulsive doses on the performance of rats in the water maze task. Eur. J. Pharmacol. 1995, 274, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ahlander, M.; Misane, I.; Schött, P.A.; Ogren, S.O. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK-801 (dizocilpine) in the rat. Neuropsychopharmacology 1999, 21, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Farina, F.R.; Commins, S. Hippocampal and prefrontal contributions to memory retrieval: Examination of immediate early gene, NMDA receptor and environmental interactions. Eur. J. Neurosci. 2020, 52, 2982–2994. [Google Scholar] [CrossRef]
- Steckler, T.; Lavreysen, H.; Oliveira, A.M.; Aerts, N.; Van Craenendonck, H.; Prickaerts, J.; Megens, A.; Lesage, A.S. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology 2005, 179, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, M.; Markou, A.; Kuczenski, R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav. Brain Res. 2011, 217, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Okuda, H.; Iwabuchi, K.; Sakurai, E.; Chen, Z.; Kato, M.; Iinuma, K.; Yanai, K. Social isolation stress significantly enhanced the disruption of prepulse inhibition in mice repeatedly treated with methamphetamine. Ann. N. Y. Acad. Sci. 2004, 1025, 257–266. [Google Scholar] [CrossRef]
- Crawley, J.N. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985, 9, 37–44. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Grandy, D.K.; Low, M.J.; Paulus, M.P.; Geyer, M.A. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999, 19, 9550–9556. [Google Scholar] [CrossRef] [PubMed]
- Sakić, B.; Szechtman, H.; Talangbayan, H.; Denburg, S.D.; Carbotte, R.M.; Denburg, J.A. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol. Behav. 1994, 56, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Njung’e, K.; Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Broekkamp, C.L.; Rijk, H.W.; Joly-Gelouin, D.; Lloyd, K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986, 126, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, Y.; Egawa, T.; Sawa, A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn. J. Pharmacol. 1995, 68, 65–70. [Google Scholar] [CrossRef]
- Borsini, F.; Podhorna, J.; Marazziti, D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology 2002, 163, 121–141. [Google Scholar] [CrossRef]
- Nicolas, L.B.; Kolb, Y.; Prinssen, E.P. A combined marble burying-locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol. 2006, 547, 106–115. [Google Scholar] [CrossRef]
- Li, X.; Morrow, D.; Witkin, J.M. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: Comparison with marble burying. Life Sci. 2006, 78, 1933–1939. [Google Scholar] [CrossRef]
- Njung’e, K.; Handley, S.L. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br. J. Pharmacol. 1991, 104, 105–112. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yatsugi, S.; Yamaguchi, T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn. J. Pharmacol. 2002, 90, 197–200. [Google Scholar] [CrossRef]
