Mucolytic Drugs Ambroxol and Bromhexine: Transformation under Aqueous Chlorination Conditions
Abstract
:1. Introduction
2. Results and Discussions
2.1. Primary Transformations of Bromhexine
2.2. Primary Transformations of Ambroxol
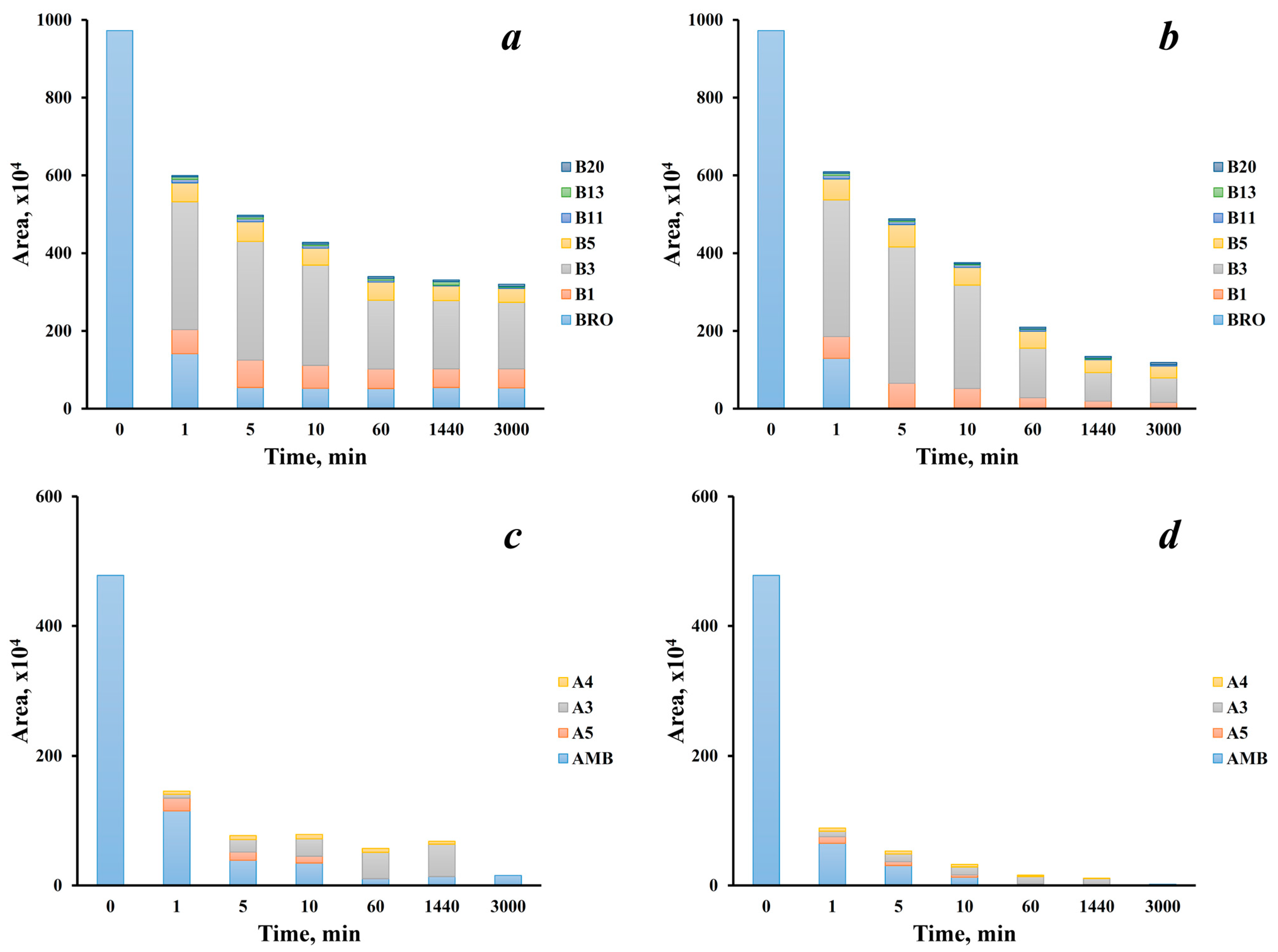
2.3. Transformation Dynamics and Effect of Active Chlorine Dosage
2.4. Deep Degradation Products
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Model Chlorination of Bromhexine and Ambroxol
3.3. Liquid Chromatography–High-Resolution Mass Spectrometry
3.4. Gas Chromatography–High-Resolution Mass Spectrometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conceicao, K.C.; Villamar-Ayala, C.A.; Plaza-Garrido, A.; Toledo-Neira, C. Seasonal behavior of pharmaceuticals and personal care products within Chilean rural WWTPs under COVID-19 pandemic conditions. J. Environ. Chem. Eng. 2023, 11, 110984. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Liu, S.; Han, J.; Li, R.; Men, J.; Li, L.; Wei, L.; Sheng, Y.; Yang, L.; et al. Occurrence and risk assessment of pharmaceuticals and personal care products (PPCPs) against COVID-19 in lakes and WWTP-river-estuary system in Wuhan, China. Sci. Total Environ. 2021, 792, 148352. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, C.; Chiavola, A.; Gioia, V.; Frugis, A.; Cecchini, G.; Ceci, C.; Spizzirri, M.; Boni, M.R. Impact of COVID19 restrictions on organic micropollutants in wastewater treatment plants and human consumption rates. Sci. Total Environ. 2022, 811, 152327. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M. The current recommended drugs and strategies for the treatment of coronavirus disease (COVID-19). Ther. Clin. Risk Manag. 2020, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, I.A.; Egbejimi, A.; Kaur, M.; Onyenaka, C.; Adebusuyi, T.; Olaleye, O.A. Inhibitory mechanism of Ambroxol and Bromhexine Hydrochlorides as potent blockers of molecular interaction between SARS-CoV-2 spike protein and human angiotensin-converting Enzyme-2. J. Mol. Graph. Model. 2022, 114, 108201. [Google Scholar] [CrossRef] [PubMed]
- Instructions for the Medical Use of Ambroxol. Ozon Pharmaceuticals, Russia. Available online: https://ozonpharm.ru/upload/iblock/34e/34e81ef3ed54650b63e44252efdfb515.pdf (accessed on 5 April 2024).
- Instructions for the Medical Use of Bromhexine. Ozon Pharmaceuticals, Russia. Available online: https://ozonpharm.ru/upload/iblock/66a/Inst.-Bromgeksin_-tab.-8mg.pdf (accessed on 5 April 2024).