- de Brouwer, G.; Fick, A.; Harvey, B.H.; Wolmarans, W. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn. Affect. Behav. Neurosci. 2019, 19, 1–39. [Google Scholar] [CrossRef]
- Lee, K.M.; Coelho, M.A.; Sern, K.R.; Class, M.A.; Bocz, M.D.; Szumlinski, K.K. Anxiolytic effects of buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress 2017, 1, 2470547017712985. [Google Scholar] [CrossRef]
- Lominac, K.D.; Oleson, E.B.; Pava, M.; Klugmann, M.; Schwarz, M.K.; Seeburg, P.H.; During, M.J.; Worley, P.F.; Kalivas, P.W.; Szumlinski, K.K. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J. Neurosci. 2005, 25, 1586–1594. [Google Scholar] [CrossRef]
- Datko, M.C.; Hu, J.H.; Williams, M.; Reyes, C.M.; Lominac, K.D.; von Jonquieres, G.; Klugmann, M.; Worley, P.F.; Szumlinski, K.K. Behavioral and Neurochemical Phenotyping of Mice Incapable of Homer1a Induction. Front. Behav. Neurosci. 2017, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin Jalfre, A.M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Jimenez Chavez, C.L.; Van Doren, E.; Matalon, J.; Ogele, N.; Kharwa, A.; Madory, L.; Kazerani, I.; Herbert, J.; Torres-Gonzalez, J.; Rivera, E.; et al. Alcohol-Drinking Under Limited-Access Procedures During Mature Adulthood Accelerates the Onset of Cognitive Impairment in Mice. Front. Behav. Neurosci. 2022, 16, 732375. [Google Scholar] [CrossRef]
- Jarrard, L.E.; Okaichi, H.; Steward, O.; Goldschmidt, R.B. On the role of hippocampal connections in the performance of place and cue tasks: Comparisons with damage to hippocampus. Behav. Neurosci. 1984, 98, 946–954. [Google Scholar] [CrossRef]
- Bimonte, H.A.; Hyde, L.A.; Hoplight, B.J.; Denenberg, V.H. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol. Behav. 2000, 70, 311–317. [Google Scholar] [CrossRef]
- Szumlinski, K.K.; Lominac, K.D.; Kleschen, M.J.; Oleson, E.B.; Dehoff, M.H.; Schwarz, M.K.; Seeburg, P.H.; Worley, P.F.; Kalivas, P.W. Behavioral and neurochemical phenotyping of Homer1 mutant mice: Possible relevance to schizophrenia. Genes Brain Behav. 2005, 4, 273–288. [Google Scholar] [CrossRef]
- Chiu, A.S.; Kang, M.C.; Huerta Sanchez, L.L.; Fabella, A.M.; Holder, K.N.; Barger, B.D.; Elias, K.N.; Shin, C.B.; Jimenez Chavez, C.L.; Kippin, T.E.; et al. Preclinical evidence to support repurposing everolimus for craving reduction during protracted drug withdrawal. Neuropsychopharmacology 2021, 46, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Huerta Sanchez, L.L.; Sankaran, M.; Li, T.L.; Doan, H.; Chiu, A.; Shulman, E.; Shab, G.; Kippin, T.E.; Szumlinski, K.K. Profiling prefrontal cortex protein expression in rats exhibiting an incubation of cocaine craving following short-access self-administration procedures. Front. Psychiatry 2023, 13, 1031585. [Google Scholar] [CrossRef]