- Wang, W.; Zhou, Z.; Ding, S.; Yang, W.; Jin, W.; Chu, W.; Xu, Z. Degradation kinetics and formation of regulated and emerging disinfection by-products during chlorination of two expectorants ambroxol and bromhexine. Water Res. 2023, 235, 119927. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Hu, Y.; Cheng, G.; Zhong, D. Quantification of the major metabolites of bromhexine in human plasma using RRLC–MS/MS and its application to pharmacokinetics. J. Pharm. Biomed. Anal. 2010, 51, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, L.; Wang, B.; Liu, C.S.; Jia, Y.; Zhai, N.; Blaney, L.; Yu, G. Efficient multiresidue determination method for 168 pharmaceuticals and metabolites: Optimization and application to raw wastewater, wastewater effluent, and surface water in Beijing, China. Environ. Pollut. 2020, 261, 114113. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.M.; Busch, W.; Krauss, M.; Schulze, T.; Brack, W. Characterization and risk assessment of seasonal and weather dynamics in organic pollutant mixtures from discharge of a separate sewer system. Water Res. 2018, 135, 122–133. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, S.; Huang, W.; Wang, F.; Fang, S.; Ge, R.; Zhang, L.; Du, W.; Fang, F.; Feng, Q.; et al. Current status of hypochlorite technology on the wastewater treatment and sludge disposal: Performance, principals and prospects. Sci. Total Environ. 2022, 803, 150085. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2017, 10, 86. [Google Scholar] [CrossRef]
- Krasner, S.W.; Westerhoff, P.; Chen, B.; Rittmann, B.E.; Amy, G. Occurrence of disinfection byproducts in United States wastewater treatment plant effluents. Environ. Sci. Technol. 2009, 43, 8320–8325. [Google Scholar] [CrossRef] [PubMed]
- Cochran, K.H.; Westerman, D.C.; Montagner, C.C.; Coffin, S.; Diaz, L.; Fryer, B.; Harraka, G.; Xu, E.G.; Huang, Y.; Schlenk, D.; et al. Chlorination of Emerging Contaminants for Application in Potable Wastewater Reuse: Disinfection Byproduct Formation, Estrogen Activity, and Cytotoxicity. Environ. Sci. Technol. 2023, 58, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.A.; Richardson, S.D.; Zhang, X.; Gonsior, M. High-molecular-weight by-products of chlorine disinfection. Nat. Water. 2023, 1, 336–347. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Galdiero, E.; Guida, M.; Luongo, G.; Liguori, R.; Di Fabio, G.; Previtera, L.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of tramadol. Molecules 2019, 24, 693. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J. To regulate or not to regulate? What to do with more toxic disinfection by-products? J. Environ. Chem. Eng. 2020, 8, 103939. [Google Scholar] [CrossRef]