- Szumlinski, K.K.; Herbert, J.N.; Mejia Espinoza, B.; Madory, L.E.; Scudder, S.L. Alcohol-drinking during later life by C57BL/6J mice induces sex- and age-dependent changes in hippocampal and prefrontal cortex expression of glutamate receptors and neuropathology markers. Addict. Neurosci. 2023, 7, 100099. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Guo, C.; Wang, H.; Sun, Y.; Wang, H.; Su, Y.A.; Li, K.; Si, T. Olanzapine Reverses MK-801-Induced Cognitive Deficits and Region-Specific Alterations of NMDA Receptor Subunits. Front. Behav. Neurosci. 2018, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Satow, A.; Maehara, S.; Ise, S.; Hikichi, H.; Fukushima, M.; Suzuki, G.; Kimura, T.; Tanak, T.; Ito, S.; Kawamoto, H.; et al. Pharmacological effects of the metabotropic glutamate receptor 1 antagonist compared with those of the metabotropic glutamate receptor 5 antagonist and metabotropic glutamate receptor 2/3 agonist in rodents: Detailed investigations with a selective allosteric metabotropic glutamate receptor 1 antagonist, FTIDC [4-[1-(2-fluoropyridine-3-yl)-5-methyl-1H-1,2,3-triazol-4-yl]-N-isopropyl-N-methyl-3,6-dihydropyridine-1(2H)-carboxamide]. J. Pharmacol. Exp. Ther. 2008, 326, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, M.; Sukhanov, I.; Maciejak, P.; Szyndler, J.; Gravius, A.; Wisłowska, A.; Płaźnik, A.; Bespalov, A.Y.; Danysz, W. Anxiolytic-like effects of mGlu1 and mGlu5 receptor antagonists in rats. Eur. J. Pharmacol. 