- Sun, X.; Wei, D.; Wang, F.; Yang, F.; Du, Y.; Xiao, H.; Wei, X.; Xiao, A. Formation of nitrogen-containing disinfection by-products during the chloramination treatment of an emerging pollutant. Chemosphere 2024, 353, 141536. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Sypalov, S.A.; Varsegov, I.S.; Shavrina, I.S.; Lebedev, A.T. Antiviral drug Umifenovir (Arbidol) in municipal wastewater during the COVID-19 pandemic: Estimated levels and transformation. Sci. Total Environ. 2022, 805, 150380. [Google Scholar] [CrossRef]
- Hu, C.Y.; Deng, Y.G.; Lin, Y.L.; Hou, Y.Z. Chlorination of bromacil: Kinetics and disinfection by-products. Sep. Purif. Technol. 2019, 212, 913–919. [Google Scholar] [CrossRef]
- Yang, Y.; Komaki, Y.; Kimura, S.Y.; Hu, H.Y.; Wagner, E.D.; Mariñas, B.J.; Plewa, M.J. Toxic impact of bromide and iodide on drinking water disinfected with chlorine or chloramines. Environ. Sci. Technol. 2014, 48, 12362–12369. [Google Scholar] [CrossRef]
- Richardson, S.D. The role of GC-MS and LC-MS in the discovery of drinking water disinfection by-products. J. Environ. Monit. 2002, 4, 1–9. [Google Scholar] [CrossRef]
- Zwiener, C.; Frimmel, F.H. LC-MS analysis in the aquatic environment and in water treatment technology—A critical review: Part II: Applications for emerging contaminants and related pollutants, microorganisms and humic acids. Anal. Bioanal. Chem. 2004, 378, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Y.; Liang, Z.F.; Wang, W.L.; Du, Y.; Hu, H.Y.; Yang, L.L.; Huang, W.C. Non-volatile disinfection byproducts are far more toxic to mammalian cells than volatile byproducts. Water Res. 2020, 183, 116080. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Popov, M.S.; Latkin, T.B.; Lebedev, A.T. Halogenated fatty amides—A brand new class of disinfection by-products. Water Res. 2017, 127, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Varsegov, I.S.; Popov, M.S.; Lebedev, A.T. Identification of novel disinfection byproducts in pool water: Chlorination of the algaecide benzalkonium chloride. Chemosphere 2020, 239, 124801. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Varsegov, I.S.; Sypalov, S.A.; Mazur, D.M.; Kosyakov, D.S.; Lebedev, A.T. Cocamidopropyl betaine—A potential source of nitrogen-containing disinfection by-products in pool water. Environ. Sci. Pollut. Res. 2024, 31, 2314–2326. [Google Scholar] [CrossRef]
- Usman, M.; Kuckelkorn, J.; Kämpfe, A.; Zwiener, C.; Wintgens, T.A.; Linnemann, V. Identification of disinfection by-products (DBP) in thermal water swimming pools applying non-target screening by LC-/GC-HRMS. J. Hazard. Mater. 2023, 449, 130981. [Google Scholar] [CrossRef]
- Richardson, S.D.; Caughran, T.V.; Poiger, T.; Guo, Y.; Crumley, F.G. Application of DNPH derivatization with LC/MS to the identification of polar carbonyl disinfection byproducts in drinking water. Ozone Sci. Eng. 2000, 22, 653–675. [Google Scholar] [CrossRef]
- Wang, W.; Moe, B.; Li, J.; Qian, Y.; Zheng, Q.; Li, X.F. Analytical characterization, occurrence, transformation, and removal of the emerging disinfection byproducts halobenzoquinones in water. Trends Anal. Chem. 2016, 85, 97–110. [Google Scholar] [CrossRef]
- Murakami, J.N.; Zhang, X.; Ye, J.; MacDonald, A.M.; Pérez, J.; Kinniburgh, D.W.; Kimura, S.Y. Formation potential and analysis of 32 regulated and unregulated disinfection by-products: Two new simplified methods. J. Environ. Sci. 2022, 117, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Schraven, E.; Koss, F.W.; Keck, J.; Beisenherz, G. Excretion, isolation and identification of the metabolites of Bisolvon. Eur. J. Pharmacol. 1967, 1, 445–451. [Google Scholar] [CrossRef]
- Lee, P.W.; Aizawa, H.; Gan, L.L.; Prakash, C.; Xhong, D. Handbook of Metabolic Pathways of Xenobiotics, 1st ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2014. [Google Scholar]
- Detenchuk, E.A.; Mazur, D.M.; Latkin, T.B.; Lebedev, A.T. Halogen substitution reactions of halobenzenes during water disinfection. Chemosphere 2020, 295, 133866. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Naiyun, G.A.O.; Yang, D. Bromate ion formation in dark chlorination and ultraviolet/chlorination processes for bromide-containing water. J. Environ. Sci. 2008, 20, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mazur, D.M.; Surmilo, A.S.; Sypalov, S.A.; Varsegov, I.S.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T. N-dealkylation of amines during water disinfection–Revealing a new direction in the formation of disinfection by-products. Chemosphere 2024, 350, 141117. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Chovelon, J.-M.; Ji, Y. Aquatic photolysis of the pharmaceutical ambroxol: The role of 2, 4-dibromoaniline chromophore and heavy atom effect of bromine. Water Res. 2022, 226, 119275. [Google Scholar] [CrossRef]
- Brauer, G. Handbook of Preparative Inorganic Chemistry; Academic Press Inc.: New York, NY, USA, 1963. [Google Scholar]
- White, G.C. Chemistry of chlorination. In Handbook of Chlorination and Alternative Disinfectants, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Sajid, M.; Alkhulaify, E.; Baig, N.; Alhooshani, K. Microextraction techniques as versatile platforms for sample preparation of disinfection byproducts from aqueous media: Advances and challenges. TrAC Trends Anal. Chem. 2023, 171, 117487. [Google Scholar] [CrossRef]



| Compound | Retention Time, min | Elemental Composition | m/z [M + H]+ | Error, ppm | m/z of Major Product Ions (Relative Intensity, %) |
|---|---|---|---|---|---|
| BRO | 10.3 | C14H20Br2N2 | 375.0067 | 0.3 | 261.8868 (100%); 292.8934 (43%); 263.8842 (28%) |