2005, 514, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Koltunowska, D.; Gibula-Bruzda, E.; Kotlinska, J.H. The influence of ionotropic and metabotropic glutamate receptor ligands on anxiety-like effect of amphetamine withdrawal in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 242–249. [Google Scholar] [CrossRef]




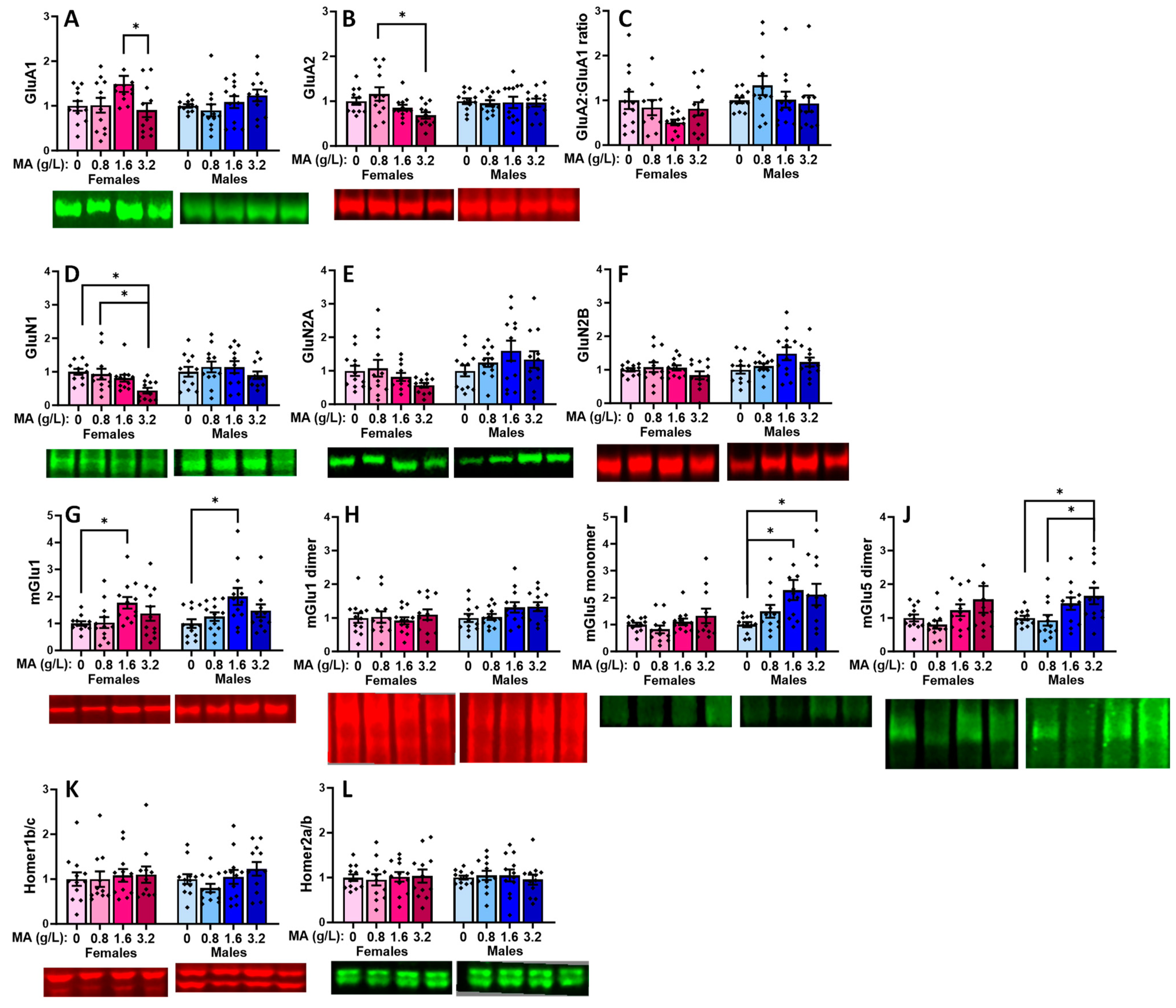



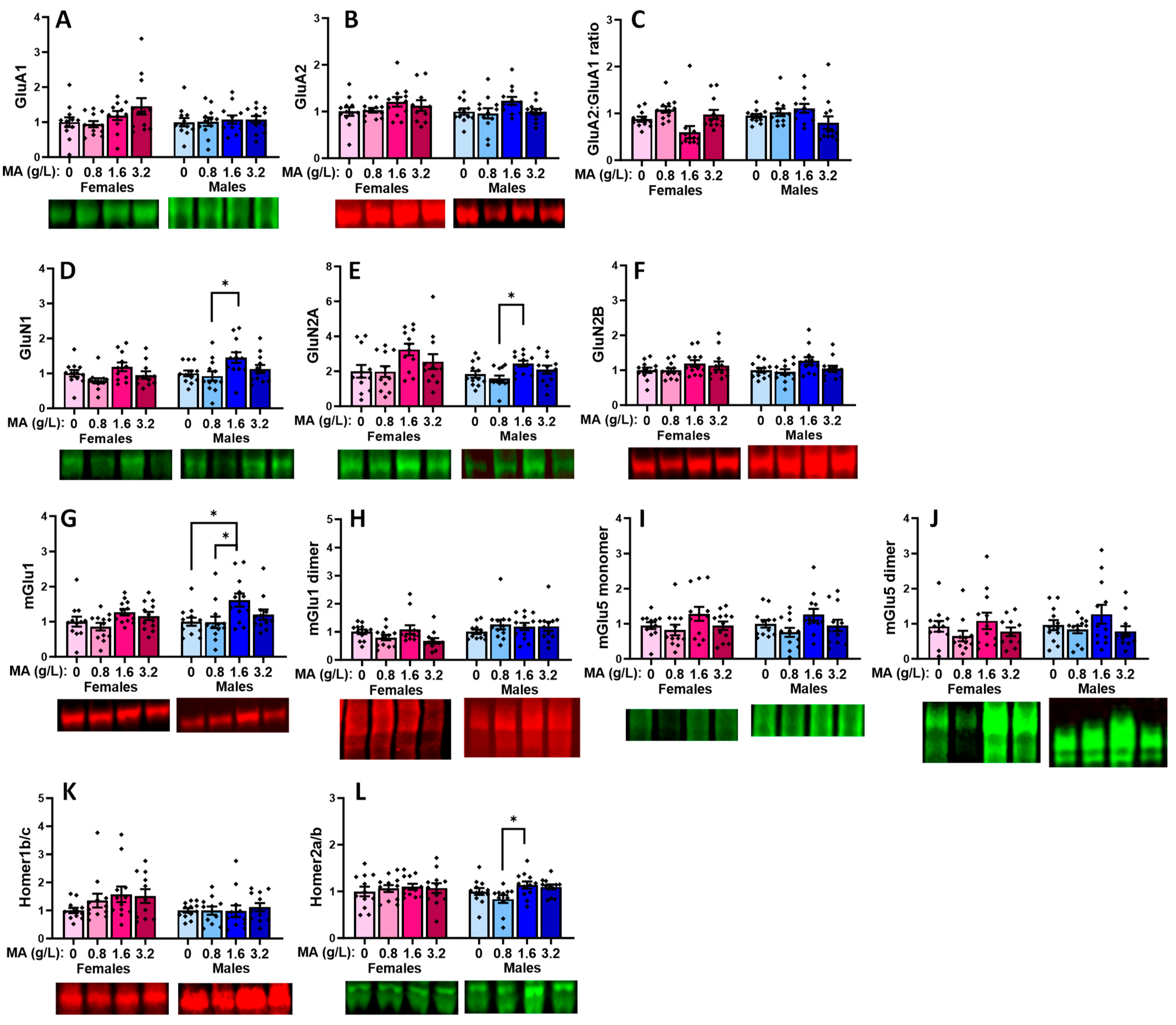

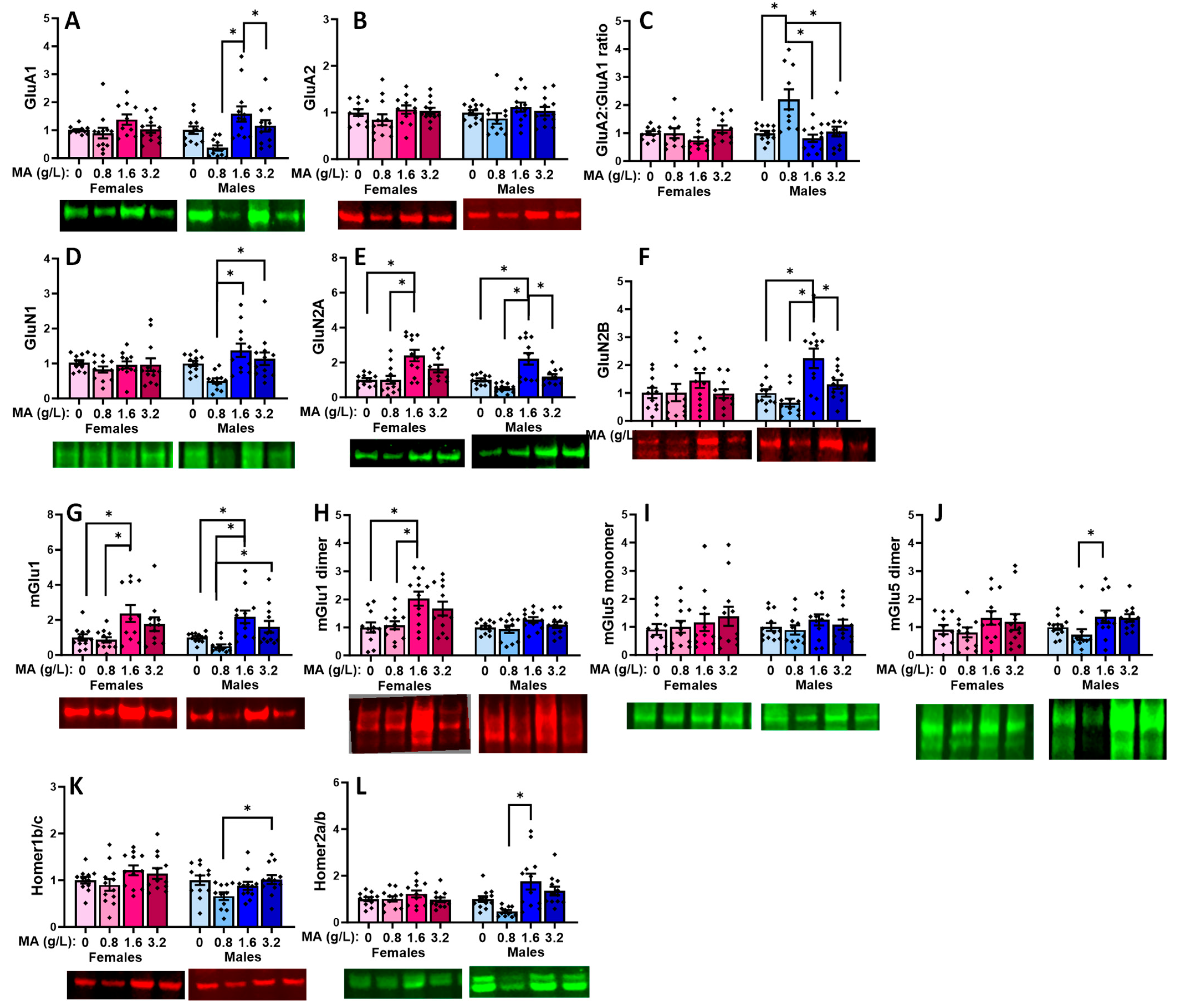
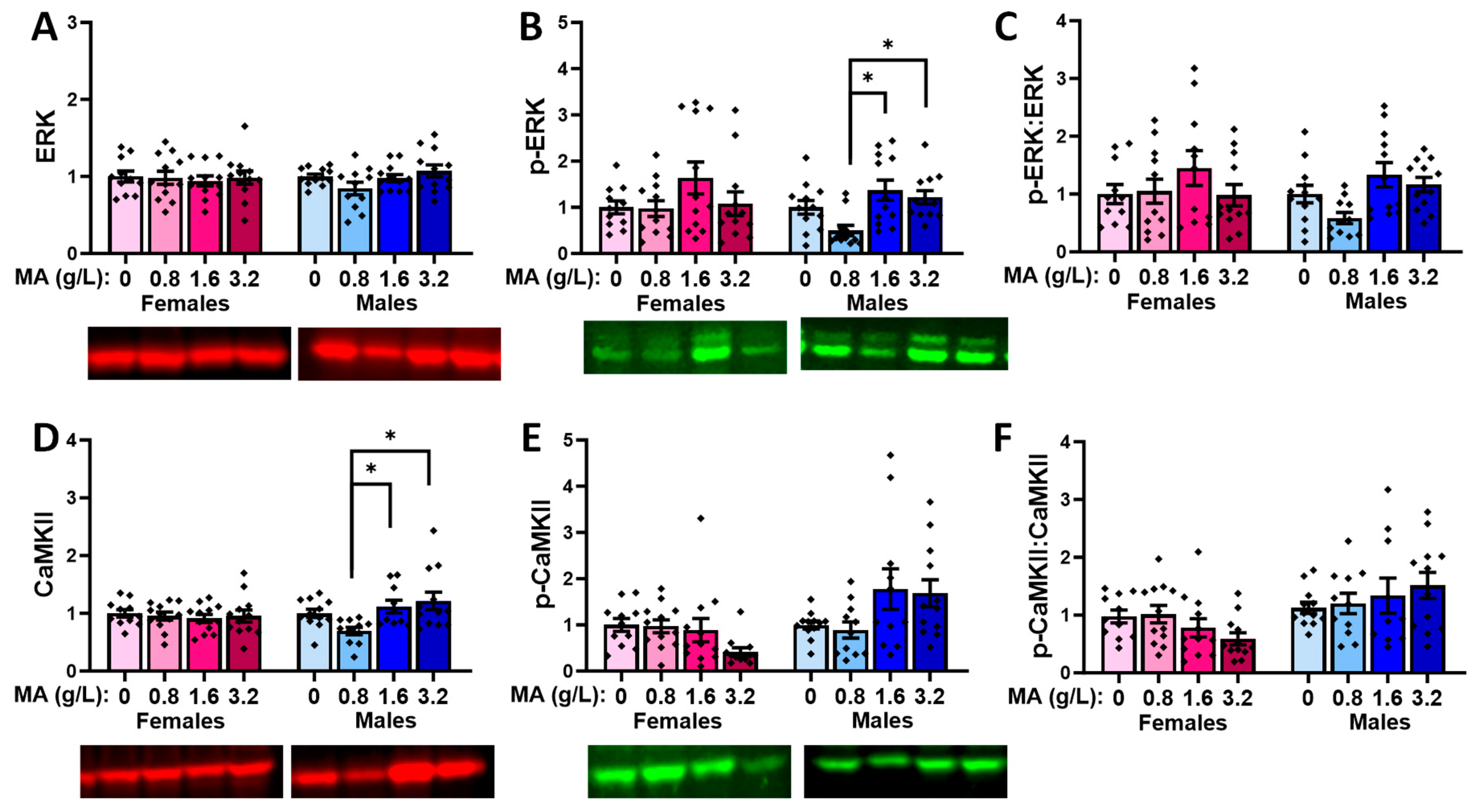

| Brain Region | vPFC | dPFC | Hippocampus | Amygdala |
|---|---|---|---|---|
| GluA1 | 1.6 > 3.2 (F) | 1.6 > 0.8 & 3.2 (M) | ||
| GluA2 | 0.8 > 3.2 (F) | |||
| GluA2:GluA1 ratio | 0, 0.8 & 3.2 < 1.6 (M) | |||
| GluN1 | 0 & 0.8 > 3.2 (F) | 0.8 < 1.6 (M) | 0.8 < 1.6 & 3.2 (M) | |
| GluN2A | 0.8 < 1.6 (M) | 0 & 0.8 < 1.6 (F) 0, 0.8 & 3.2 < 1.6 (M) | ||
| GluN2B | 0, 0.8 & 3.2 < 1.6 (M) | |||
| mGlu1 monomer | 0 < 1.6 | 0 < 1.6 (F) 0 & 0.8 < 1.6 (M) | 0 & 0.8 < 1.6 (M) | 0 & 0.8 < 1.6 (F) 0 & 0.8 < 3.2 (M) 3.2 > 0.8 (M) |
| mGlu1 dimer | 0 & 0.8 < 1.6 (F) | |||
| mGlu5 monomer | 0 < 1.6 & 3.2 (M) | 0 < 1.6 & 3.2 (F) | ||
| mGlu5 dimer | 0 < 3.2 0.8 < 3.2 (M) | 0.8 < 1.6 (M) | ||
| Homer1b/c | 0.8 < 3.2 (M) | |||
| Homer2a/b | 0.8 < 1.6 (M) | 0.8 < 1.6 (M) | ||
| ERK | 0.8 < 1.6 & 3.2 (M) | |||