| B1 | 9.8 | C14H18Br2N2 | 372.9910 | −0.9 | 261.8868 (100%); 112.1131 (12%); 182.9677 (8%) |
| B2 | 13.7 | C14H17Br2ClN2 | 406.9525 | 2.0 | 324.8740 (100%); 283.8472 (13%); 293.8312 (7%) |
| B3 | 9.6 | C14H18BrClN2 | 329.0411 | −1.1 | 217.9374 (100%); 112.1135 (13%); 246.9638 (4%) |
| B4 | 9.2 | C14H18Cl2N2 | 285.0911 | −3.4 | 173.9874 (100%); 112.1130 (24%); 203.0139 (4%) |
| B5 | 9.2 | C14H16Br2N2 | 370.9745 | −1.6 | 274.8945 (14%); 328.9291 (8%); 194.9676 (7%) |
| B6 | 8.9 | C14H16Br2N2 | 327.0250 | −2.5 | 230.9441 (19%); 284.9787 (12%); 151.0185 (3%) |
| B7 | 8.2 | C14H17BrN2O | 309.0591 | −1.9 | 226.9814 (100%); 148.0633 (23%); 211.9575 (5%) |
| B8 | 9.1 | C14H17ClN2O | 265.1093 | −3.1 | 183.0322 (100%); 148.0636 (15%); 168.0086 (5%) |
| B9 | 8.7 | C14H16Br2N2O | 386.9694 | −2.1 | 311.9011 (9%); 337.9181 (3%); 110.0974 (3%) |
| B10 | 8.5 | C14H16BrClN2O | 343.0207 | −1.0 | 260.9426 (100%); 182.0239 (33%); 181.0159 (10%) |
| B11 | 6.6 | C14H21BrN2O | 313.0903 | −2.2 | 199.9711 (100%); 114.1287 (15%); 171.9711 (4%) |
| B12 | 6.2 | C14H21ClN2O | 269.1418 | 1.0 | 156.0208 (100%); 114.1283 (50%); 128.0255 (8%) |
| B13 | 10.2 | C13H18Br2N2 | 360.9898 | −3.5 | 261.8860 (100%); 182.9675 (8%); 104.0504 (2%) |
| B14 | 9.8 | C13H18BrClN2 | 317.0405 | −2.7 | 217.9366 (100%); 139.0185 (5%) |
| B15 (AMB) | 7.2 | C13H18Br2N2O | 376.9860 | 1.4 | 261.8874 (100%); 182.9678 (6%) |
| B16 | 6.1 | C7H5Br2N | 261.8849 | −3.2 | 182.9662 (35%); 181.9584 (19%); 155.9565 (15%) |
| B17 | 6.4 | C7H5BrClN | 217.9367 | −0.8 | 104.0512 (30%); 139.0184 (22%); 138.0118 (17%) |
| B18 | 9.2 | C7H5Cl2N | 173.9870 | −0.5 | - * |
| B19 | 6.8 | C8H10Br2N2 | 292.9273 | −3.2 | 261.8863 (100%); 263.8842 (25%); 182.9677 (16%); 194.9674 (10%) |
| B20 | 15.7 | C8H6Br2N2 | 288.8967 | 1.2 | 209.9786 (100%); 208.9712 (70%); 273.8735 (5%) |
| B21 | 9.2 | C8H7BrN2O | 226.9815 | −0.2 | 148.0615 (100%); 147.0552 (65%); 211.9584 (50%) |
| Compound | Retention Time, min | Elemental Composition | m/z [M + H]+ | Error, ppm | m/z of Major Product Ions (Relative Intensity, %) |
|---|---|---|---|---|---|
| AMB | 7.2 | C13H18Br2N2O | 376.9866 | 2.0 | 261.8874 (100%); 182.9678 (6%) |
| A1 | 6.7 | C13H18BrClN2O | 333.0364 | −0.1 | 217.9364 (100%); 230.9931 (1%); 194.9721 (1%) |
| A2 | 3.0 | C13H19BrN2O2 | 315.0701 | 0.4 | 199.9702 (100%); 171.9758 (4%) |
| A3 | 16.1 | C13H14Br2N2O | 372.9553 | 2.0 | 261.8850 (30%); 288.8955 (10%); 194.9669 (6%) |
| A4 | 16.2 | C13H14Br2N2O2 | 388.9495 | 0.6 | 290.8758 (100%); 211.9571 (12%); 370.9368 (1%) |
| A5 | 6.1 | C7H5Br2N | 261.8860 | −0.6 | 182.9670 (30%); 181.9594 (12%); 104.0503 (11%) |
| A6 | 5.5 | C7H5BrClN | 217.9363 | −1.7 | 139.0183 (35%); 104.0503 (19%); 112.0060 (11%) |
| N | Retention Time, s | Elemental Composition | Assumed Compound | % of the Total Peak Area of the Detected DBPs | |
|---|---|---|---|---|---|
| BRO | AMB | ||||
| THMs | |||||
| 1 | 176 | CHCl3 | Trichloromethane | 2.5 | 3.7 |
| 2 | 267 | CHBrCl2 | Bromodichloromethane | 22.6 | 10.7 |
| 3 | 381 | CHBr2Cl | Dibromochloromethane | 54.3 | 64.5 |
| 4 | 495 | CHBr3 | Tribromomethane | 12.0 | 12.4 |
| DHANs | |||||
| 5 | 283 | C2HCl2N | Dichloroacetonitrile | 0.1 | 0.3 |
| 6 | 407 | C2HBrClN | Bromochloroacetonitrile | 0.2 | 1.3 |
| 7 | 527 | C2HBr2N | Dibromoacetonitrile | 0.1 | 0.8 |
| HAEs | |||||
| 8 | 442 | C3H4Cl2O2 | Methyl ester dichloroacetic acid | 0.03 | 0.1 |
| 9 | 539 | C3H4BrClO2 | Methyl ester bromochloroacetic acid | 0.1 | 1.1 |
| 10 | 627 | C3H4Br2O2 | Methyl ester dibromoacetic acid | 0.1 | 2.0 |
| Haloanilines | |||||
| 11 | 1000 | C6H4Cl3N | 2,4,6-trichloroaniline | 2.7 | 0.1 |
| 12 | 1073 | C6H4BrCl2N | 4-Bromo-2,6-dichloroaniline | 0.8 | - |
| 13 | 1077 | C6H4BrCl2N | 2-Bromo-4,6-dichloroaniline | 0.6 | 0.2 |
| 14 | 1144 | C6H4Br2ClN | 2,6-Dibromo-4-chloroaniline | 0.2 | 0.01 |
| 15 | 1148 | C6H4Br2ClN | 2,4-Dibromo-6-chloroaniline | 1.2 | 0.5 |
| 16 | 1713 | C7H4Br2ClNO | 2,4-Dibromo-6-chloro-2-aminobenzaldehyde | 0.02 | 0.1 |
| 17 | 1217 | C6H4Br3N | 2,4,6-tribromoaniline | 0.6 | 0.1 |
| 18 | 1123 | C6H5BrClN | 4-bromo-2-chloroaniline | 0.1 | 0.3 |
| 19 | 1195 | C6H5Br2N | 2,4-dibromoaniline | 1.3 | 0.1 |
| 20 | 1118 | C7H7BrClNO | 3-Bromo-5-chloro-2-methoxyaniline | 0.2 | 0.8 |
| 21 | 1185 | C7H7Br2NO | 2,6-dibromo-4-methoxyaniline | - | 0.5 |
| Halobenzenes | |||||
| 22 | 933 | C6H3Br2Cl | 1,3-dibromo-5-chlorobenzene | 0.04 | 0.1 |
| Haloindazoles | |||||
| 23 | 1318 | C8H6BrClN2 | 5-Bromo-7-chloro-2-methylindazole | 0.1 | 0.1 |
| 24 | 1383 | C8H6Br2N2 | 5,7-Dibromo-2-methylindazole | 0.1 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sypalov, S.A.; Varsegov, I.S.; Ulyanovskii, N.V.; Lebedev, A.T.; Kosyakov, D.S. Mucolytic Drugs Ambroxol and Bromhexine: Transformation under Aqueous Chlorination Conditions. Int. J. Mol. Sci. 2024, 25, 5214. https://doi.org/10.3390/ijms25105214
Sypalov SA, Varsegov IS, Ulyanovskii NV, Lebedev AT, Kosyakov DS. Mucolytic Drugs Ambroxol and Bromhexine: Transformation under Aqueous Chlorination Conditions. International Journal of Molecular Sciences. 2024; 25(10):5214. https://doi.org/10.3390/ijms25105214
Chicago/Turabian StyleSypalov, Sergey A., Ilya S. Varsegov, Nikolay V. Ulyanovskii, Albert T. Lebedev, and Dmitry S. Kosyakov. 2024. "Mucolytic Drugs Ambroxol and Bromhexine: Transformation under Aqueous Chlorination Conditions" International Journal of Molecular Sciences 25, no. 10: 5214. https://doi.org/10.3390/ijms25105214