| p-ERK | 0.8 > 1.6 & 3.2 | 0.8 < 1.6 & 3.2 (M) | ||
| p-ERK:ERK ratio | ||||
| CaMKII | 0.8 < 1.6 & 3.2 (M) | |||
| p-CaMKII | 1.6 < 3.2 (F) 0, 0.8 & 1.6 < 3.2 (M) | 1.6 < 3.2 (F) 0, 0.8 & 1.6 < 3.2 (M) | ||
| p-CaMKII:CaMKII ratio | 0 & 1.6 < 3.2 | 0 & 1.6 < 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denning, C.J.E.; Madory, L.E.; Herbert, J.N.; Cabrera, R.A.; Szumlinski, K.K. Neuropharmacological Evidence Implicating Drug-Induced Glutamate Receptor Dysfunction in Affective and Cognitive Sequelae of Subchronic Methamphetamine Self-Administration in Mice. Int. J. Mol. Sci. 2024, 25, 1928. https://doi.org/10.3390/ijms25031928
Denning CJE, Madory LE, Herbert JN, Cabrera RA, Szumlinski KK. Neuropharmacological Evidence Implicating Drug-Induced Glutamate Receptor Dysfunction in Affective and Cognitive Sequelae of Subchronic Methamphetamine Self-Administration in Mice. International Journal of Molecular Sciences. 2024; 25(3):1928. https://doi.org/10.3390/ijms25031928
Chicago/Turabian StyleDenning, Christopher J. E., Lauren E. Madory, Jessica N. Herbert, Ryan A. Cabrera, and Karen K. Szumlinski. 2024. "Neuropharmacological Evidence Implicating Drug-Induced Glutamate Receptor Dysfunction in Affective and Cognitive Sequelae of Subchronic Methamphetamine Self-Administration in Mice" International Journal of Molecular Sciences 25, no. 3: 1928. https://doi.org/10.3390/ijms25031928





